Abstract
Background and Purpose
Previous studies demonstrated that intravascular injection of bone marrow stromal cells (BMSCs) significantly improved neurological functional recovery in a rat model of intracerebral hemorrhage (ICH). To further investigate the fate of transplanted cells, we examined the effect of male rat BMSCs administered to female rats after ICH.
Methods
Twenty-seven female Wistar rats were subjected to ICH surgery. At 24 hours after ICH, these rats were randomly divided into three groups, of which each was intravenously injected with 1 ml phosphate-buffered saline (PBS) or 0.5 million or 1 million male rat BMSCs in PBS. To evaluate the neurological functional outcome, each rat was subjected to a series of behavioral tests (mNSS and corner turn test) at 1, 7 and 14 days after ICH. The rats were then anesthetized intraperitoneally, sacrificed and the brain tissues were processed at Day 14 after ICH. Immunohistochemistry and in situ hybridization were employed to identify cell specific markers.
Results
The male rat BMSCs significantly improved the neurological functional outcome and also significantly diminished tissue loss when intravenously transplanted into the rats after ICH. Immunoassay for BrdU and neuronal markers demonstrated a significant increase in the number of BrdU-positive cells, which indicated endogenous neurogenesis, and a significant increase in the number of cells positive for immature neuronal markers. In situ hybridization showed that more BMSCs resided around the hematoma of the rats treated with the 1 million cell dose compared to the 0.5 million cell dose group. In addition, a subfraction of Y chromosome-positive cells were co-immunostained with the neuronal marker (MAP-2) or the astrocyte marker (GFAP).
Conclusions
Male rat BMSCs improve neurological outcome and increases histochemical parameters of neurogenesis when administered to female rats after ICH. This study has shown that the intravenously administered male rat BMSCs enter the brain, migrate to the perihematomal area and express parenchymal markers.
Keywords: Bone marrow stromal cells, intracerebral hemorrhage, rat
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a common and devastating form of brain injury, with much worse outcome than ischemic stroke, and with a death rate close to 50% within 30 days.1 ICH usually results from rupture of an arteriosclerotic small artery which has been weakened primarily because of chronic arterial hypertension. Currently there are no satisfactory therapeutic options for ICH. Measures aimed at decreasing elevated intracranial pressure have limited effectiveness. Empirically, immediate surgical removal of the hematoma from the brain can relieve pressure within the skull, and therefore surgical evacuation of ICH has been selectively practiced for decades. However, the benefit of rapid surgical intervention does not appear universally to result in favorable outcome. In fact, the results from a recently conducted large prospective clinical trial did not support surgical intervention.5
Much attention has been focused on neuroprotective and neurorestorative therapies, which include early rapid use of procoagulant therapy to stop hemorrhage expansion, pharmacologic agents designed to prevent secondary neuronal injury and bone marrow stromal cell (BMSC) transplantation.1,8 BMSCs have been a potential cell therapy source for various medical conditions in recent years.3,6,17 Compared to embryonic and other adult stem cells, BMSCs are readily accessible, amenable to manipulation, and are easily expanded in culture. Utilization of an adult tissue would circumvent the legal and ethical issues surrounding the use of embryonic and fetal tissues. Additionally, BMSCs have exhibited neuroectodermal traits in the brain, and promoted significant functional recovery in a number of disease-specific animal models.10,19
We have recently documented the beneficial effects of human BMSC infusion in male rats subjected to intracerebral hemorrhage. When preceded by mannitol infusion, human BMSCs are more effectively recruited to the injury site and promote better neurological recovery at a dose of 3 × 106 cells.21 To further investigate the fundamental concern of the fate of the BMSCs after intravenous injection, we have studied the paradigm of the male rat BMSCs using the ICH animal model in female rats. In situ hyridzation of the Y chromosome of BMSCs in the female rat is a highly reliable method for determining their localization and phenotypic expression.
