Abstract
Water is the natural medium for protein folding, which is also used in all in vitro studies. In the present work, we posed, and answered affirmatively, a question of whether it is possible to fold correctly a typical protein in a nonaqueous solvent. To this end, unfolded and reduced hen egg-white lysozyme was refolded and reoxidized in glycerol containing varying amounts of water. The unfolded/reduced enzyme was found to regain spontaneously substantial catalytic activity even in the nearly anhydrous solvent; for example, the refolding yield in 99% glycerol was still some one-third of that in pure water, and one-half of that was regained even in 99.8% glycerol. The less than full recovery of the enzymatic activity in glycerol is, as in water, because of competing protein aggregation during the refolding. Lysozyme reoxidation in glycerol was successfully mediated by two dissimilar oxidizing systems, and the refolding yield was markedly affected by the pH of the last aqueous solution before the transfer into glycerol. No recovery of the lysozyme activity was observed when the refolding/reoxidation reaction was carried out in the denaturing solvent dimethyl sulfoxide. This study paves the way for a systematic investigation of the solvent effect on protein folding and demonstrates that water is not a unique milieu for this process.
The protein folding problem remains one of the key unresolved issues in biochemistry (1, 2). Specifically, despite massive research efforts and much recent progress, it is still unclear exactly how a disordered polypeptide chain spontaneously folds into a uniquely structured, biologically active protein molecule (3–6).
The surrounding solvent, water, is thought to be involved inextricably in the protein folding process (7–9). Directly testing this concept experimentally would require replacing water as a medium for protein folding with a nonaqueous solvent and determining whether the protein molecule can still fold into its native conformation. This solvent replacement strategy should also yield penetrating insights into the mechanism of protein folding, as it has into the mechanisms of organic reactions (10) and enzymatic specificity (11). In addition, such an approach may shed light on intriguing observations concerning protein folding in vacuo (12–14).
Studies of protein folding in nonaqueous solvents have been prevented by the common knowledge that proteins are insoluble in almost all organic solvents and that those few that do dissolve proteins are strong denaturants, such as dimethyl sulfoxide (DMSO) (15). However, our recent findings have greatly expanded the range of protein-dissolving solvents by revealing the importance of the protein having been lyophilized from aqueous solution of the “right” pH (16, 17). Consequently, it has become possible to address systematically the effect of the solvent on the protein folding process. As a first step toward this end, in the present work we have investigated the refolding/reoxidation of unfolded/reduced hen egg-white lysozyme in glycerol and observed a substantial recovery of the enzymatic activity even in the essentially anhydrous solvent.
MATERIALS AND METHODS
Hen egg-white lysozyme (EC 3.2.1.17; thrice crystallized, dialyzed, and lyophilized), dried Micrococcus lysodeikticus cells, dl-DTT, reduced and oxidized glutathiones (GSH and GSSG, respectively), 2-mercaptoethanol, urea, iodoacetamide, 5,5′-dithio-bis(2-nitrobenzoic acid), and DMSO (at least 99.9% pure) were purchased from Sigma. Sodium selenite was from Aldrich, and glycerol (at least 99.9% pure, 0.02% water content) was from Mallinckrodt. All other chemicals were obtained from commercial suppliers and were, as those above, of analytical grade or purer.
Unfolded and reduced lysozyme was prepared by a slightly modified procedure of Saxena and Wetlaufer (18). The native enzyme was dissolved in 0.1 M Tris⋅HCl aqueous buffer, pH 8.5, containing 8 M urea, 50 mM DTT, and 10 mM EDTA at 20 mg/ml (100 mg/ml when the refolding was examined in 99.8% glycerol and also when the refolding yield was examined as a function of lysozyme concentration). Its concentrations were determined spectrophotometrically at 280 nm, with the extinction coefficients of 2.63 (mg of protein)⋅ml−1⋅cm−1 for native and 2.37 for unfolded lysozyme (19). After a 7-hr incubation at room temperature, the solution was filtered through a 0.22-μm Millipore filter to remove traces of insolubles, and the pH was adjusted to 8.2 (unless stated otherwise).
