Abstract
Manufacturers of vaccines and other biologicals are under increasing pressure from regulatory agencies to develop production methods that are completely animal-component-free. In order to comply with this demand, alternative cell culture substrates to those now on the market, primarily collagen or gelatin, must be found. In this paper, we have tested a number of possible substitutes including recombinant collagen, a 100-kDa recombinant gelatin fragment and a peptide derived from a cell-binding region of type I collagen. The small 15-amino acid peptide did not support attachment of human fibroblasts in monolayer culture. The 100-kDa gelatin fragment supported cell attachment in monolayer culture, but was significantly less active than intact porcine gelatin. Recombinant type I collagen was as successful in promoting cell attachment as native collagen, and both were more effective than porcine gelatin. Based on these data, dextran microspheres were treated with the same attachment proteins—porcine gelatin, native collagen, or recombinant collagen. The same trends were observed as in monolayer culture. Concentrations of the recombinant collagen (as well as native collagen) supported cell attachment on dextran microspheres at concentrations as low as 0.01 μg/cm2. Treatment of the dextran with a low level of polyethylenimine, a cationic moiety, further enhanced attachment when used in conjunction with the low concentration of recombinant collagen. Where there was increased cell attachment, increased proliferation followed. We are confident, based on these findings, that a fully recombinant substitute could replace gelatin in current microcarrier preparations without losing the cell growth benefits provided by the native protein.
Keywords: Microcarrier, Animal component-free, Recombinant collagen, Dextran, PEI
Introduction
Microcarriers in current use for the large-scale cultivation of anchorage-dependent mammalian cells have one of two types of surface coatings—either cationic moieties such as diethylaminoethylamine (DEAE; Van Wezel 1967) and trimethylamine (TMA; Varani et al. 1998) or extracellular matrix molecules such as native collagen and gelatin (Varani et al. 1989). Cells attach efficiently through electrostatic interactions to microcarriers with surface DEAE or TMA groups. If the surface charge is too high, though, subsequent cell growth is retarded and, eventually, the substrate is toxic to the cells. Initial cell attachment to collagen or gelatin, primarily through cell surface integrin receptors (Geiger et al. 2001), is slower than to charged surfaces. However, once integrin receptors are engaged, intracellular signaling events stimulate cytoskeletal reorganization and cell proliferation (Takai et al. 2006). When the substrate surface does not contain collagen, gelatin, or some other matrix component, serum attachment factors (primarily fibronectin or vitronectin; Evans et al. 2004; van Wachem et al. 1985) can bind to the microcarrier surface and promote cell attachment as well as integrin binding and activation. Obviously, this source of attachment factors is obviated when serum-free media formulations are utilized. Alternatively, if the cells are able to attach through electrostatic charge, then they can secrete matrix components and utilize these to promote integrin-mediated binding. Unfortunately, the high electrostatic charge necessary for efficient cell attachment to microcarriers in the high shear–stress environment of a bioreactor is inhibitory to matrix production (Varani et al. 1989). Manufacturers of vaccines and other biologicals are under increasing pressure to implement animal-product-free production methods (Directive 86/609/EEC in the European Union; Convention ETS 123 of the Council of Europe; Letter to Manufacturers of Biological Products: Recommendations Regarding Bovine Spongiform Encephalopathy. FDA, 2000 Update 2002). Completely synthetic media are now available to support long-term growth of many of the cells used in bio-manufacturing. When all animal products have been removed from the culture medium, the use of a substrate containing native collagen or gelatin is not acceptable. With this in mind, the present study was carried out to assess the capacity of recombinant collagen and gelatin as well as synthetic collagen-derived peptides to replace their native counterparts as microcarrier surface coatings and to support cell attachment and growth on microcarriers.
Materials and Methods
The following materials were used in the preparation of the coated substrates:
Recombinant human collagen type I (rhCollagen; FibroGen Inc; South San Francisco, CA), supplied as 3.33 mg/ml in water.
Recombinant 100-kDa human gelatin (rhGelatin; Fibro-Gen Inc.) supplied as a lyophilized powder and reconstituted as 5 mg/ml in 0.01 N glacial acetic acid (AcOH; Fisher Scientific; Fairlawn, NJ).
Rat tail type I collagen (BD Bioscience, Bedford, MA) supplied as 8.4 mg/ml in 0.02 N AcOH.
