Abstract
While soluble fms-like tyrosine kinase-1 (sFlt-1), an antagonist of vascular endothelial growth factor and placental growth factor has been implicated in the pathogenesis of hypertension during preeclampsia (PE), the mechanisms whereby enhanced sFlt-1 production leads to hypertension remain unclear. Both sFlt-1 and endothelin-1 production are elevated in women with PE and in placental ischemic animal models of PE, however, the importance of endothelin-1 and sFlt-1 interactions in control of blood pressure during pregnancy is unknown. The purpose of this study was to determine the role of endothelin-1 in mediating sFlt-1-induced hypertension in pregnant rats. To achieve this goal, sFlt-1 (3.7μg/kg/day for 6 days) was infused into normal pregnant rats (NP) and pregnant rats treated with a selective endothelin type A receptor antagonist, ABT 627 (5 mg/kg/day for 6 days). Plasma concentration of sFlt-1 increased from 735±34 pg/ml in NP rats to 2498±645 pg/ml, (p<0.05) with infusion of sFlt-1. Arterial pressure increased from 100±1 mmHg in NP rats to 122±3 mmHg, (p<0.05) in sFlt-1 infused rats. Chronic increases in plasma sFlt-1 in NP rats increased preproendothelin mRNA expression in the renal cortices ≈ 3 fold. In addition, chronic ETA blockade completely abolished the blood pressure response to sFlt-1 in pregnant rats (104±3 vs. 100±1 mmHg, p<0.05), while the ETA receptor antagonist had no effect on arterial pressure in NP rats (105±2 vs. 100±1mm Hg). In conclusion, this study demonstrates that endothelin-1, via endothelin type A receptor activation, plays an important role in mediating the hypertension in response to excess sFlt-1 during pregnancy.
Keywords: Pregnancy, preeclampsia, endothelial factors, placenta, nitric oxide
INTRODUCTION
Preeclampsia, a pregnancy-specific disease of the maternal vasculature, occurs in 5–10% of pregnancies within the United States,1 with the incidence of the disease having risen 40% within the last decade.2 While it is the leading cause of maternal and perinatal death and morbidity,3 the mechanisms underlying the pathophysiology of the disease remains unclear. Over the past decade, there has been ample animal data to suggest placental ischemia as an important initiating event of the disease 4–7, although other causes than hypoxia leading to augmented placental oxidative stress in humans are recently suggested by Burton et al. 8 The hypertension in response to placental ischemia is believed to result from an imbalance of pro- and anti-angiogenic factors such as vascular endothelial growth factor (VEGF) and soluble fms-like tyrosine kinase-1 (sFlt-1).9–12 In support of the angiogenic imbalance hypothesis are studies demonstrating that women with preeclampsia have increased serum sFlt-1 concentrations and decreased circulating levels of VEGF and placental growth factor (PlGF) as compared to women with normotensive pregnancies. 11,13,14 This angiogenic imbalance is then believed to lead to endothelial dysfunction, systemic vasoconstriction, and hypertension. Further support for the angiogenic imbalance concept are studies demonstrating that adenovirus overexpression of sFlt-1 or chronic administration of sFlt-1 into normal pregnant rats produce significant endothelial dysfunction, hypertension, and proteinuria.13,15
While sFlt-1 is thought to play an important role in increasing blood pressure and causing endothelial dysfunction in preeclampsia or in response to placental ischemia in pregnant rats, the physiological mechanism whereby this angiogenic imbalance increases arterial pressure in pregnant rats are unclear. One possible mechanism whereby chronic excess sFlt-1 may result in elevations in mean arterial pressure (MAP) in pregnant rats is through the potent vasoconstrictor endothelin-1 (ET-1). ET-1 has been found to be elevated in women with preeclampsia.16–18 In an animal model of reduced uterine perfusion (RUPP), serum and placental sFlt-1 levels are significantly increased in response to RUPP in pregnant rats.19 Associated with the increase in sFlt-1, renal cortical and medullary expression of preproendothelin (preproET-1) are increased compared to control pregnant rats.20 Furthermore, blockade of the endothelin type A (ETA) receptor markedly attenuated the rise in mean arterial pressure in response to reductions in uterine perfusion pressure.20 Thus, activation of the endothelin system appears to play an important role in mediating increases in blood pressure in response to placental ischemia in pregnant rats.