Refolding of lysozyme was performed in 0.1 M Tris⋅HCl aqueous buffer, pH 8.2, or a mixture of this buffer with an organic solvent. Either the equimolar mixture of GSH and GSSG (6 mM each) (18) or 0.2 mM sodium selenite and 3.6 mM 2-mercaptoethanol under aerobic conditions (20) (no EDTA added) were used as the oxidant. The refolding reaction was started by a 100-fold dilution of the unfolded/reduced lysozyme into the refolding system [a 500-fold dilution was used with glycerol containing 0.2% (vol/vol) water]. After a vigorous 30-s agitation on a Vortex mixer, the solution was incubated at 30°C for 40 hr unless stated otherwise. When the refolding yield was examined as a function of lysozyme concentration, the solution of the unfolded/reduced enzyme was diluted before refolding/reoxidation. In these experiments, to keep concentrations of other components of that mixture the same as in the basic procedure, we raised the initial concentration of DTT to 72 mM.
GSH and GSSG used for enzyme reoxidation in organic solvent systems were lyophilized from the pH 8.2 aqueous solution. In water, the observed refolding yield was the same with or without this lyophilization (when the pH dependence of the refolding yield was studied, both the pH of the Tris buffer was adjusted to the desired value and the GSH/GSSG mixture was lyophilized from that pH).
The final water content in the refolding system included the water brought in with the unfolded/reduced lysozyme sample. For water contents below 3%, direct water determination after refolding was also independently conducted by using Fischer titration (21). The measured values were in a good agreement with the calculated ones.
The S-alkylation of free sulfhydryl groups after refolding was carried out by adding 30 mM iodoacetamide to a glycerol solution of lysozyme, followed by a 20-hr incubation at 30°C. The complete disappearance of the free sulfhydryl groups was verified by Ellman’s titration (22).
Refolding yields were determined by measuring the catalytic activity of the refolded/reoxidized lysozyme either in water or in 99% glycerol. For the former case, the refolded enzyme was diluted 50–300 times into the assay mixture containing a prefiltered 0.16 mg/ml suspension of M. lysodeikticus cells, and a change in absorbance at 450 nm because of the enzymatic cell disintegration was measured at 25°C (18, 23). Organic solvents, urea, DTT, EDTA, and oxidizing agents in the concentrations equal to those introduced with the refolded lysozyme did not affect the aqueous activity measurement.
When activity of the lysozyme refolded in 99% glycerol was determined directly in that solvent, M. lysodeikticus cells were suspended in aqueous 0.1 M phosphate buffer, pH 6.2, and then diluted 100-fold with glycerol. The resultant suspension was mixed 9:1 with a glycerol solution of the refolded enzyme to the final cell concentration of 0.16 mg/ml. Changes in the absorbance at 450 nm were monitored during the subsequent incubation of the sample at 50°C (this elevated temperature was used to lower the viscosity of the glycerol system). A calibration curve was obtained separately with the native lysozyme introduced by a 100-fold dilution of its solution in aqueous 0.1 M Tris buffer, pH 8.2, into glycerol; urea, DTT, EDTA, and the GSH/GSSG mixture were also present in the same concentrations as those introduced with the refolded enzyme. Comparison of the lysozyme activity in water and in glycerol was carried out at 50°C as outlined above, except that the enzyme aqueous solution pH was 6.2 and no components of the reoxidation mixture were present.
Specific activity of lysozyme refolded in 99% glycerol was determined as follows. After the S-alkylation and extensive dialysis against a 50 mM aqueous phosphate buffer, pH 6.2, containing 0.15 M NaCl at 4°C (dialysis membrane tubing with a 3.5-kDa cut-off), the resultant enzyme solution was concentrated by using a Centriprep-3 concentrator (Amicon) with a 3.0-kDa cut-off and analyzed by gel filtration chromatography on a Superose 12 (HR 10/30; Pharmacia Biotech) column with the same buffer as the eluent and a flow rate of 0.3 ml/min. The peak with the retention volume corresponding to that of the native lysozyme was collected, and its enzymatic activity was determined as outlined above.
