Abstract
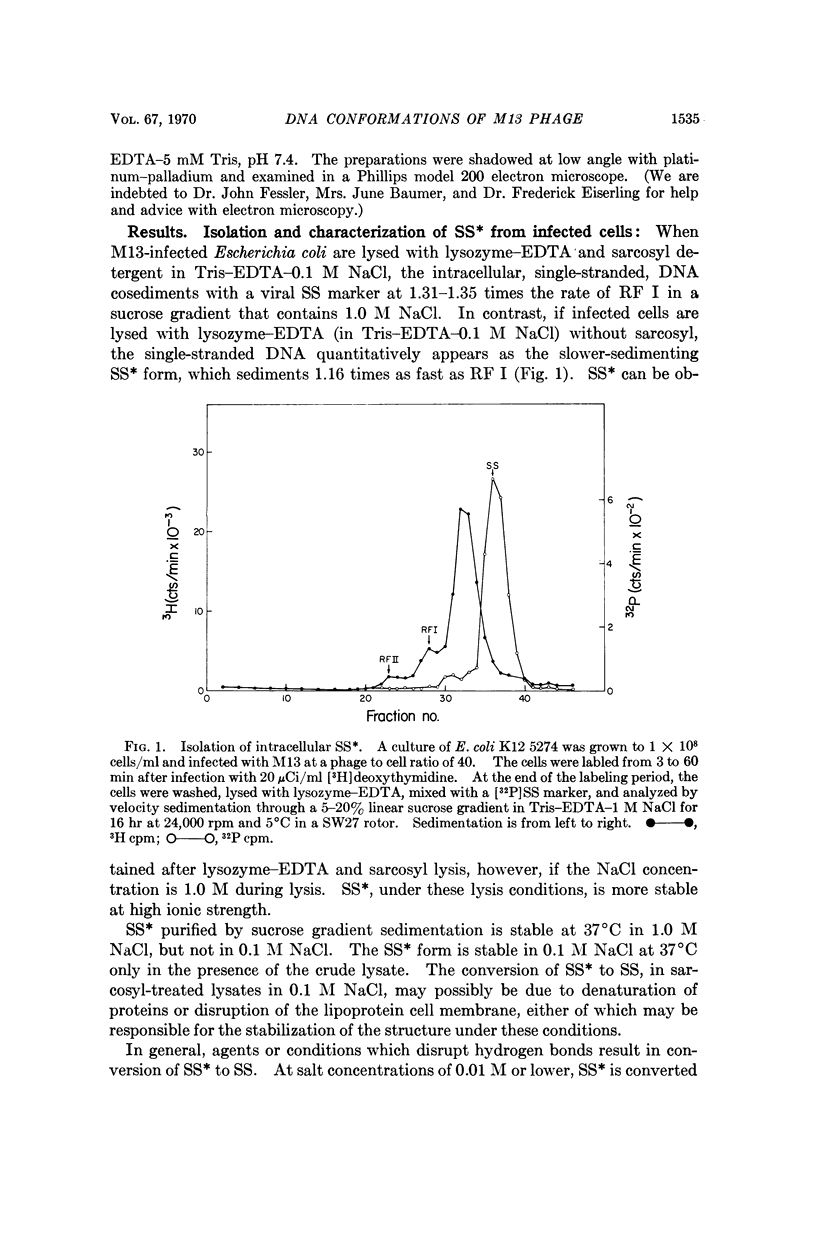
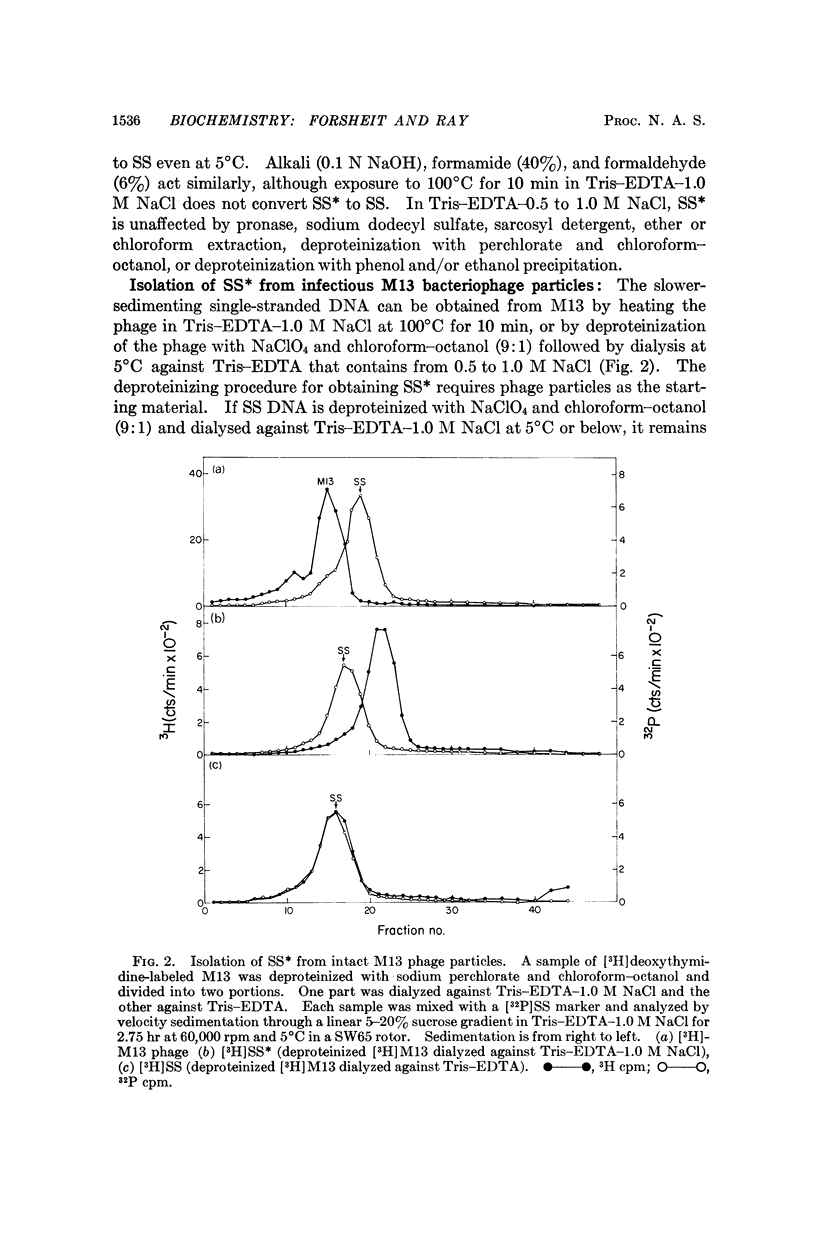
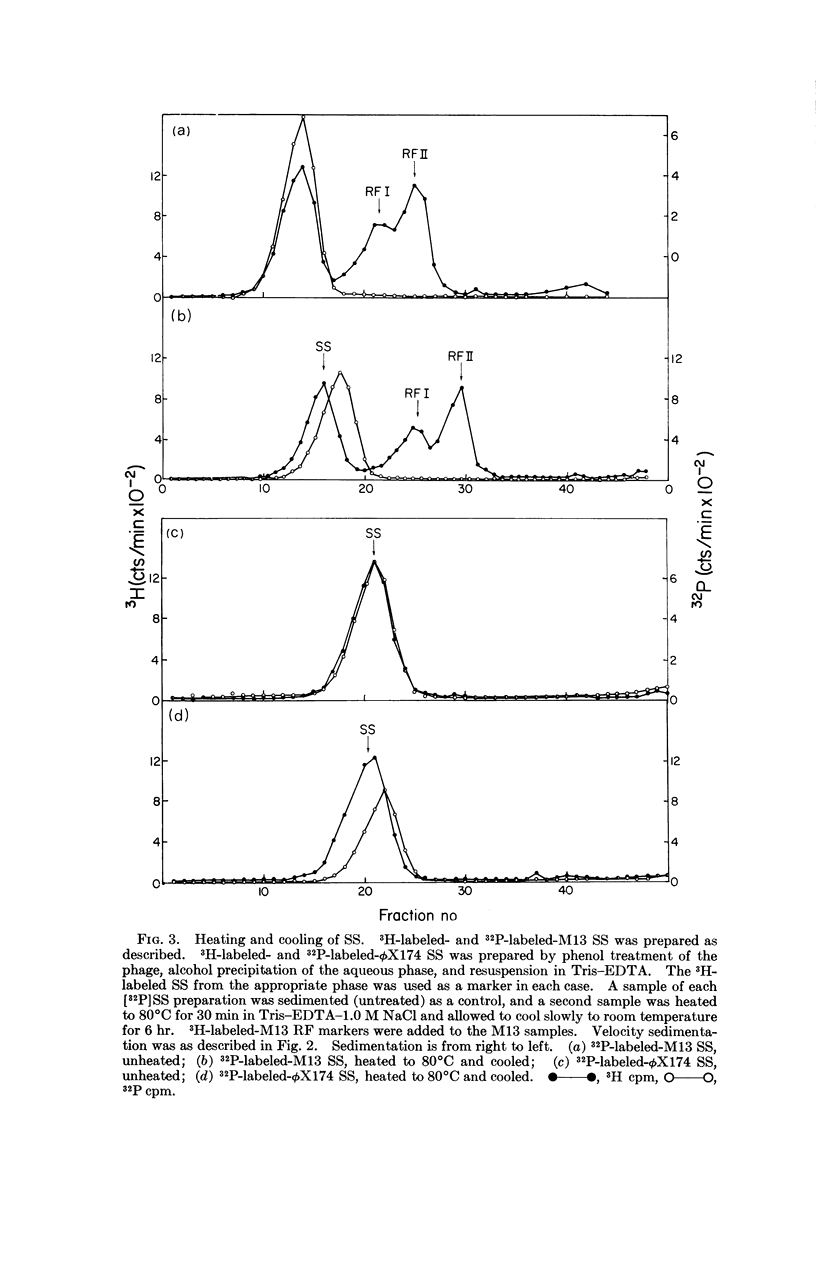
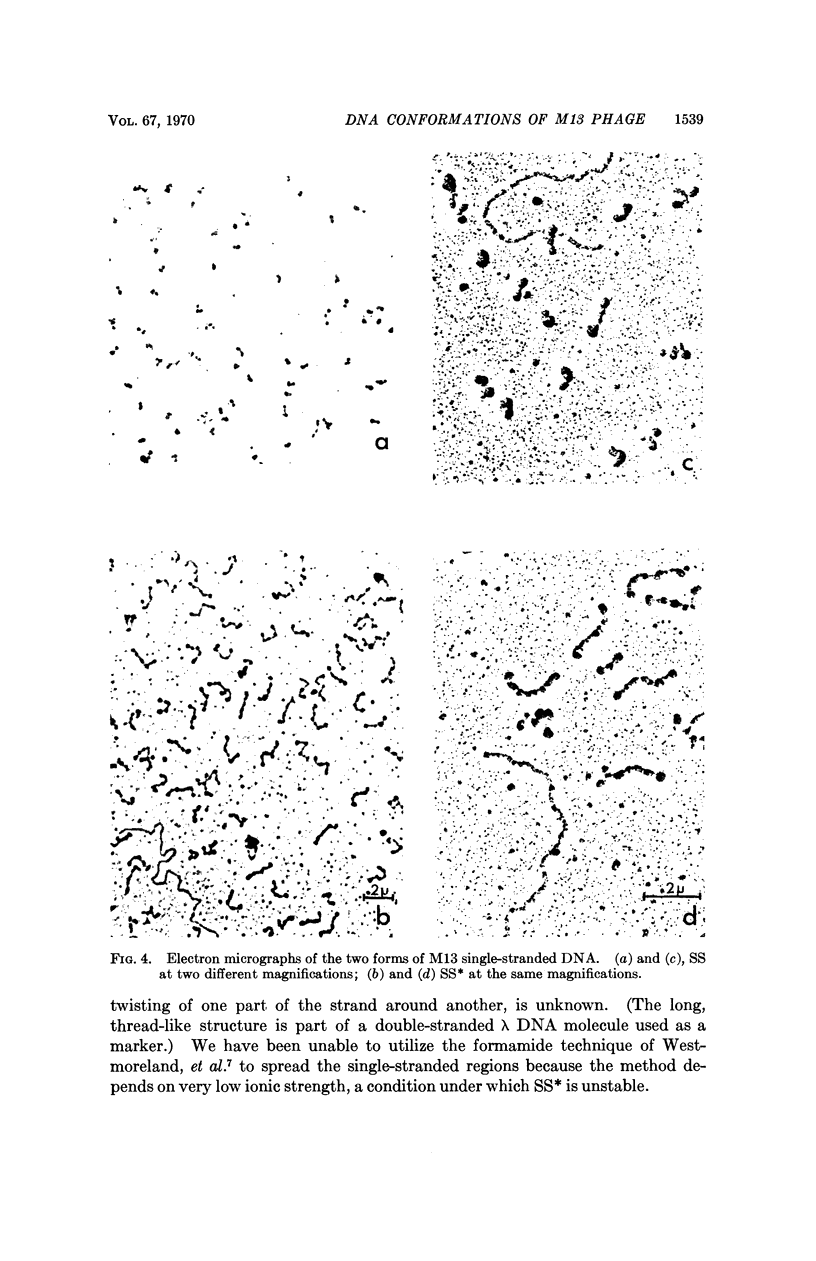
At least two conformations of M13 single-stranded DNA have been demonstrated by measuring differences in sedimentation coefficient and by direct visualization in the electron microscope. Which form is obtained from infected cells and/or intact phage depends on the pH, ionic strength, and temperature. The slower-sedimenting form can be converted to the faster-sedimenting, single-stranded form by low ionic strength, alkali treatment, formamide, or formaldehyde, but not