Abstract
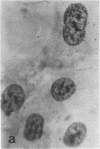
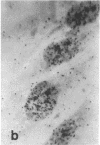
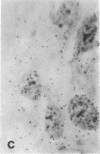
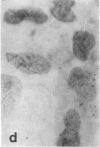
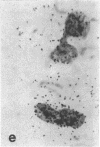
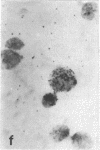
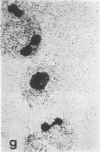
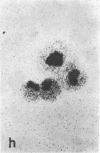
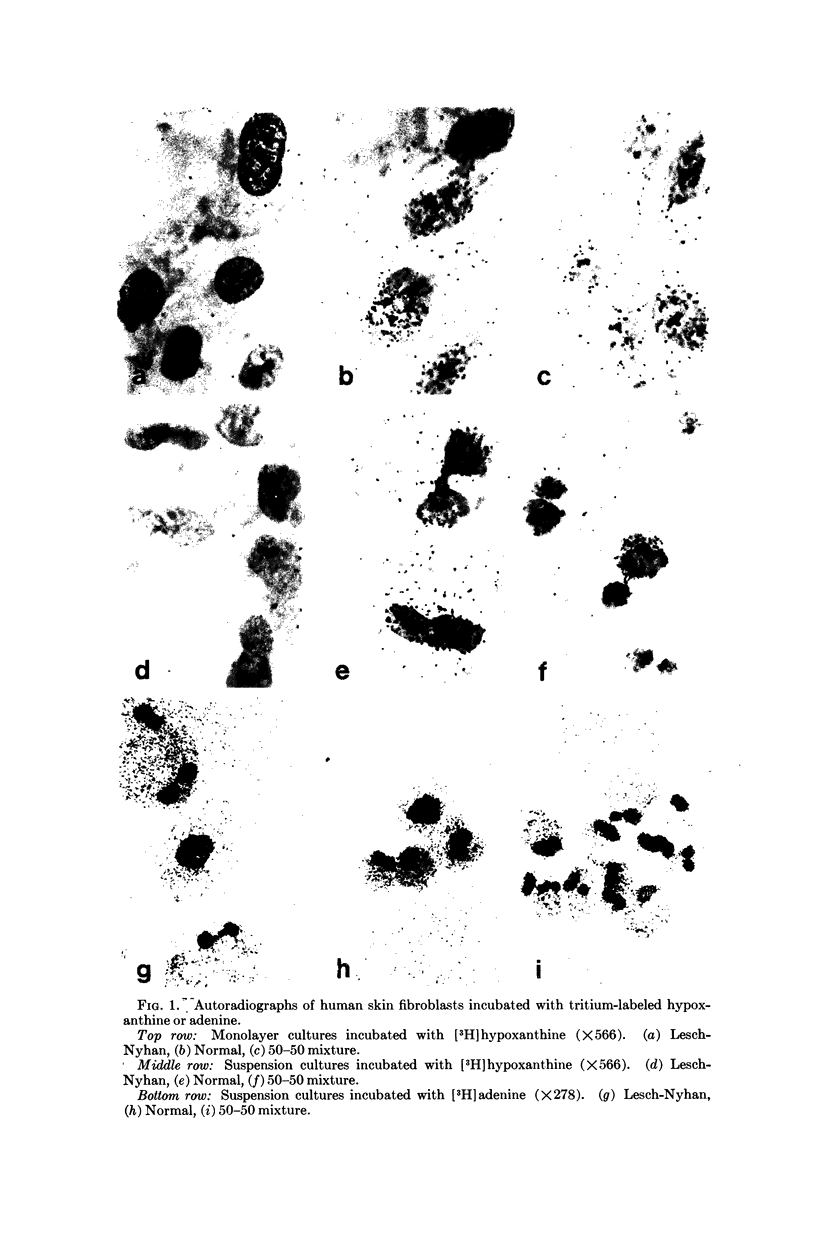
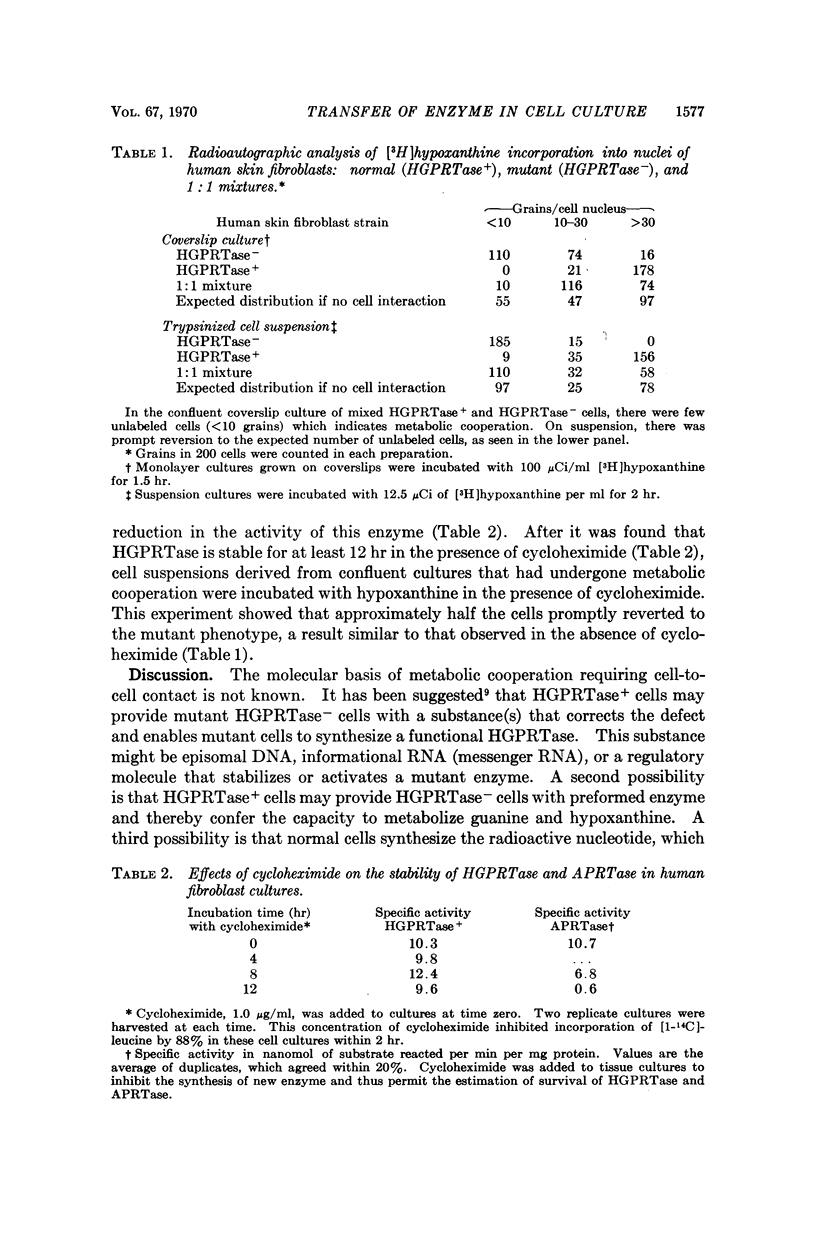
Tissue culture fibroblasts derived from patients with Lesch-Nyhan disease (congenital hyperuricosuria) have a reduced IMP:pyrophosphate phosphoribosyltransferase (EC 2.4.2.8) activity and therefore incorporate, as detected by radioautography, much smaller amounts of tritiated hypoxanthine or guanine into cell nuclei and cytoplasm than do normal cells. However, Lesch-Nyhan cells grown in close contact with normal fibroblasts incorporate these purines. This phenomenon, which requires cell to cell contact for correction of the mutant phenotype, has been called metabolic cooperation. After separation of Lesch-Nyhan cells from normal cells, there is a prompt reversion to the mutant phenotype although the transferase is stable under these conditions for many hours.