RESULTS AND DISCUSSION
As a model protein for this study, we selected hen egg-white lysozyme, whose folding has been extensively investigated (18, 23–28). The general experimental methodology we used to examine lysozyme’s folding was a classical one (18): The protein was unfolded and reduced in aqueous solution containing urea and DTT, followed by dilution into a denaturant-free solvent system containing the oxidizing mixture of oxidized and reduced glutathiones; after an incubation, the lysozyme activity was assayed in water and/or in the solvent system itself. Because the unfolded and reduced lysozyme is devoid of enzymatic activity, its recovery (henceforth referred to as the refolding yield) is a quantitative measure of lysozyme’s refolding/reoxidation.
When aqueous solution was used as a medium in the refolding/reoxidation phase of the aforementioned process, the refolding yield was 38 ± 1% (Table 1). This value is in the range of those obtained in the literature under comparable conditions (18, 23, 25, 27, 29). The less than full recovery of the lysozyme activity on refolding/reoxidation is because of the competing reaction of nonspecific aggregation (23, 25, 27, 28).
Table 1.
The enzymatic activity yield upon refolding/reoxidation of hen egg-white lysozyme in various glycerol–water mixtures
| Concentration in the mixture, % (vol/vol)
|
Refolding yield, %† | |
|---|---|---|
| Water* | Glycerol | |
| 100 | 0 | 38 ± 1 |
| 80 | 20 | 61 ± 3‡ |
| 60 | 40 | 61 ± 4‡ |
| 40 | 60 | 42 ± 6 |
| 20 | 80 | 33 ± 2 |
| 10 | 90 | 32 ± 1 |
| 1 | 99 | 12 ± 1 |
| 0.2 | 99.8 | 5.8 ± 0.5 |
Lysozyme (20 mg/ml except for the 99.8% glycerol experiment, where it was 100 mg/ml) was first unfolded and reduced in a buffered aqueous solution (0.1 M Tris⋅HCl, pH 8.5) containing 8 M urea, 50 mM DTT, and 10 mM EDTA. After a 7-hr incubation at room temperature, the pH was adjusted to 8.2, and an aliquot was withdrawn and diluted 100-fold (500-fold for the 99.8% glycerol experiment) with the reoxidation mixture. The latter always contained 6 mM each oxidized and reduced glutathione (both had been lyophilized from a pH 8.2 aqueous solution). Following a 40-hr incubation at 30°C, an aliquot was withdrawn and assayed for the lysozyme activity in water (50 mM phosphate buffer, pH 6.2) containing suspended cells of M. lysodeikticus as a substrate.
Aqueous solution containing 0.1 M Tris⋅HCl, pH 8.2. The low water contents, 1% and 0.2% (vol/vol), were independently confirmed by Fischer titration (see Materials and Methods).
All measurements were done at least in quadruplicate, and the numbers provided in the table are the mean values and the standard deviations from them.
We have previously found lysozyme to be highly soluble in glycerol (and in many other hydrophilic, polar organic solvents) (16). Having chosen this solvent as a replacement for water in the protein refolding reaction, we first examined the refolding yield of lysozyme in various water–glycerol mixtures. The data obtained, presented in Table 1, are striking in that the refolding yield of lysozyme in 90% glycerol is similar to that in water. Moreover, even in 99% glycerol the refolding yield is still nearly one-third of that in the pure aqueous solvent.
Fig. 1 depicts the results of a more detailed examination of the refolding yield as a function of glycerol concentration in the low water, 90–99% range. It can be seen that this dependence is not threshold-like but gradual, suggesting significant lysozyme refolding yields even at water contents below 1%. For technical reasons, clear from the Materials and Methods, it is impossible to carry out the refolding experiments in that concentration range under the same conditions as in Table 1 and Fig. 1. However, under somewhat distinct but still comparable refolding conditions, even in 99.8% glycerol lysozyme was found to regain one-seventh of the catalytic activity compared with water (Table 1). In all subsequent experiments, the refolding/reoxidation of lysozyme was studied in 99% glycerol.
Figure 1.