Although the angiogenic imbalance is known to lead to endothelial dysfunction, the importance of ET-1 in mediating the elevations in blood pressure during sFlt-1-induced hypertension in pregnant rats is unknown. Therefore, the purpose of this study was to determine the role of ET-1 in mediating the hypertension in sFlt-1-induced hypertension in pregnant rats. To achieve this goal, we examined the effect of sFlt-1 on ET-1 production in normal pregnant and sFlt-1 hypertensive pregnant rats. We also compared the blood pressure responses in both control and experimental groups treated with a selective ETA receptor antagonist, ABT627.
METHODS
All studies were performed in timed-pregnant Sprague-Dawley rats purchased from Harlan Inc (Indianapolis Ind). Animals were housed in a temperature-controlled room (23°C) with a 12:12 light: dark cycle. All experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for the use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center.
Experimental Design
sFlt-1 (Recombinant mouse VEGF R1/Flt-1 Fc Chimera, Cat. No. 471-F1) was infused at a rate of 3.7μg/kg/day for 6 days (in sterile saline) beginning on day 13 of gestation via mini-osmotic pump (model 2001; Alzet Scientific Corporation, Palto Alto, CA) into normal pregnant rats (n=11) and in pregnant rats orally treated (drinking water) with at ETA receptor antagonist (ABT627, 5 mg/kg/day for 6 days starting on day 13 of gestation). The infusion rate used has been shown to increase plasma sFlt-1 concentrations approximately threefold and decreased free VEGF by 30%,19 comparable to levels observed in the reduced uterine perfusion pressure (RUPP) animal. Normal pregnant/control groups were fitted with a vehicle filled mini-osmotic pump. Rats were also surgically instrumented with arterial catheters for arterial pressure measurements on day 19. On day 19 of gestation, mean arterial pressure was measured, blood samples were collected, kidneys, placentas and aortas were harvested, and litter size and pup weights were recorded.
Measurement of Arterial Pressure in Chronically Instrumented Conscious Rats
Arterial pressure was determined in all groups of rats on day 19 of gestation. Pregnant rats were catheterized on day 18 of gestation under a short-acting anesthetic, with isoflurane delivered by an anesthesia apparatus. A catheter of V-3 tubing (SCI) was inserted into the carotid artery for blood pressure monitoring. The catheter was tunneled to the back of the neck and exteriorized after implantation. On day 19 of gestation, pregnant rats were placed in individual restraining cages for arterial pressure measurements. Arterial pressure was monitored with a pressure transducer (Cobe III Transducer CDX Sema) and was recorded continuously for two 20-minute periods after 30 minutes of stabilization. Rats were anesthetized using isoflurane delivered by an anesthesia apparatus for blood and tissue collection.
Determination of Plasma sFlt-1 Levels
Circulating sFlt-1 concentrations were measured using a commercial ELISA kit available from R&D Systems (Quantikine) according to the manufacturer’s directions. The assay displayed a sensitivity level of 9.8 pg/ml and interassay variability of <10% and intra-assay of <10%.
Determination of Kidney, Placental, and Aortic Preproendothelin mRNA Levels
The cortex and medulla of the kidneys were separated immediately after harvesting, quickly frozen in liquid nitrogen (LN2), and stored in −80°C. Total RNA was extracted using the RNeasy Protect Mini Kit (Qiagen) after the cortex, medulla, and placenta were crushed in LN2 with a mortar and pestle. Isolation procedure was then performed as outlined in the instructions provided by the manufacturer. Genomic DNA was digested with DNAseI following instructions outlined by Ambion. RNA was quantified spectrophotometrically using an Eppendorf BioPhotometer. cDNA was synthesized from 5μg of RNA with Invitrogen’s Superscript II reverse-transcriptase using the following primers: preproendothelin forward 1: CTAGGTCTAAGCGATCCTTG and preproendothelin reverse 1: TCTTTGTCTGCTTGGC, supplied by custom primers from Invitrogen. Real-time polymerase chain reaction (PCR) was performed using the BioRad Sybre Green supermix and iCycler using a nested forward primer; preproendothelin forward 2: CTAGGTCTAAGCGATCCTTG and the reverse primer outlined. Invitrogen’s reverse-transcription PCR primer control kit was used to amplify β-actin transcripts as control. Levels of mRNA expression were calculated using the mathematical formulas for delta/delta CT recommended by Applied Biosystems (Applied Biosystems User Bulletin, No. 2, 1997).