Methods
Animal ICH Hemorrhage Model
All animals received humane care in compliance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System. Adult female Wistar rats weighing 270 to 320 g were anesthetized and maintained at 37°C throughout the surgical procedure using a feedback-regulated water heating pad. Under a dissecting scope, a PE50 catheter is inserted into the right femoral artery 1–2 cm through a small puncture and prepared for collecting the blood. The stereotactic frame measurements were used to guide the site of the craniectomy (3.5 mm lateral to midline, 0.5 mm anterior to bregma, depth 5.5 mm below the surface to midline). Once the dura was penetrated with the stereotactic drill, attention was turned to collecting the blood (approximately 0.3 cc). The blood was placed into a 1 cc syringe with 26G½ needle that is loaded on the stereotactic frame. The micropipette was inserted into the craniectomy site and advanced 5 mm into the brain. Then 0.1 cc of blood was infused at a rate of 10 µl per minute. To prevent blood from backing up, a piece of bone wax was used to close the craniectomy site and keep the blood in the brain.
Experimental Groups
BMSCs, provided by Cognate Therapeutics (Sunnyvale, CA), were suspended in phosphate buffered saline (PBS) at 0.5 × 106 /ml. Twenty- seven ICH rats were randomly divided into three experimental groups. Group 1, which consisted of 9 rats, included slow delivery of 1 ml PBS into the tail vein. Group 2, which consisted of 9 rats, included delivery of 0.5 × 106 BMSCs into the tail vein. Group 3, which consisted of 9 rats, included delivery of 1 × 106 BMSCs into the tail vein. All animals received daily intraperitoneal injections of 100 mg/kg bromodeoxyuridine (BrdU; a thymidine analog, which labels newly synthesized DNA) (Sigma, St. Louis, MO) starting at 24 hours after ICH and subsequently for 13 consecutive days.
Functional Tests
The modified neurological severity score (mNSS) and the corner turn test were performed at 1, 7, and 14 days after ICH.25 The mNSS is a composite of motor (i.e., muscle status and abnormal movement), sensory (i.e., visual, tactile, and proprioceptive), beam balance, and reflex tests. In this ICH model, injury in the right hemisphere striatum of rats causes sensory and motor functional deficits with elevated scores on motor, sensory, and beam balance tests in the early phase after injury.
The corner turn test was developed for measuring long-term functional recovery in the rat.25 The test is sensitive to unilateral cortical injury because it reflects multiple asymmetries, including postural, vibrissae sensory, and fore- and hindlimb use asymmetries, which all combine to bias turning. An uninjured rat randomly turns left or right, whereas an injured rat preferentially turns toward the unimpaired (right) side. We recorded the number of right turns from 10 trials for each test and used the results for statistical analysis.
Histology and Immunohistochemistry
Fourteen days after ICH, the rats were anesthetized intraperitoneally with ketamine and xylazine and perfused transcardially with saline solution, followed by 4% paraformaldehyde. Their brains were promptly removed and further fixed in 4% paraformaldehyde for 1 to 2 days at room temperature. The cerebral tissues were cut into 7 equally spaced (2 mm) coronal blocks (A, B, C, D, E, F, G), and then processed for paraffin sectioning. A series of adjacent 6-um-thick sections were cut from each block in the coronal plane and were stained with hematoxylin and eosin. The sections were traced by the Global Laboratory Image analysis system (Data Translation, Marlboro, MA). The area of preserved striatum on the side of the hemorrhage was subtracted from that of the contralateral hemisphere, thus reckoning the percentage of tissue loss from the ICH, when comparing treated to untreated animals.
The brain tissue residing on block C was the primary site of ICH injury and therefore block C was specifically selected for the immunohistochemistry study. Every 40th coronal section from this block was used for immunohistochemical staining with the same antibody. Sections were first blocked in a Tris-buffered saline containing 1% BSA. Sections were then incubated with the primary antibodies for localization of BrdU (1:100; a marker for proliferation cells), TUJ1 (1:5,000; a marker for immature neurons), DCX (1:50; a marker for migrating neuroblasts), and synaptophysin (1:1000; a marker for presynapic plasticity and synaptogenesis). To visualize the cellular colocalization of BrdU-specific and cell-type-specific markers in the same cells, double staining was used. Each coronal section was first treated with the primary BrdU monoclonal antibody and followed with FITC goat anti-mouse IgG for 30 minutes. After a brief washing, the sections were incubated with TUJ1 monoclonal antibody and subsequently with rabbit anti-mouse Cy5 IgG for 30 minutes. All immunostainings were performed at the same time with two negative controls (i.e., the omission of primary antibody and the use of preimmune serum) for quality control of the immunoassaying procedure.