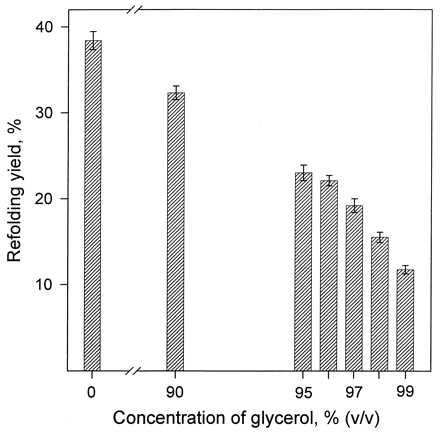
The enzymatic activity yield on refolding/reoxidation of hen egg-white lysozyme in glycerol containing small fractions of water and in the pure aqueous solvent. Lysozyme was unfolded and reduced in aqueous solution, refolded and reoxidized in one of the glycerol–water mixtures depicted by the shaded bars, and finally assayed in aqueous solution against dried cells of M. lysodeikticus. For experimental conditions, see Materials and Methods and the legend to Table 1. At each glycerol concentration, the refolding yield (i.e., the recovery of the lysozyme activity) was independently measured at least in quadruplicate; the heights of the shaded bars correspond to the mean values, and the error bars correspond to the standard deviations in these experiments.
One might wonder whether the refolding/reoxidation of lysozyme in glycerol described above really occurs in that solvent as opposed to afterward, in water, during the assay of the enzymatic activity. We verified this fact in two independent experiments. In the first, the refolding yield was measured as a function of the time of incubation in glycerol, where the time of the subsequent aqueous activity assay was constant. The results obtained, shown in Table 2, reveal that the longer the incubation in glycerol, the higher the refolding yield. In particular, no appreciable recovery of the lysozyme activity was detected after a very short (1-min) incubation in glycerol. In the second experiment, we blocked any putative free thiols in lysozyme after a 40-hr incubation in glycerol, thereby preventing any possibility of the subsequent reoxidation of the protein in water. This was accomplished by adding an S-alkylating agent, iodoacetamide, in a large molar excess with respect to both lysozyme’s half-cystines and the free thiols of the DTT and GSH potentially still present. A titration with Ellman’s reagent (22) confirmed the absence of free thiols after this treatment. Nevertheless, when the treated mixture in glycerol was then assayed in water (where no reoxidation would be feasible now because of the lack of protein SH groups), the enzymatic activity observed was the same as after a 40- or 60-hr incubation in glycerol with no S-alkylation (Table 2). All these data indicate unequivocally that lysozyme indeed correctly refolds/reoxidizes in glycerol.
Table 2.
The dependence of the enzymatic activity yield of hen egg-white lysozyme on the time of refolding/reoxidation in 99% (vol/vol) glycerol
| Time | Refolding yield, %* |
|---|---|
| 1 min | <0.2 |
| 15 min | 0.78 ± 0.35 |
| 30 min | 1.1 ± 0.3 |
| 60 min | 1.6 ± 0.2 |
| 12 hr | 8.4 ± 0.4† |
| 40 hr | 12 ± 1 |
| 60 hr | 12 ± 1 |
For experimental conditions, see the legend to Table 1.
See the corresponding footnote to Table 1, except that all experiments were done at least in triplicate.
Essentially the same refolding yield was obtained when the refolding/reoxidation of lysozyme was carried out at 37°C; however, upon increasing the temperature to 50°C, the refolding yield dropped by a factor of 3.