Porcine gelatin (Sigma; type A, G1890) dissolved in 0.01 N AcOH at 50°C for 30 min and then filter-sterilized.
A 15-amino acid synthetic peptide (NH2-Gly-Thr-Pro-Gly-Pro-Gln-Gly-Ile-Ala-Gly-Gln-Arg-Gly-Val-Val-COOH) derived from a cell-binding region of type I collagen (Bhatnagar and Qian 1992; Scaria et al. 1989). In addition, two other modified versions of this peptide were tested. The first was synthesized with five additional lysine residues at the C terminus in order to increase the cationic charge and electrostatic adhesion. The second had two added valine residues at the C terminus to increase hydrophobicity. Peptides were synthesized at the University of Michigan Protein Structure Facility (Ann Arbor, MI). All were prepared as 10 mg/ml in water.
Bovine serum albumin (BSA; Sigma 8806) prepared as a 1% solution in Dulbecco’s phosphate-buffered saline (DPBS; Gibco, Grand Island, NY) and filter-sterilized.
Polyethylenimine (PEI; no. 181978; Sigma-Aldrich Inc., St. Louis, MO) with an average molecular weight of 750,000 by LS, prepared as 10 mg/ml solution in water.
Glutaraldehyde solution (Electron Microscopy Sciences, Hatfield, PA).
Protein treatment of polystyrene surface for monolayer culture
A 24-well non-tissue culture-treated plate (BD Biosciences, Bedford, MA) was coated with the proteins and peptides outlined above in 0.5 ml of 0.01 N acetic acid. Deposition was accomplished by leaving the lid off the plate overnight in a laminar flow hood. The wells were then treated with 5 mg/cm2 BSA in 0.5 ml/well Hanks balanced salt solution (HBSS; Gibco) for 30 min and washed once in HBSS.
Dextran microsphere preparation and treatment
One gram of G-100 Sephadex (Pharmacia Biotech, Uppsala, Sweden) was swelled overnight in 100 ml sterile water at room temperature and then washed three times to remove small particles. Protein solutions were prepared and added to microspheres in a 15-ml sterile polypropylene tubes (Corning Inc., Corning, NY) in 7 ml of 0.01 N AcOH and rotated overnight at room temperature on a Dynal Rotator (Dynal Inc., New Hyde Park, NY). Protein solutions with PEI were treated in the same way, although microspheres coated with PEI alone were treated in water. The microspheres were allowed to settle, the protein solution removed, and then glutaraldehyde cross-linked by adding 7 ml of a 0.9% glutaraldehyde solution in 89% DPBS:11% water. PEI-only-treated microspheres were fixed in 0.9% glutaraldehyde in water. The microspheres were rotated overnight, washed ten times with sterile water, again rotated in 7 ml water overnight, and further washed five times with water.
Cells
Low passage human dermal fibroblasts obtained from neonatal foreskin were used in this study. The cells were grown in monolayer culture using Dulbecco’s modified minimal essential medium (Gibco) supplemented with nonessential amino acids and 10% fetal bovine serum as culture medium (Hyclone Laboratories, Logan, UT). Fibroblasts were maintained and experiments were performed at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were subcultured by exposure to 0.05% trypsin/0.53 mM ethylenediaminetetraacetic acid (trypsin–EDTA; Gibco) as needed. Cell attachment studies were conducted using animal-component-free media, a modified version of keratinocyte growth media (mKGM; Lonza, Walkersville, MD). The KGM additives, amphotericin and pituitary extract, were not added. The media was supplemented with 50 μg/ml gentamycin sulfate (Gibco) and to a final calcium concentration of 1.55 mM (calcium chloride; Sigma-Aldrich). For studies, typsin-detached cells were trypsin-inactivated with trypsin neutralizing solution (Lonza), filtered through a 40-μm cell strainer (BD Biosciences), and centrifuged for 7 min at 400×g. The fibroblast were then resuspended in mKGM and added to the protein-treated microspheres or treated polystyrene wells.
Cell attachment to microspheres or treated polystyrene wells
To ascertain the potential efficacy of various protein treatments, in addition to testing another substrate, cell attachment studies were conducted on treated polystyrene wells. Cells prepared for treated polystyrene wells were added at 2×104 cells/well in 0.5 ml mKGM supplemented with 10 mM HEPES (Gibco).