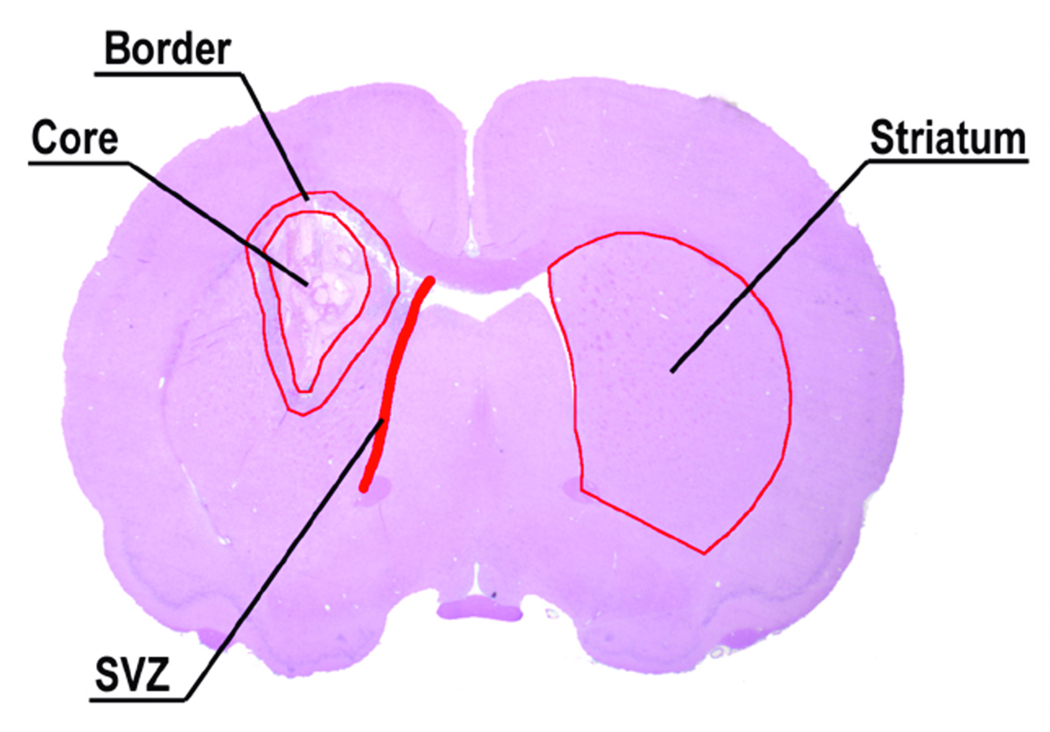
For semiquantitative measurements of synaptophysin, BrdU, TUJ1 and DCX, all of the immunostaining sections were digitized under a 20 X objective lens (Olympus BX40; Olympus Optical Co., Tokyo, Japan) by using a 3D-CCD color video camera (model DXC-970MD; Sony Corp., Tokyo, Japan) interfaced with the MCID image analysis system (Imaging Research, Inc., St. Catharine’s, ON, Canada). Synaptophysin was measured in the striatum region. TUJ1 and DCX were measured at the subventricular zone (SVZ). BrdU-positive cells were counted at the boundary zone of the hemotoma. A representative ICH coronal section illustrates these regions (i.e., damaged striatum core, boundary zone, SVZ and striatum) (Fig. 1). For synaptophysin, TUJ1 and DCX, data are presented as a percentage of the immunopositive areas in each field divided by the total areas in the field (628 × 480 µm2).
Fig. 1.
A representative H&E stained coronal section from the level of the anterior commissure of a rat brain. The detected areas of the injured hemisphere recognized under the microscope are the core hemorrhage area, the boundary zone, the subventricular zone (SVZ) and striatum.
In Situ Hybridization
A coronal section from block C was selected and staining was performed using fluorescent in situ hybridization (FISH) as described. Briefly, a PCR product of 459 bp genomic DNA fragment specific for rat Y chromosome was prepared and labeled with digoxigenin using a Random Primer DIG labeling and Detection Kit (Boehringer Mannheim, Indianapolis, IN, USA). The slides were digested with 50 mg/mL proteinase K for 20 minutes at 37°C. Hybridizations were performed in a 50% formaldehyde hybridization buffer at 50°C for 40 hours. The digoxigenin-labeled Y chromosome positive BMSCs were visualized using the fluorescent labeled antibody against digoxigenin (Boehringer Mannheim). The slides were then counterstained with propidium iodide for nuclear staining and mounted with antifade solution and coverslips. Control slides from each animal received identical staining preparation, except that the probe or the antidigoxigenin antibodies were omitted during the procedure to serve as negative contrasts. Y chromosome-positive cells were counted along the boundary of the hematoma.