Inspection of Table 2 shows that a 40-hr incubation in glycerol at 30°C is quite sufficient to attain the maximal refolding yield, 12 ± 1%, under the conditions used. The same value, albeit faster, was reached at 37°C. A question arises as to why this refolding yield is far below 100%. In water, where the situation is similar, the unrecovered enzymatic activity is, as mentioned above, lost to an aggregation of unfolded/reduced lysozyme molecules, which competes with the desired refolding/reoxidation reaction (23, 25, 27, 28). Because the former reaction is intermolecular and the latter intramolecular, lowering protein concentration during the refolding should disfavor the aggregation and thus increase the refolding yield. This has been observed in water both previously (25, 27, 28, 30) and herein (Fig. 2, curve a). It seemed reasonable to assume that the same competing aggregation was responsible for the incomplete recovery of the lysozyme activity during refolding in glycerol. This assumption is confirmed by the data represented by curve b in Fig. 2; it is seen that as the lysozyme concentration is lowered, the refolding yield markedly rises to reach nearly 40% at 0.02 mg/ml lysozyme. Further confirmation comes from the observation that the specific activity of the monomeric FPLC fraction of the lysozyme refolded/reoxidized in glycerol was nearly the same as that of the native enzyme—i.e., no misfolded protein was detected. As to why the refolding yield in glycerol is lower than in water (Fig. 2, curves b and a), it is likely because of a slower reoxidation of lysozyme relative to its aggregation; oxidation of free thiols (like those in the reduced enzyme) should be slower in organic solvents than in water because of their higher pKa values (31, 32).
Figure 2.
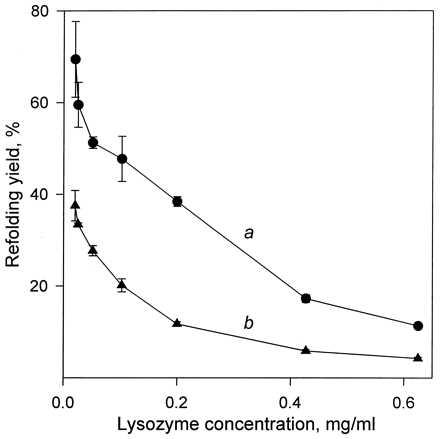
The dependence of the recovery of the lysozyme activity upon refolding/reoxidation on the protein concentration during this reaction in aqueous solution (curve a) and in 99% (vol/vol) glycerol (curve b). For experimental conditions, see Materials and Methods and for those in b also the legend to Table 1. Each data point shown was obtained at least in triplicate, and the mean values were plotted with error bars corresponding to the standard deviations from the mean.
In contrast to water, because there is no pH in the traditional sense in glycerol (33), it was of interest to determine whether the pH of the aqueous solution immediately before the 100-fold dilution with glycerol affects the refolding yield. To this end, we varied that pH from 7.2 to 10.2. The refolding yield was found to be the same for pH 8.2 and 9.2 but dropped 2-fold for pH 10.2 and another 2-fold for pH 7.2. These findings indicate that both reduced lysozyme and other components of the reoxidation mixture in glycerol exhibit a “pH memory” of their last aqueous solution, as do enzymes suspended in organic solvents (34).
To test the generality of the refolding/reoxidation phase, we examined it by using another oxidation system, sodium selenite (Na2SeO3) and 2-mercaptoethanol under aerobic conditions (20). The refolding yield for this system in 99% glycerol turned out to be even higher than in Table 1 under otherwise identical conditions, namely 27 ± 2%. Therefore, the glutathione oxidizing mixture, used by us thus far, is not unique herein.
To ascertain whether any lysozyme-dissolving nonaqueous solvent can act as a medium for the correct refolding/reoxidation of the protein, we replaced glycerol with DMSO. As can be seen in Table 3, in contrast to glycerol (Table 1), even in 70 or 80% DMSO the refolding yield was zero. This is probably because of the propensity of DMSO to denature proteins (15), thus resulting in a random-coiled (instead of a native-like) conformation of lysozyme during refolding and hence a nonnative, scrambled disulfide pairing. In our future studies, we will examine other lysozyme-dissolving solvents as refolding media.
Table 3.