Statistical Analysis
All data are expressed as mean ± standard error. Differences between control and experimental groups were analyzed using unpaired t-tests. Data were considered statistically different at p values <0.05. Blood pressure comparisons for multigroup and multifactorial analyses were performed using ANOVA with Student Newman Keul’s Test Poc Hoc test.
RESULTS
Plasma sFlt-1 Levels and Arterial Pressure Responses in Control and sFlt-1-Treated Pregnant Rats
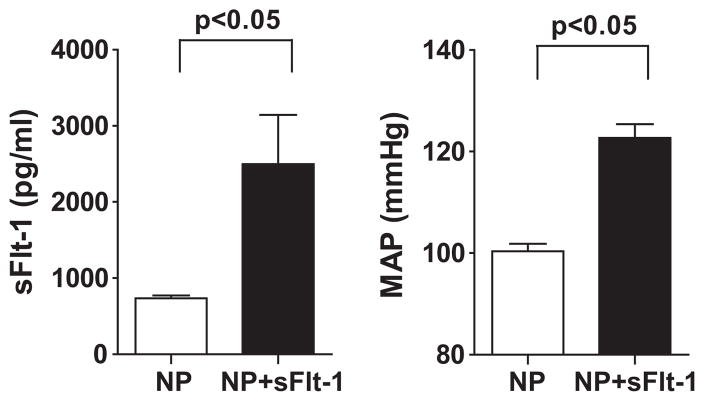
A significant elevation in sFlt-1 plasma concentrations was achieved in sFlt-1-treated pregnant rats. An approximate 3.5 fold increase in circulating levels of sFlt-1 (2498±645 pg/ml) was reached after chronic infusion compared to normal pregnant rats (735±34 pg/ml) (Figure 1). Associated with the elevated plasma sFlt-1 concentrations were significant elevations in mean arterial pressure, 122±2 mmHg, compared to control rats, 100±1 mmHg on day 19 of gestation.
Figure 1.
Plasma concentrations of sFlt-1 in normal pregnant (NP) and sFlt-1-treated (NP+sFlt-1) pregnant rats (*p<0.05 vs. NP rats). Data expressed as mean±SEM.
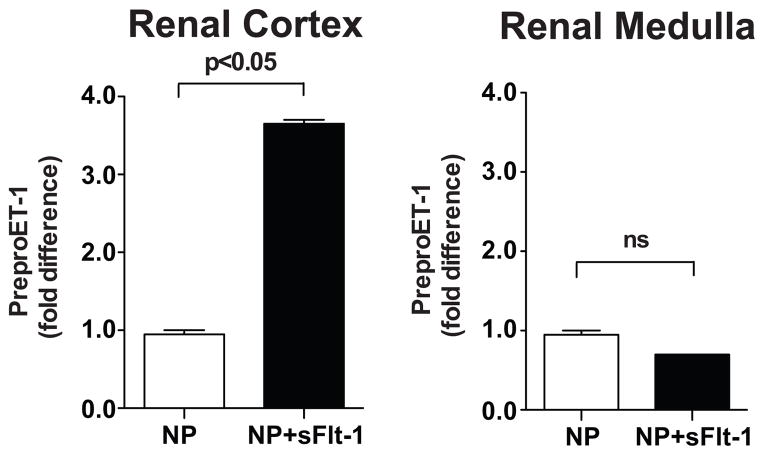
Preproendothelin mRNA levels in the Kidney of Normal Pregnant and sFlt-1-Treated Pregnant Rats
Preproendothelin in the renal medulla did not differ significantly between sFlt-1-treated pregnant rats and normal pregnant rats. However, preproendothelin mRNA levels increased ≈ 3-fold in the renal cortex of sFlt-1-treated pregnant rats compared to normal pregnant rats (Figure 2).
Figure 2.
Tissue preproedothelin mRNA levels, determined by real time PCR levels, of the renal cortices and medulla of normal pregnant rats (NP) and sFlt-1-treated pregnant rats (NP+sFlt-1). Data expressed as mean±SEM.