by exposure to 100°C in 1.0 M NaCl. The ability to assume either conformation appears to be a function of the nucleic acid alone. Whether or not these different conformations are of biological significance is still unknown.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. S., Schekman R. W. Replication of bacteriophage M13. 3. Identification of the intracellular single-straned DNA. J Mol Biol. 1969 Aug 14;43(3):645–647. doi: 10.1016/0022-2836(69)90365-9. [DOI] [PubMed] [Google Scholar]
- Ray D. S., Schekman R. W. Replication of bacteriophage M13. I. Sedimentation analysis of crude lysates of M13-infected bacteria. Biochim Biophys Acta. 1969 Apr 22;179(2):398–407. [PubMed] [Google Scholar]
- SALIVAR W. O., TZAGOLOFF H., PRATT D. SOME PHYSICAL-CHEMICAL AND BIOLOGICAL PROPERTIES OF THE ROD-SHAPED COLIPHAGE M13. Virology. 1964 Nov;24:359–371. doi: 10.1016/0042-6822(64)90173-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schaller H., Voss H., Gucker S. Structure of the DNA of bacteriophage fd. II. Isolation and characterization of a DNA fraction with double strand-like properties. J Mol Biol. 1969 Sep 28;44(3):445–458. doi: 10.1016/0022-2836(69)90372-6. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Sinsheimer R. L. Initial kinetics of degradation of MS2 ribonucleic acid by ribonuclease, heat and alkali and the presence of configurational restraints in this ribonucleic acid. J Mol Biol. 1968 Jun 28;34(3):453–465. doi: 10.1016/0022-2836(68)90172-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Effects of the conformation of single-stranded DNA on renaturation and aggregation. J Mol Biol. 1969 Apr;41(2):199–209. doi: 10.1016/0022-2836(69)90385-4. [DOI] [PubMed] [Google Scholar]
- Westmoreland B. C., Szybalski W., Ris H. Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science. 1969 Mar 21;163(3873):1343–1348. doi: 10.1126/science.163.3873.1343. [DOI] [PubMed] [Google Scholar]