To visualize the cellular colocalization of the Y chromosome and phenotypical markers in the same cell, the FITC-conjugated antibody and DAB staining were employed for double labeling. Each coronal section was first treated with cell-type-specific antibodies, MAP-2 for neurons and GFAP for astrocytes, then visualized with DAB staining. Subsequently, FISH staining was employed for the identification of male BMSCs.23
Statistical Analysis
Data are presented as mean ± standard error (SE). Statistical analyses were performed using SPSS software (version 11.5, SPSS, Inc., Chicago, IL). Statistical comparisons of functional scores, tissue loss, immunohistochemical and FISH results, between two different treatment groups were made using two-tailed Student’s t tests. P values of less than 0.05 were considered statistically significant.
Results
Neurological functional tests
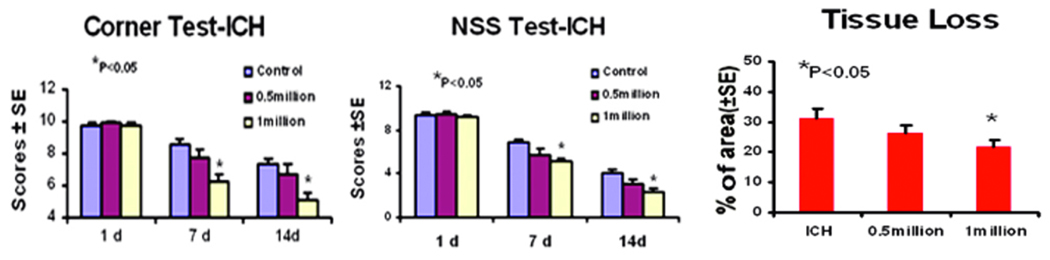
As evaluated by both mNSS and corner turn test, all ICH-induced female rats had similar neurological impairments at Day 1 after ICH. At Days 7 and 14 after ICH, the 1 × 106 BMSC treatment group showed significant improvement of neurological functions over the control group (Fig. 2). Rats in the half million group showed a trend of improvement but with no statistical significance.
Fig. 2.
Neurological function tests and relative striatal tissue loss percentage of the ICH side. A and B: Quantitative bar graph illustrating the results of the corner turn test and mNSS for Groups 1, 2, and 3 (saline controls, the 0.5 × 106 BMSC group, and the 1 × 106 BMSC group). C: Bar graph showing the amount of striatal tissue in the ICH region relative to the contralateral normal region in the 3 groups (ICH indicates the control group).
Histology and Immunohistochemistry
The data for the percentage of striatal tissue loss on the side of hemorrhage is presented in Figure 1. The area of tissue loss as a percentage of the normal contralateral side is as follows: control, 30 ± 2.7%; the lower dose group, 28 ± 4 %; and the higher dose group, 22 ± 1.5% (p < 0.05). Consequently, the one million dosage group showed significantly reduced tissue loss when compared to the untreated group 2 weeks after the ICH. The half million dosage group also showed the same trend, but with no significant difference when compared to the control group.