The treated microspheres (10 cm2 per well) were prepared for cell attachment by first gently rinsing in keratinocyte basal medium (KBM; Lonza). Then, they were transferred to a six-well plate (non-tissue culture treated; Becton Dickinson Labware, Franklin Lakes, NJ) in 2.2 ml m-KGM. To inhibit cell or bead attachment to the plate, the plates were previously coated with poly 2-hydroxyethyl methacrylate (Sigma-Aldrich) in 95% ethyl alcohol and dried overnight. The plates were rinsed once in KBM before use. Fibroblasts were prepared as before and added to the wells containing microspheres at 3×105 cells/well in a total volume of 2.5 ml mKGM. The cultures were gently swirled every 30 min for 2 h, and the next day, the media was changed.
Assessment of cell attachment and growth on coated polystyrene wells and dextran microspheres
At various time points, cells on polystyrene wells or dextran microspheres were visually assessed to estimate percent attached and spread. To count cells attached to microspheres, at 18 or 72 h, the media were removed and the microspheres washed in DPBS (calcium/magnesium-free). At 18 h, nearly all microspheres that had cells were joined with other microspheres through cell-based adhesions. These cell-coated microspheres were separated from the non-attached cells (single cells or cell clumps) by collection with a 100-μm cell strainer (BD Bioscience). The small microspheres that were lost through the filter had no or very few cells attached. Cells were subsequently separated from the microspheres by treating them overnight in cold trypsin–EDTA, filtering through a 40-μm cell strainer and counting on Z2 Coulter particle counter (Beckman Coulter, Fullerton, CA).
Confocal fluorescence microscopy
Monolayer cultures were washed and then fixed with 4% formaldehyde for 20 min. After fixation, the samples were washed two times with wash buffer (0.05% Tween-20 in DPBS) followed by permeabilization with 0.1% Triton X-100 for 10 min. The samples were again washed and then exposed to a blocking solution consisting of 1% BSA in DPBS for 30 min. Next, they were treated with a monoclonal antibody to vinculin in blocking solution for 1 h. After three subsequent washing steps with DPBS (5 min each), cells were treated with Alexa Fluor 488-conjugated secondary antibody (Invitrogen, Carlsbad, CA) in blocking solution and incubated for 45 min. Samples were concomitantly stained for actin expression by incubation with Alexa Fluor 546-phalloidin simultaneously with the secondary antibody. Following three additional washing steps, the cells were rinsed once with wash buffer and coverslips mounted with Prolong Gold Anti-fade medium containing 4′,6-diamidino-2-phenylindole (Invitrogen). Cells were examined with a Zeiss LSM 510 confocal microscope using a 63× (C-Apochr) NA=1.2 water immersion objective lens. Laser excitation wavelengths included 364, 488, and 543 nm scanned in sequence by the line method.
Scanning electron microscopy
Microspheres with attached cells were fixed in 4% Sorenson’s buffered glutaraldehyde. Post-fixation in 1% osmium tetroxide buffered in s-collidine was followed by en bloc staining with uranyl acetate. Dehydration was in a graded series of ethanol, followed by critical point drying from absolute ethanol through liquid carbon dioxide. Specimens were then mounted on stubs and conductive-coated with gold in a dc sputter coater. Following this, specimens were examined using an ISI Super IIIA scanning electron microscope. Scanning electron microscopy services were provided on a fee-for-service basis through the Morphology Core Laboratory in the Department of Cellular and Developmental Biology at the University of Michigan.