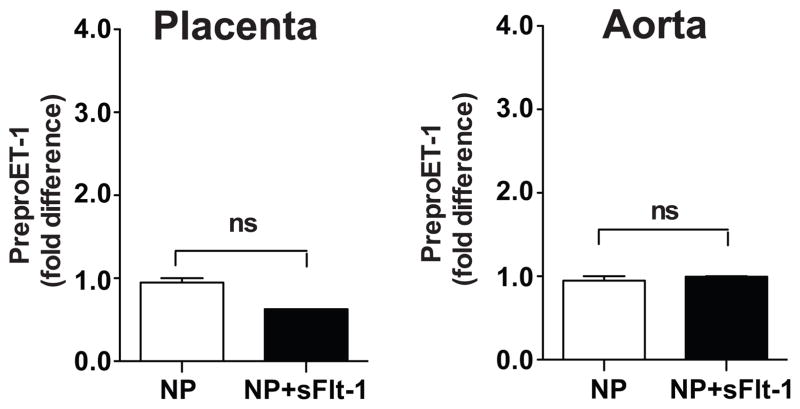
Preproendothelin mRNA levels in Placenta and Aorta in Normal Pregnant and sFlt-1-Treated Pregnant Rats
Preproendothelin in the placenta or aorta did not differ significantly between sFlt-1-treated pregnant rats and normal pregnant rats (Figure 3).
Figure 3.
Tissue preproedothelin mRNA levels in aorta and placenta determined by real time PCR. Data shown as fold difference between CT of β-actin vs. CT of preproendothelin-1, and statistically analyzed using un-paired t-test.
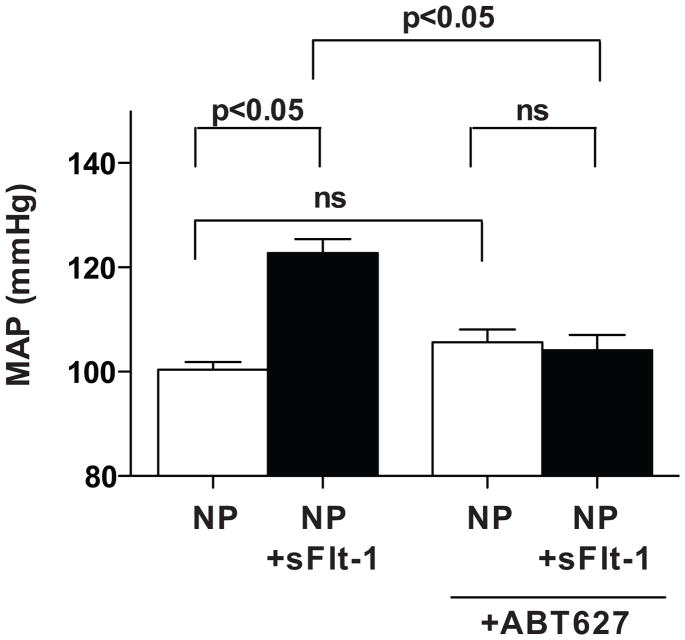
Arterial Pressure Responses to sFlt-1 in Normal Pregnant Rats and ETA Receptor Antagonist-Treated Pregnant Rats
Chronic infusion of sFlt-1 at a rate of 3.7μg/kg/day for 6 days into pregnant rats resulted in significant elevations in mean arterial pressure on day 19 of gestation. Pregnant rats treated with sFlt-1 and ETA receptor antagonist showed significant reductions in mean arterial pressure of 104±3 mmHg compared to sFlt-1-treated pregnant rats. Treatment of normal pregnant rats with ETA receptor antagonist alone had no effect on mean arterial pressure (105±2 mmHg) compared to normal pregnant rats (100±1 mmHg) (Figure 4).
Figure 4.
Mean arterial pressure in response to sFlt-1 infusion (NP+sFlt-1) and treatment with an endothelin type A receptor antagonist (ABT627) in normal pregnant (NP) rats. All data are expressed as mean±SEM.