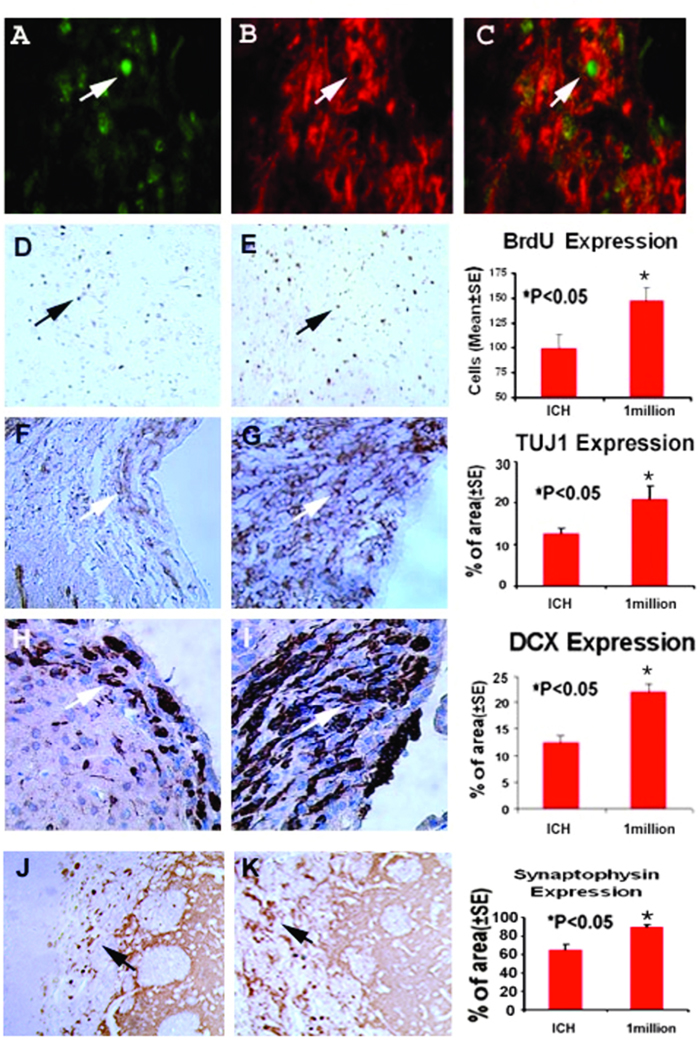
Typical immunostainings for synaptophysin, DCX and TUJ1 in the control group and Group 3 are presented in Fig. 3. Statistical analysis indicated that staining for all three neural markers in both treated groups was significantly increased, suggesting that BMSCs enhance synapse formation, neuronal migration and neuronal formation in the subventricular zone ipsilateral to the ICH, respectively. Consistent with the results of the neurological functionstudies, Group 3 specimens exhibited a larger staining area for all 3 markers. Immunoassay for BrdU was also performed. Both groups treated with BMSCs demonstrated significantly increased BrdU-positive cells around the injury site when compared with the ICH rats injected with PBS. Further, double staining for BrdU and TUJ1 identified a subpopulation of cells co-stained with both BrdU and TUJ1, suggesting newly formed immature neurons in the region of the subventricular zone in rats treated with BMSCs (Fig. 3).
Fig. 3.