Results
Cell attachment to recombinant collagen, recombinant 100 kDa fragment, and collagen-derived peptides in monolayer culture
In the first series of experiments, wells of a 24-well plastic culture dish were coated with varying concentrations (0.01–10 μg/cm2) of recombinant collagen or the recombinant 100 kDa gelatin fragment. The synthetic peptides (15-AA, 17-AA, and 20-AA) were coated onto wells in parallel. Native rat tail collagen and porcine skin gelatin served as positive controls. Following treatment with the adhesion proteins, the wells were exposed to 5 mg/cm2 BSA to block cell attachment to areas of exposed polystyrene and to act as a negative control. Human dermal fibroblasts were added to the wells in serum-free, protein-free culture medium and assessed for attachment and spreading. The middle panel of Fig. 1 demonstrates the time course for attachment and spreading on wells coated with 0.1 μg of each protein or peptide per square centimeter of surface area. The lower panel presents dose–response data with each moiety. Firstly, we observed that recombinant collagen and native collagen were indistinguishable in their ability to support fibroblast attachment and spreading (in terms of both dose–response and time course). Fibroblast attachment and spreading on either substrate occurred more rapidly and to a greater extent than it did on porcine-derived gelatin, which is the surface coating in the most commonly used microcarriers on the market today. Native gelatin was, in turn, a “better” substrate than the 100-kDa recombinant gelatin fragment. The 15-AA synthetic peptide, and the modified versions with additional valine or lysine residues, did not support attachment or spreading under the conditions used.
Figure 1.
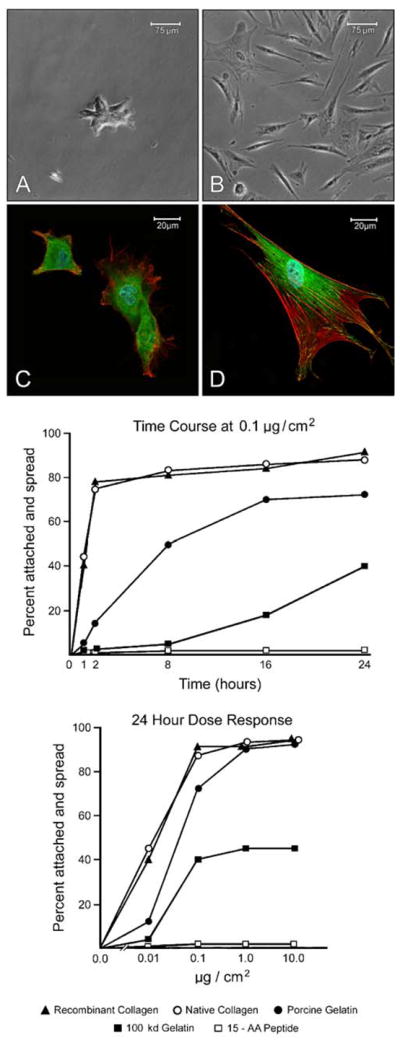
Attachment and spreading of human dermal fibroblasts on coated polystyrene culture dishes. Lower graph: Wells of a 24-well polystyrene dish were coated with varying concentrations of each moiety. Human dermal fibroblasts were added at time zero. At 24 h, cell attachment and spreading was assessed. Upper graph: Using one concentration of the moieties (0.1 μg/cm2), percentage of cells that were attached and spread at each time point was assessed. Values shown are means based on duplicate samples in a single experiment. The experiment was carried out two times with similar results. Photographic inserts: A) Phase-contrast micrograph showing a small aggregate of partially spread fibroblasts on BSA-coated polystyrene plastic. Magnification, 93× B) Phase-contrast micrograph showing well-spread and spindle-shaped fibroblasts on a polystyrene plastic coated with 0.1 μg/cm2 of recombinant collagen (18 h). C) Confocal microscopy showing cells on BSA-coated polystyrene plastic. There are few focal adhesions and no detectable actin stress fibers. Magnification, 300×. D) Confocal microscopy showing a cell on recombinant collagen-coated polystyrene plastic. Actin stress fibers (red fluorescence) are evident. These radiate from focal adhesions (punctate yellow green fluorescence) at the cell boundary.
The photographic inserts (upper panel of Fig. 1) show the appearance of dermal fibroblasts on recombinant collagen in comparison to the same cells on BSA-coated control wells after 18-h incubation. At the phase-contrast microscopic level, it can be seen that only a few cells have attached to the surface coated with BSA (A). These cells are tightly packed together and not fully spread. In contrast, cells that are attached to recombinant collagen have developed a flattened shape or spindle-shaped appearance (B). By confocal fluorescence microscopy, cells on recombinant collagen (D) can be seen to have well-developed focal adhesions as indicated by punctate staining for vinculin (green fluorescence). Actin stress fibers (red fluorescence) radiate from the focal adhesions throughout the cell. In contrast, cells on BSA demonstrate neither well-developed focal adhesions nor actin stress fibers (C).