The enzymatic activity yield upon refolding/reoxidation of hen egg-white lysozyme in various mixtures of DMSO and water
In the foregoing experiments, unfolded/reduced lysozyme was refolded/reoxidized in glycerol (or another solvent system) and then diluted at least 50-fold with a buffered aqueous suspension of dried M. lysodeikticus cells to assay for the enzymatic activity. A more straightforward (although difficult) refolding test would involve measuring the enzymatic activity in the reoxidation solvent itself. This, however, would require that native lysozyme (i.e., that which had not undergone the unfolding/reduction-refolding/reoxidation cycle) be catalytically active in glycerol. We conducted the corresponding experiment and found that lysozyme, first dissolved in an aqueous buffer (pH 6.2) and then transferred into glycerol to end up in a suspension of M. lysodeikticus cells in 99% glycerol, was in fact capable of digesting them even in this solvent. Although the lysozyme activity in 99% glycerol was found to be much below (approximately 600 times) that in water, it was sufficient to assay the refolding yield directly in that solvent.
First, we measured the enzymatic activity of the native lysozyme in 99% glycerol containing all the components of the reoxidation mixture in their respective concentrations. Then we determined the enzymatic activity of the reoxidized lysozyme in this system. Comparison of the two showed that the refolded enzyme’s activity was 15 ± 2% of that of the native lysozyme. This refolding yield measured in glycerol is the same within the experimental error as that (Table 1) obtained by assaying the enzyme in water. These data indicate that reduced lysozyme not only reoxidizes into the correctly S—S paired structure in 99% glycerol but also refolds into a catalytically active conformation in this organic solvent. This conclusion was further confirmed by the finding that when lysozyme was unfolded, without reduction, in 8 M aqueous urea and then transferred to 99% glycerol, its catalytic activity immediately reached that of the never unfolded enzyme. (Note that when the reduced and unfolded enzyme was placed in 99% glycerol containing no oxidizing agent, it exhibited no catalytic activity.)
In closing, this work demonstrates the basic feasibility of studying how the solvent affects the protein folding process not merely by varying the composition of the aqueous solution but by replacing it with various organic media. In addition, our findings show that although water is the natural milieu for protein folding, it is not truly indispensable or unique, because at least one common and typical protein, lysozyme, correctly refolds in a nonaqueous solvent.
Acknowledgments
We are grateful to the U.S. Department of Energy and the Biotechnology Process Engineering Center at Massachusetts Institute of Technology for financial support.
ABBREVIATIONS
- DMSO
dimethyl sulfoxide
- GSH
reduced glutathione
- GSSG
oxidized glutathione
References
- 1.Gierasch L M, King J, editors. Protein Folding. Washington, DC: Am. Assoc. Adv. Sci.; 1990. [Google Scholar]
- 2.Creighton T E, editor. Protein Folding. New York: Freeman; 1992. [Google Scholar]
- 3.Kim P S, Baldwin R L. Annu Rev Biochem. 1990;59:631–660. doi: 10.1146/annurev.bi.59.070190.003215. [DOI] [PubMed] [Google Scholar]
- 4.Nall B K, Dill K A, editors. Conformations and Forces in Protein Folding. Washington, DC: Am. Assoc. Adv. Sci.; 1991. [Google Scholar]
- 5.Merz K M Jr, LeGrand S M, editors. The Protein Folding Problem and Tertiary Structure Prediction. Boston: Birkhauser; 1994. [Google Scholar]
- 6.Dill K A, Bromberg S, Yue K Z, Fiebig K M, Yee D P, Thomas P D, Chan H S. Protein Sci. 1995;4:561–602. doi: 10.1002/pro.5560040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rupley J A, Careri G. Adv Protein Chem. 1991;41:37–172. doi: 10.1016/s0065-3233(08)60197-7. [DOI] [PubMed] [Google Scholar]