DISCUSSION
Inadequate invasion of trophoblasts into the maternal spiral is believed to play an important role in the etiology behind pregnancy-induced hypertension. Over the past decade, there has been ample animal data to suggest placental ischemia as an important initiating event of the disease 5–8, although other causes than hypoxia leading to augmented placental oxidative stress in humans are recently suggested by Burton et al 8 The hypertension in response to placental oxidative stress is believed to result from the release of soluble, placental factors into the maternal circulation. These factors are then believed to act on the maternal endothelium and to result in systemic vasoconstriction.5,21 One such factor that is known to be upregulated during preeclampsia is the anti-angiogenic factor soluble fms-like tyrosine kinase-1, or sFlt-1. sFlt-1 is a member of the pro-angiogenic VEGF family and analogous to theFlt1 receptor, however, the lack of the transmembrane C-terminus makes the sFlt-1 receptor soluble. sFlt-1 acts to sequester free VEGF and lead to an angiogenic imbalance weighted toward an anti-angiogenic state.11,13,14 VEGF is a regulator of normal endothelial cell function, and it is suggested that a decrease in circulating levels of free VEGF leads to the abnormal endothelial cells and resultant endothelial cell activation and dysfunction associated with PE.21 We, and others, have shown that chronic excess sFlt-1 infusion into normal pregnant rats results in significant elevations in mean arterial pressure that is associated with decreased levels of VEGF and PlGF, proteinuria, and reductions in renal function.13,19 Consistent with these findings, we report in this study that a 2–3 fold increase in sFlt-1 in pregnant rats increased blood pressure approximately 20 mmHg. The current study extends previous findings by examining the role of the endothelin-1 in increasing the blood pressure in response to sFlt-1.
While sFlt-1 increases blood pressure, there is compelling evidence to suggest an important role for endothelin in PE. ET-1 is a known by-product of endothelial dysfunction, and has been found to be elevated ≈ 2 to 3 fold in the plasma of some, but not all, women with preeclampsia.16,17 In addition, recent data from our laboratory shows that a reduced uterine perfusion pressure (RUPP) model of PE in pregnant rats results in significant elevations in mean arterial pressure and renal preproET production.20 Furthermore, selective blockade of the ETA receptor completely abolished the hypertension in response to chronic reduced utero-placental perfusion in these animals.
Collectively, these data strongly suggest the endothelin system may play an important role in mediating the hypertension in response to chronic excess sFlt-1 infusion into pregnant rats. To test the hypothesis we first examined whether sFlt-1 induced hypertension activated the endothelin-1 system. In response to chronic excess sFlt-1 infusion into pregnant rats we observed a ≈ 3.5 fold increase in cortical preproET expression compared to normal pregnant rats. There was no significant difference in medullary, placental, or aortic preproET mRNA expression in sFlt-1 hypertensive and normal pregnant rats. Treatment with a selective ETA receptor antagonist, ABT627, significantly reduced blood pressure in sFlt-hypertensive pregnant rats while having no effect on normotensive, normal pregnant rats.
Since the endothelium is a major target organ for the actions of VEGF, it is likely that alterations in the production of endothelium derived relaxing factors such as nitric oxide, may also play a role in the hypertensive response to sFlt-113. Although the relative importance of nitric oxide in mediating the increase in blood pressure in response to sFlt-1 has yet to be fully elucidated, Facemire and colleagues recently reported that administration of a specific antibody against the major VEGF receptor, VEGFR2, to normal mice caused an increase in blood pressure that was associated with significant reductions in the expression of endothelial and neuronal nitric oxide synthases in the kidney.22 To further establish a role for reduced nitric oxide synthesis in the hypertension caused by blocking VEGFR2, they reported that L-NAME administration abolished the difference in blood pressure between the vehicle- and anti-VEGFR2-treated groups. Further support for an interaction between nitric oxide and antiangiogenic factors are the clinical findings of Sandrim et al that nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia.23 While these results suggest that reducing nitric oxide production and/or availability may be one mechanism underlying hypertension caused by anti-angiogenic agents targeting VEGF, it remains to be determined whether sFlt-1 induced hypertension during pregnancy is associated with reductions in nitric oxide production. Moreover, it is possible that reductions in nitric oxide synthesis may be in part responsible for the increase in ET-1 in response to sFlt-1 since nitric oxide is known to be a potent inhibitor of ET-1 production.