Fibroblast attachment to and proliferation on recombinant collagen-coated dextran microspheres
Based on the results from the monolayer culture experiments, dextran microspheres (150- to 250-μm diameter) were coated with recombinant collagen at concentrations ranging from 0.01 to 10 μg/cm2 of surface area. Binding of the recombinant protein to the microspheres was carried out as described in “Materials and Methods”. Native collagen and gelatin served as controls as did BSA alone. Dermal fibroblasts were then added to the spheres (3×104 cells/cm2). The percentage of cells that were attached to each substrate was estimated at 18 h. Essentially, no cells were attached to uncoated or BSA-coated dextran spheres at any concentration. Likewise, few cells attached to spheres coated with native gelatin at 0.01 μg/cm2. With coatings at 0.1 μg/cm2, approximately 1×104 cells/cm2 were attached to the porcine gelatin-coated microspheres at 18 h and 2×104 cells/cm2 were attached to spheres coated with either recombinant collagen or native collagen. At the higher concentrations (1 and 10 μg/cm2), all proteins were equally active, with approximately two thirds of the cells attached at 18 h (2×104 cells/cm2; not shown).
Cells were allowed to grow until day 3 in the serum-free, protein-free medium, at which time they were harvested and counted. As seen in Fig. 2, total cell yields of 4×104 cells/cm2 were achieved on both recombinant collagen and its native counterpart. One cell doubling during the 2-d period is consistent with results on plastic tissue culture flasks under the same conditions. A maximal response was observed on both substrates at concentrations as low as 0.1 μg/cm2 of surface area. The number of cells recovered from collagen-coated spheres at 0.01 μg/cm2 was 3.5×104 cells/cm2 (a reduction in growth of approximately 15%), while the porcine gelatin-coated spheres supported minimal growth. Again, there was no difference between the two collagen preparations. The photographic insert to Fig. 2 shows phase-contrast and scanning electron micrographic images of dermal fibroblasts on BSA-coated and recombinant collagen-coated dextran spheres (day 3). Attached and spread cells can be seen on the collagen-coated spheres, while the surface of the BSA-coated spheres is barren of cells. Arrows in panel A point toward aggregates of cells which form under conditions in which there is no suitable substratum to which the cells can attach.
Figure 2.
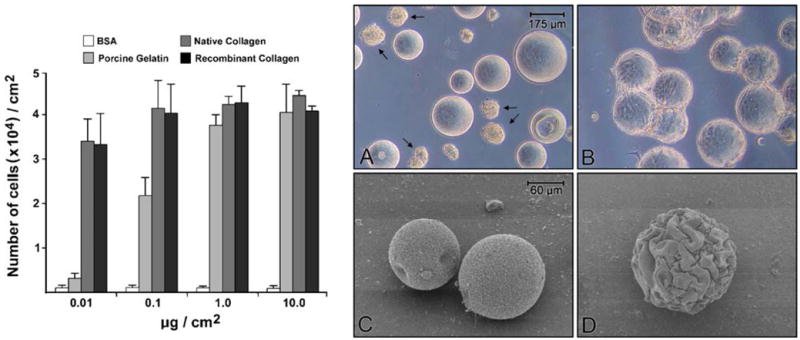
Human dermal fibroblast growth on coated dextran microspheres. Dextran microspheres were coated with varying concentrations of each moiety as described in “Materials and Methods”. Human dermal fibroblasts (3×104 per cm2) were added at time zero. After 3 d, cells were harvested and counted. Values shown are means and standard deviation based on duplicate experiments with duplicate samples per data point. Photographic inserts: Phase-contrast (A and B; magnification, 43×) and scanning electron microscopic (C and D; magnification 133×) appearance of dermal fibroblasts on coated dextran microspheres on day 3. Virtually no attached cells are seen by either photographic technique with BSA-coated dextran spheres (A and C). Dextran microspheres coated with recombinant collagen (0.1 μg/cm2) are covered with cells. Arrows in A show clumps of cells that are still present in the culture after three days of incubation. Virtually all of the cells in such clumps are non-viable.