Immunostaining of the control and one million BMSCs group sections showing the quantitative immunoreactivities of Brdu, TUJ1, synaptophysin, and DCX. A higher number of cells per area are stained to Brdu, TUJ1, synaptophysin and DCX. Quantitative immunoreactivities for the two groups are presented as bar graphs on the right side of each panel. Colocalization of BrdU and TUJ1 in a subpopulation of cells near the injured region of the 1 million BMSC group is shown on the bottom panel. Arrows indicate cells positively stained for both BrdU and TUJ1.
In Situ Hybridization
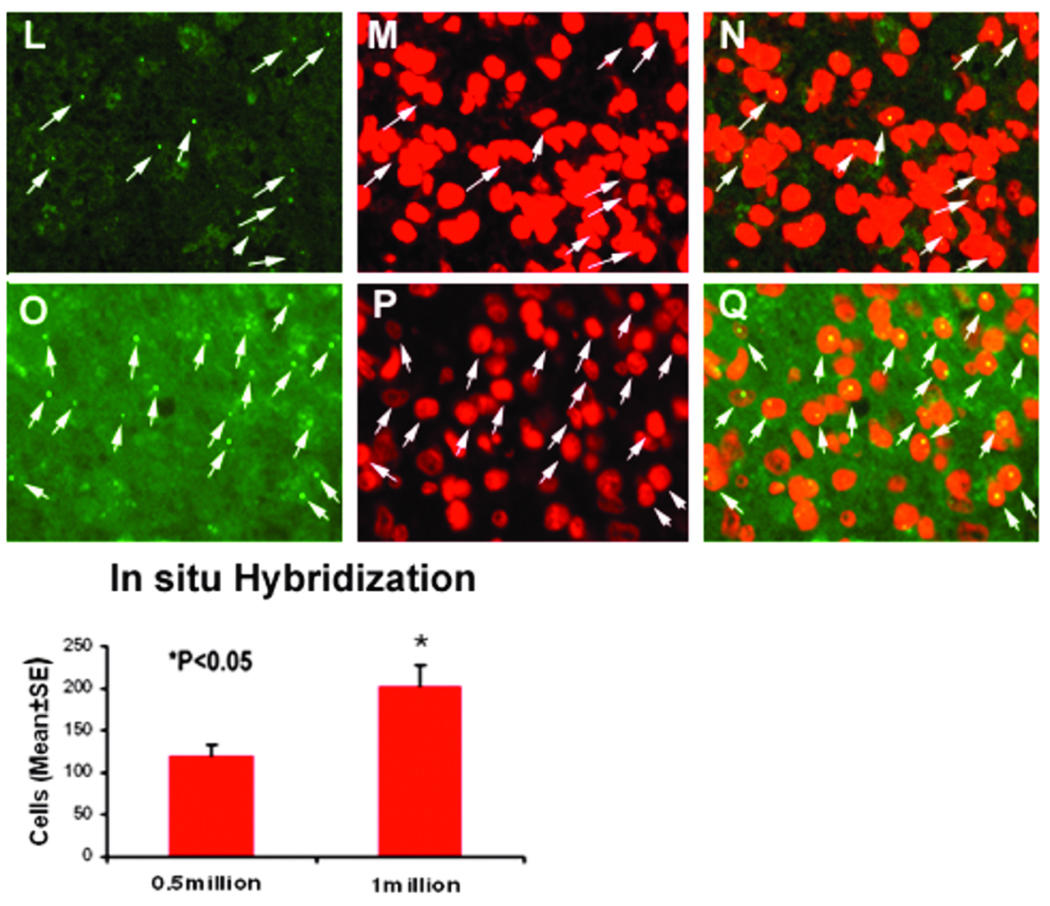
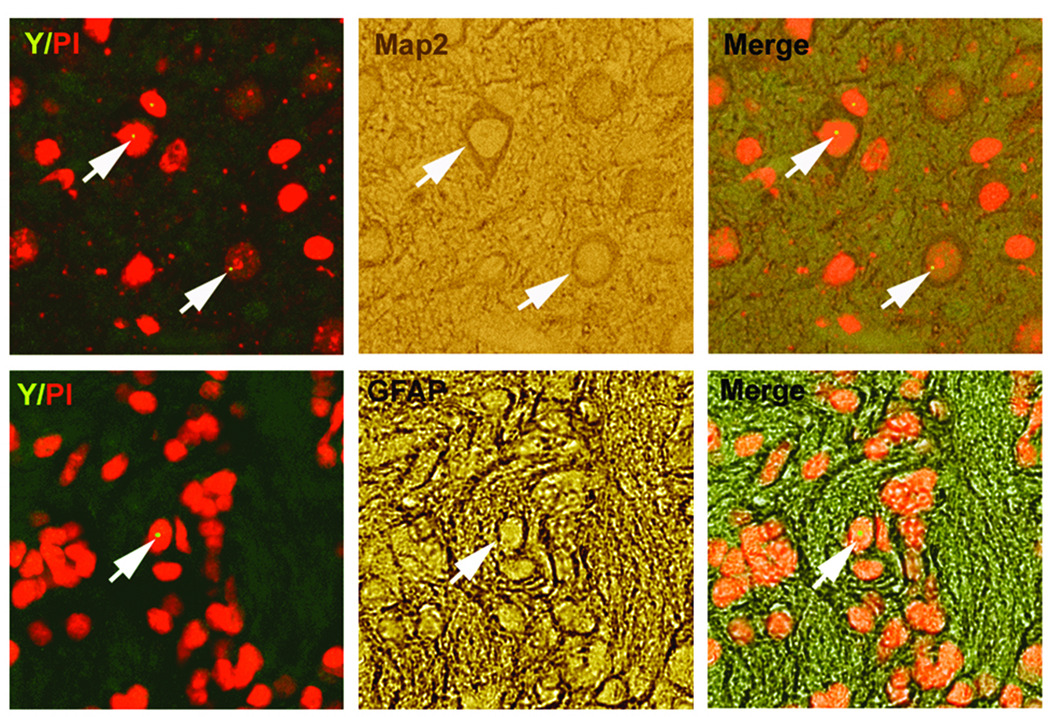
To track the fate of administered BMSCs, Y chromosome-positive cells were identified using DNA in situ hybridization. In both treated groups, dose-dependent Y chromosome-positive staining was observed. The numbers of Y chromosome-positive cells in the ipsilateral hemisphere of Group 3 (the higher-dose group) were significantly greater than those of Group 2. No Y chromosome-positive cells were observed in Group 1 (the PBS control group) (Fig. 4). Double staining also revealed that a small number of Y chromosome-positive cells expressed either the neuronal marker MAP-2 (3.64% ± 1.7%) or the astrocytic marker GFAP (8.39% ± 2.1%) (Fig. 5).