These results are most compatible with the transfer from normal to mutant fibroblasts of the product of the normal enzyme, a nucleotide or a nucleotide derivative, rather than the transfer of the transferase or informational macromolecules leading to the synthesis of the enzyme. Metabolic cooperation may provide a mechanism for maintaining normal cell function in the heterozygote in vivo. Evidence has been presented previously that selection of normal cells, presumably during embryogenesis, also provides a means for achieving normal function in the heterozygote.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman P. H., Balis M. E., Dancis J. A method for the prenatal diagnosis of congenital hyperuricemia. J Pediatr. 1969 Sep;75(3):488–491. doi: 10.1016/s0022-3476(69)80279-9. [DOI] [PubMed] [Google Scholar]
- Berman P. H., Balis M. E., Dancis J. Diagnostic test for congenital hyperuricemia with central nervous system dysfunction. J Lab Clin Med. 1968 Feb;71(2):247–253. [PubMed] [Google Scholar]
- Bürk R. R., Pitts J. D., Subak-Sharpe J. H. Exchange between hamster cells in culture. Exp Cell Res. 1968 Oct;53(1):297–301. doi: 10.1016/0014-4827(68)90380-7. [DOI] [PubMed] [Google Scholar]
- COX R. P., MACLEOD C. M. Alkaline phosphatase content and the effects of prednisolone on mammalian cells in culture. J Gen Physiol. 1962 Jan;45:439–485. doi: 10.1085/jgp.45.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis J., Berman P. H., Jansen V., Balis M. E. Absence of mosaicism in the lymphocyte in X-linked congenital hyperuricosuria. Life Sci. 1968 Jun 15;7(12):587–591. doi: 10.1016/0024-3205(68)90079-9. [DOI] [PubMed] [Google Scholar]
- Dancis J., Cox R. P., Berman P. H., Jansen V., Balis M. E. Cell population density and phenotypic expression of tissue culture fibroblasts from heterozygotes of Lesch-Nyhan's disease (inosinate pyrophosphorylase deficiency). Biochem Genet. 1969 Dec;3(6):609–615. doi: 10.1007/BF00485483. [DOI] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. Hurler and Hunter syndromes: mutual correction of the defect in cultured fibroblasts. Science. 1968 Nov 1;162(3853):570–572. doi: 10.1126/science.162.3853.570. [DOI] [PubMed] [Google Scholar]
- Frost P., Weinstein G. D., Nyhan W. L. Diagnosis of Lesch-Nyhan syndrome by direct study of skin specimens. JAMA. 1970 Apr 13;212(2):316–318. [PubMed] [Google Scholar]
- Fujimoto W. Y., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency: activity in normal, mutant, and heterozygote-cultured human skin fibroblasts. Proc Natl Acad Sci U S A. 1970 Mar;65(3):577–584. doi: 10.1073/pnas.65.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 1967 Apr 28;156(3774):525–528. doi: 10.1126/science.156.3774.525. [DOI] [PubMed] [Google Scholar]
- LEIBMAN K. C., HEIDELBERGER C. The metabolism of P32-labeled ribonucleotides in tissue slices and cell suspensions. J Biol Chem. 1955 Oct;216(2):823–830. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Migeon B. R., Der Kaloustian V. M., Nyhan W. L., Yough W. J., Childs B. X-linked hypoxanthine-guanine phosphoribosyl transferase deficiency: heterozygote has two clonal populations. Science. 1968 Apr 26;160(3826):425–427. doi: 10.1126/science.160.3826.425. [DOI] [PubMed] [Google Scholar]
- Neufeld E. F., Fratantoni J. C. Inborn errors of mucopolysaccharide metabolism. Science. 1970 Jul 10;169(3941):141–146. doi: 10.1126/science.169.3941.141. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Balis M. E., Piomelli S., Berman P. H., Dancis J. Elevated AMP pyrophosphorylase activity in congenital IMP pyrophosphorylase deficiencey (Lesch-Nyhan disease). J Lab Clin Med. 1969 Nov;74(5):732–741. [PubMed] [Google Scholar]
- SUBAK-SHARPE H. BIOCHEMICALLY MARKED VARIANTS OF THE SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Exp Cell Res. 1965 Apr;38:106–119. doi: 10.1016/0014-4827(65)90432-5. [DOI] [PubMed] [Google Scholar]
- Salzmann J., DeMars R., Benke P. Single-allele expression at an X-linked hyperuricemia locus in heterozygous human cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):545–552. doi: 10.1073/pnas.60.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe H., Bürk R. R., Pitts J. D. Metabolic co-operation between biochemically marked mammalian cells in tissue culture. J Cell Sci. 1969 Mar;4(2):353–367. doi: 10.1242/jcs.4.2.353. [DOI] [PubMed] [Google Scholar]