Enhancement of fibroblast attachment to recombinant collagen-coated dextran microspheres by PEI
The data presented in Fig. 2 demonstrate that a concentration of collagen (either recombinant or native) as low as 0.01 μg/cm2 of surface area is sufficient to support fibroblast attachment to dextran spheres and to support proliferation of the attached cells. However, at this low collagen concentration, initial attachment and overall growth were reduced as compared to what was observed at higher collagen concentrations. Initial cell attachment to the substrate is one of the critical variables in determining the outcome in microcarrier culture, particularly in large-scale bioreactors where shear–stress is high (Bueno et al. 2007). Because of this, experiments were carried out to determine if a cationic moiety could be coupled to the dextran spheres along with a low concentration of recombinant collagen in order to enhance attachment. Cationic moieties are well known to enhance cell substrate attachment. However, high concentrations of the same agents inhibit cell proliferation and are ultimately toxic (Fischer et al. 1999; Ahlemeyer et al. 2000; Neu et al. 2005; Hunter 2006). The key therefore is to identify a concentration of an appropriate cationic moiety that is sufficiently high to boost initial attachment but not so high as to be growth-inhibitory. Figure 3 summarizes results from experiments in which different concentrations of PEI were examined in conjunction with the low concentration of recombinant collagen (0.01 μg/cm2). Several things are worth noting. First, at the higher concentrations of PEI (0.1 and 0.5 μg/cm2), in conjunction with the recombinant collagen, initial attachment was stimulated over that seen with recombinant collagen alone. However, both concentrations proved to be inhibitory to cell growth (under serum-free conditions), and the number of cells recovered from the microspheres at 18 h had fallen to near zero (open and closed squares in the middle panel of the figure). When the level of PEI was reduced to 0.01 μg/cm2, the moiety alone (closed triangles) was too weak to provide meaningful cell substrate attachment. However, when this low concentration of PEI was used together with 0.01 μg/cm2 of recombinant collagen, cell attachment occurred more rapidly than it did to spheres coated only with recombinant collagen and was sustainable to promote cell proliferation. This was evident at 18 h, with an increased cell yield (lower panel of Fig. 3). At day 3, maximal growth was comparable to that achieved on recombinant collagen alone.
Figure 3.
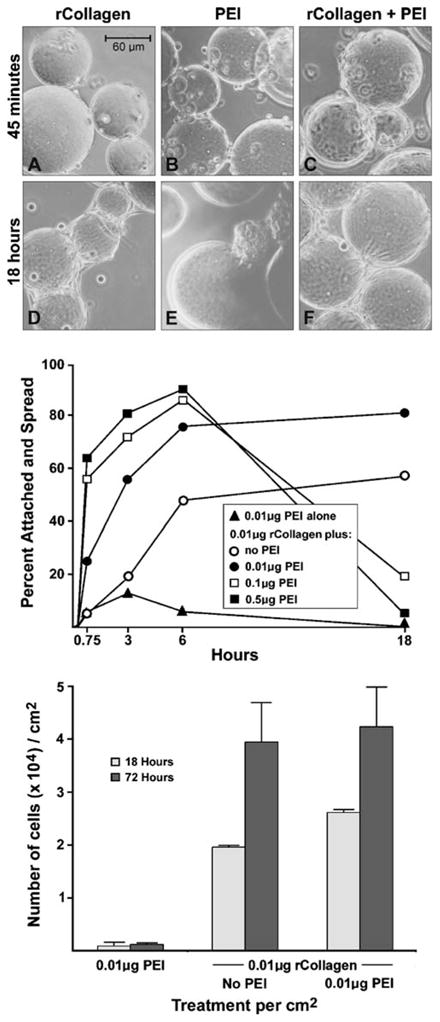
Cell attachment, spreading and growth of human dermal fibroblasts on dextran microspheres coated with a combination of PEI and recombinant collagen. Dextran microspheres were coated with varying concentrations of recombinant collagen and PEI as described in “Materials and Methods”. Upper graph: Human dermal fibroblasts (3×104 per cm2) were added at time zero. The percentage of cells that were attached and spread during the first 18 h of incubation were determined. Values shown are means based on duplicate samples in a single experiment. The experiment was carried out two times with similar results. Lower graph: At 18 h and 3 d, cells were harvested and counted. Values shown are means and standard deviation based on duplicate experiments with duplicate samples per data point. Photographic inserts: A–C) Phase-contrast micrograph (magnification, 150×) showing cell attachment at 45 min after addition of the cells to dextran microspheres coated with 0.01 μg/cm2 of recombinant collagen and/or 0.01 μg/cm2 of PEI. D–F) Phase-contrast micrograph showing cell attachment to the same dextran microspheres at 18 h. The presence of PEI enhances early attachment of cells (45-min time point) but does not inhibit cell number at 18 h when combined with 0.01 μg/cm2 of recombinant collagen.