- 8.Englander S W. Science. 1993;262:848–849. doi: 10.1126/science.8235606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maddox J. Nature (London) 1994;370:13. doi: 10.1038/370013a0. [DOI] [PubMed] [Google Scholar]
- 10.Reichardt C. Solvents and Solvent Effects in Organic Chemistry. Weinheim, Germany: VCH; 1988. [Google Scholar]
- 11.Wescott C R, Klibanov A M. Biochim Biophys Acta. 1994;1206:1–9. doi: 10.1016/0167-4838(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 12.Wood T D, Chorush R A, Wampler F M, Little D P, O’Connor P B, McLafferty F W. Proc Natl Acad Sci USA. 1995;92:2451–2454. doi: 10.1073/pnas.92.7.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolynes P G. Proc Natl Acad Sci USA. 1995;92:2451–2454. doi: 10.1073/pnas.92.7.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shelimov K B, Clemmer D E, Hudgins R R, Jarrold M F. J Am Chem Soc. 1997;119:2240–2248. [Google Scholar]
- 15.Singer S J. Adv Protein Chem. 1962;17:1–68. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]
- 16.Chin J T, Wheeler S L, Klibanov A M. Biotechnol Bioeng. 1994;44:140–145. doi: 10.1002/bit.260440120. [DOI] [PubMed] [Google Scholar]
- 17.Bromberg L E, Klibanov A M. Proc Natl Acad Sci USA. 1995;92:1262–1266. doi: 10.1073/pnas.92.5.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxena V P, Wetlaufer D B. Biochemistry. 1970;9:5015–5022. doi: 10.1021/bi00827a028. [DOI] [PubMed] [Google Scholar]
- 19.Wetlaufer D B, Johnson E R, Clauss L M. In: Lysozyme. Osserman E F, Canfield R E, Beychok S, editors. New York: Academic; 1974. pp. 269–280. [Google Scholar]
- 20.Dillard C J, Tappel A L. J Inorg Biochem. 1986;28:13–20. doi: 10.1016/0162-0134(86)80019-8. [DOI] [PubMed] [Google Scholar]
- 21.Laitinen H A, Harris W E. Chemical Analysis. 2nd Ed. New York: McGraw-Hill; 1975. pp. 361–363. [Google Scholar]
- 22.Ellman G L. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Rozema D, Gellman S H. Biochemistry. 1996;35:15760–15771. doi: 10.1021/bi961638j. [DOI] [PubMed] [Google Scholar]
- 24.Epstein C J, Goldberger R F. J Biol Chem. 1963;238:1380–1383. [PubMed] [Google Scholar]
- 25.Goldberg M E, Rudolph R, Jaenicke R. Biochemistry. 1991;30:2790–2797. doi: 10.1021/bi00225a008. [DOI] [PubMed] [Google Scholar]
- 26.Dobson C M, Evans P M, Radford C V. Trends Biochem Sci. 1994;19:31–37. doi: 10.1016/0968-0004(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 27.Raman B, Ramakrishna T, Rao C M. J Biol Chem. 1996;271:17067–17072. doi: 10.1074/jbc.271.29.17067. [DOI] [PubMed] [Google Scholar]
- 28.Hevehan D L, De Bernardez Clark E. Biotechnol Bioeng. 1997;54:221–230. doi: 10.1002/(SICI)1097-0290(19970505)54:3<221::AID-BIT3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Fischer B, Sumner I, Goodenough P. Arch Biochem Biophys. 1993;306:183–187. doi: 10.1006/abbi.1993.1498. [DOI] [PubMed] [Google Scholar]
- 30.Maeda Y, Koga H, Yamada H, Ueda T, Imoto T. Protein Eng. 1995;8:201–205. doi: 10.1093/protein/8.2.201. [DOI] [PubMed] [Google Scholar]
- 31.Szajewski R P, Whitesides G M. J Am Chem Soc. 1980;102:2011–2026. [Google Scholar]
- 32.Snyder G H. J Biol Chem. 1984;259:7468–7472. [PubMed] [Google Scholar]
- 33.Bates R G. Determination of pH. New York: Wiley; 1964. [Google Scholar]
- 34.Klibanov A M. Trends Biochem Sci. 1989;14:141–144. doi: 10.1016/0968-0004(89)90146-1. [DOI] [PubMed] [Google Scholar]
- 35.Sawano H, Koumoto Y, Ohta K, Sasaki Y, Segawa S, Tachibana H. FEBS Lett. 1992;303:11–14. doi: 10.1016/0014-5793(92)80466-t. [DOI] [PubMed] [Google Scholar]
- 36.Maeda Y, Yamada H, Ueda T, Imoto T. Protein Eng. 1996;9:461–465. doi: 10.1093/protein/9.5.461. [DOI] [PubMed] [Google Scholar]