In summary, we found that chronic excess sFlt-1 infusion into pregnant rats was associated with significant elevations in mean arterial pressure and a ≈ 3.5 fold increase the cortical preproET-1 mRNA expression. Chronic treatment with a selective ETA receptor antagonist significantly reduced mean arterial pressure in sFlt-1 hypertensive pregnant rats, while having no effect on normal pregnant rats. Based on these findings, ET-1 appears to play an important role in mediating the hypertension produced by chronic, excess sFlt-1 in pregnant rats.
PERSPECTIVES
Preeclampsia, which affects 5% to 10% of all pregnancies in the United States, is a multi-systemic disorder of pregnancy that is associated with hypertension and endothelial dysfunction. Despite being one of the leading causes of maternal and perinatal morbidly and mortality, the pathophysiological mechanisms underlying the hypertension during preclampsia is unknown. While sFlt-1, an endogenous antagonist of vascular endothelial growth factor and placental growth factor has been implicated in the pathogenesis of hypertension during preeclampsia, the mechanisms whereby enhanced sFlt-1 production leads to endothelial dysfunction and hypertension remain unclear. Both sFlt-1 and endothelin-1 production are elevated in women with PE and in placental ischemic animal models of PE, however, the importance of endothelin-1 and sFlt-1 interactions in control of blood pressure during pregnancy has been unknown. The data from the current study suggests that elevated preproET-1 expression in sFlt-1 hypertensive pregnant rats appears to mediate the hypertension seen in this model.
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH grant HL51971.
Footnotes
DISCLOSURE
The authors have no conflicts of interest to disclose.
References
- 1.Noris M, Perico N, Remuzzi G. Mechanisms of Disease: pre-eclampsia. Nat Clin Prac Nephrology. 2005;1:98–114. doi: 10.1038/ncpneph0035. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 3.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 4.Wang A, Rana S, Karumanchi SA. Preeclampsia: The Role of Angiogenic Factors in Its Pathogenesis. Physiology. 2009;24:147–158. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- 5.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of Hypertension During Preeclampsia Linking Placental Ischemia with Endothelial Dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 7.LaMarca BD, Gilbert JS, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension. 2008;51:982–988. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 10.Maynard SE, Venkatshea S, Thadhani R, Karumanchi SA. Soluble fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 11.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 12.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFlt-1. Kidney International. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 13.Maynard SE, Min YH, Merchan J, Lim KH, Li J, Mondal S, Liberman TA, Morgan TP, Sellke FW, Stillman IE, Epstein EH, Sukhatme VP, Karumanchi AS. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;11:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine R, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating Angiogenic Factors and the Risk of Preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 15.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens. 2009;22:564–568. doi: 10.1038/ajh.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekker GA, Kraayenbrink AA, Aeeman GG, van Kamp GJ. Increased plasma levels of the novel vasoconstrictor peptide endothelin in severe preeclampsia. Eur J Obstet Gynecol Reprod Biol. 1991;40:215–220. doi: 10.1016/0028-2243(91)90120-a. [DOI] [PubMed] [Google Scholar]
- 17.Nova A, Shibai BM, Barton JR, Mercer BM, Mitchell MD. Maternal plasma levels of endothelin are increased in pre-eclampsia. Am J Obstet Gynecol. 1991;165:724–727. doi: 10.1016/0002-9378(91)90317-k. [DOI] [PubMed] [Google Scholar]
- 18.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion pressure in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 20.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin Type A Receptor Blockade Attenuates the Hypertension in Response to Chronic Reductions in Uterine Perfusion Pressure. Hypertension. 2001;37:485–589. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 21.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens. 1991;4:700–708. doi: 10.1093/ajh/4.8.700. [DOI] [PubMed] [Google Scholar]
- 22.Facemire CS, Nixon AB, Griffiths R, Hurtwitz H, Coffman TM. Vascular Endothelial Growth Factor Receptor 2 Controls Blood Pressure by Regulating Nitric Oxide Synthase Expression. Hypertension. 2009;54:652–658. doi: 10.1161/HYPERTENSIONAHA.109.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandrim VC, Palei AC, Metzger IF, Gomes VA, Cavalli RC, Tanus-Santos JE. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. Hypertension. 2009;52:402–407. doi: 10.1161/HYPERTENSIONAHA.108.115006. [DOI] [PubMed] [Google Scholar]