These findings are depicted in the photographic insert. At the 45-min time, there were more cells attached to microspheres coated with PEI or PEI with recombinant collagen (B and C, respectively) than there were to spheres coated only with collagen (A). At the 18-h time, however, cells were well spread on the microspheres containing recombinant collagen (without or with PEI) (D and F, respectively), while on dextran microspheres coated only with PEI, cells had already detached (E).
Discussion
Manufacturers of vaccines and biologicals are under increasing pressure from regulatory agencies to develop production methods that are completely animal-product-free (Directive 86/609/EEC in the European Union; Convention ETS 123 of the Council of Europe; Letter to Manufacturers of Biological Products: Recommendations Regarding Bovine Spongiform Encephalopathy. FDA, 2000 Update 2002). In order to comply with this demand, alternative substrates to those now on the market that contain gelatin (Cytodex III microcarriers sold by GE Healthcare and collagen-coated polystyrene microcarriers manufactured by Solohill Engineering) must be found. In this study, we have tested a number of possible substitutes including a fully recombinant (yeast expression system) collagen, a 100-kDa recombinant gelatin fragment produced in the same expression system and three fully synthetic peptides derived from a cell-binding region of type I collagen (Scaria et al. 1989; Bhatnagar and Qian 1992). The peptides were virtually without activity, while the 100-kDa gelatin fragment supported cell attachment in the monolayer cultures and promoted attachment and growth when coupled to dextran microspheres. However, the 100-kDa gelatin fragment was less active than was native porcine gelatin under the same conditions. Finally, recombinant type I collagen and native type I collagen were equally effective in promoting cell attachment, and both were more effective than the porcine gelatin preparation currently used in commercially available microcarrier preparations. The differences between the two collagen preparations and gelatin were most notable at low protein concentrations. While the relatively low effectiveness of gelatin can be overcome by using higher concentrations of this inexpensive animal-derived moiety, our data indicate that even with a high concentration of gelatin on the surface of the microsphere, results will be no better than attainable with low collagen concentrations. Thus, it is reasonable to suggest that a low concentration of collagen (either native or recombinant) will provide a surface that is at least as good as can be expected from currently available gelatin surface microspheres. Based on these data, we are confident that it will be possible to replace native gelatin in current microcarrier preparations with a fully recombinant substitute without losing the cell growth benefits provided by the native counterpart.
Several points are worth discussing in more detail. Firstly, can microcarriers coated with recombinant collagen function as well as (or better than) existing products under large-scale “real world” manufacturing conditions? All we can say at this point is that microcarrier products currently in use were successfully tested using the same small-scale assay approaches to predict activity at bioreactor scale (Varani et al. 1989, 1998). There is no a priori reason to believe that it will be different with recombinant collagen as the coating.
Secondly, can these microcarriers be produced economically? This will depend ultimately on the necessary effort to manufacture the product at industrial scale. Each of the steps outlined here is readily scalable. There are no intermediary drying steps or long-term incubations. All of the reactions take place in aqueous buffers. The end-product can be dried and autoclaved. Thus, this procedure should lend itself to large-scale production methods. Cost will depend primarily on the price per unit of the recombinant collagen and the amount of protein needed per unit of microcarrier surface area. Of note is that proteins elaborated in yeast systems can usually be scaled up with only incrementally increased costs. The major determinant may ultimately turn out to be not so much the cost of the recombinant moiety, per se, but the amount needed per unit of substrate surface area. The data presented here indicate that as little as 0.01 μg/cm2 may be sufficient. At that amount, recombinant collagen at $300 per gram would add no more than a few pennies per gram of microcarrier to the price of existing products.
Finally, there is the use of PEI to support attachment to the microspheres containing a low concentration (0.01 μg/cm2) of the recombinant collagen. PEI, like other cationic moieties, provides strong cell attraction through an electrostatic charge mechanism (Varani et al. 1998). The problem with cationic surfaces is that at high concentrations growth inhibition and cytotoxicity are observed (Fischer et al. 1999; Ahlemeyer et al. 2000; Neu et al. 2005; Hunter 2006). Fortunately, after examining multiple concentrations of PEI in conjunction with 0.01 μg/cm2 of recombinant collagen, we were able to find a combination that promoted initial cell attachment without compromising cell yields at day 3. Capacity to increase cell attachment at low collagen concentration is likely to be important in bioreactor-scale culture systems where shear stress reduces effectiveness of cell substrate collisions (Bueno et al. 2007).
In summary, the data presented here suggest that current large-scale cell culture operations which use porcine gelatin-coated dextran microcarriers could employ, without a loss of performance, microcarriers with a low concentration of recombinant collagen. Microcarriers with a recombinant collagen surface could be part of the response to regulatory pressure for animal-product-free manufacturing in the vaccine and biologicals industry.
Acknowledgments
We thank Bruce Donohoe of the University of Michigan Department of Cell and Developmental Biology for his confocal microscopy expertise and limitless patience. This study was supported in part by a subcontract from SoloHill Engineering, Inc., Ann Arbor, MI through SBIR grant GM 070195 from the USPHS.
References
- Ahlemeyer B, Fischer D, Kissel T, Krieglstein J. Staurosporine-induced apoptosis in cultured chick embryonic neurons is reduced by polyethylenimine of low molecular weight used as a coating substrate. Neurosci Res. 2000;374:245–253. doi: 10.1016/S0168-0102(00)00128-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar RS, Qian JJ. A synthetic peptide related to collagen supports cell attachment and migration. Trans Orthop Res Soc. 1992;17:106. [Google Scholar]
- Bueno EM, Laevsky G, Barabino GA. Enhancing cell seeding of scaffolds in tissue engineering through manipulation of hydrodynamic parameters. J Biotechnol. 2007;1293:516–531. doi: 10.1016/j.jbiotec.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Evans MDM, Pavon-Djavid G, Helary G, Legeais JM, Migonney V. Vitronectin is significant in the adhesion of lens epithelial cells to PMMA polymers. J Biomed Mater Res. 2004;69A:469–476. doi: 10.1002/jbm.a.30017. [DOI] [PubMed] [Google Scholar]
- Fischer D, Bieber T, Li YX, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–1279. doi: 10.1023/A:1014861900478. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Hunter AC. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv Drug Deliv Rev. 2006;5814:1523–1531. doi: 10.1016/j.addr. 2006.09.008. [DOI] [PubMed] [Google Scholar]
- Neu M, Fischer D, Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J Gene Med. 2005;7:992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- Scaria PV, Sorensen KR, Bhatnagar RS. Expression of a reactive molecular perspective within the triple helical region of collagen. Am Peptide Symp. 1989;11:605–607. [Google Scholar]
- Takai E, Landesberg R, Katz RW, Hung CT, Guo XE. Substrate modulation of osteoblast adhesion strength, focal adhesion kinase activation, and responsiveness to mechanical stimuli. Mol Cell Biol. 2006;3:1–12. [PubMed] [Google Scholar]
- Van Wachem PB, Beugeling T, Feijen J, Bantjes A, Detmers JP, Van Aken WG. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials. 1985;66:403–418. doi: 10.1016/0142-9612(85)90101-2. [DOI] [PubMed] [Google Scholar]
- Van Wezel AL. Growth of cell-strains and primary cells on microcarriers in homogenous culture. Nature. 1967;216:64–65. doi: 10.1038/216064a0. [DOI] [PubMed] [Google Scholar]
- Varani J, Fligiel SEG, Inman DR, Helmreich DL, Bendelow MJ, Hillegas WJ. Substrate-dependent differences in production of extracellular matrix molecules by squamous carcinoma cells and diploid fibroblasts. Biotechnol Bioeng. 1989;33:1235–1241. doi: 10.1002/bit.260331003. [DOI] [PubMed] [Google Scholar]
- Varani J, Piel F, Josephs S, Beals TF, Hillegas WJ. Attachment and growth of anchorage-dependent cells on a novel, charged-surface microcarrier under serum-free conditions. Cytotechnology. 1998;28:101–109. doi: 10.1023/A:1008029715765. [DOI] [PMC free article] [PubMed] [Google Scholar]