Fig. 4.
Characterization of in vivo BMSCs by fluorescent in situ hybridization to identify Y-chromosome specific male BMSCs. Y-chromosome positive cells were found substantially along the boundary of the hematoma of the 0.5 million BMSC group (L-N) and 1.0 million BMSC group (O-Q). Bar graphs of quantitative Y-chromosome positive cells in the 0.5 million BMSC group relative to 1.0 million BMSC group are shown on the bottom panel.
Fig. 5.
Double-immunohistochemical staining of in vivo male BMSCs with neuronal and astrocytic markers. Immunofluorescent staining shows that a small number of Y chromosome-positive cells (left panels) expressing phenotypically the neuronal marker (microtubule-associated protein-2, MAP-2) (upper-right panel), or astrocytic marker (glial fibrillary acidic protein, GFAP) (lower-right panel) along the boundary zone of lesion. All the Y-chromosomes colocalize with propidium iodide (PI)-stained nuclei, showing the specificity of fluorescent in situ hybridization for the Y-chromosomes.
Discussion
The present study addressed two major findings. First, following our previous study of human BMSCs in the ICH animal model,19 this report demonstrated that rat BSMC administration can increase markers of neurogenesis, improve neurological functional outcome and significantly reduce tissue loss. Second, double staining illustrated that a portion of the Y chromosome-positive rat male BMSCs can express the neuronal marker (MAP-2) and the astrocyte marker (GFAP) near the region of injury, demonstrating that the intravenously administered BMSCs can enter the brain, migrate to the perihematomal area and express neuronal and astrocytic phenotypes in vivo.
Intracerebral hemorrhage is a type of deadly intracranial bleeding that can increase intracranial pressure and potentially crush delicate brain tissue or reduce local blood supply. The ongoing bleeding and the sustained growth of the local hematoma create both physical and chemical factors that cause an immediate and destructive mass lesion. Perihematomal injury and the subsequent inflammatory cascade activated by the coagulation products and the toxic blood breakdown products lead to perihematomal edema and disruption of the blood-brain barrier (BBB), resulting in neurological deterioration. Continued bleeding and hematoma growth during the first hours in patients with ICH are common and are believed to be one of the primary determinants of both mortality and poor functional outcome.1 Ideal experimental animal models of ICH should mimic these pathophysiological features of ICH in human beings. Currently, two models of experimentally induced ICH have been adopted for investigating ICH: collagenase and autologous blood injection.16 The autologous blood induced ICH rat model has been demonstrated to be reproducible in our studies.19–21
Previously, we have documented the effectiveness of human BMSCs in improving neurological functional recovery. Preadministration of mannitol can increase the efficacy of low-dose intraarterial BMSCs. In this study, we have used male rat BMSCs in female rats after experimentally induced ICH to substantiate our early experiments. With DNA in Situ Hybridization specifically to differentiate the Y chromosome, the donor cells can be easily tracked from the brain sections. In the perilesion of ICH brain, significantly more transplanted cells in the 1.0 × 106 cell dose group were detected than that of the 0.5 × 106 dose group, suggesting a dose-dependence of BMSC migration to the injury site. Notably more Y chromosome-positive cells accumulate in the boundary zone of the hematoma than in the subventricular zone (SVZ). On the other hand, cells positively stained with DCX and TUJ1 are mainly localized in the SVZ. The mechanisms behind this may relate to the fact that a hematoma induces both physical and chemical damage to the blood-brain barrier and causes upregulation of cell adhesion molecules.7 Consequently, the transplanted cells were preferentially recruited along the margin of the hematoma. At the same time, these recruited BMSCs can release certain neurotrophic and other growth factors to accelerate proliferation, migration and differentiation of neuronal progenitor cells in the adult rat subventricular zone ipsilateral to ICH.21
The results of both BrdU staining and in situ hybridization demonstrated that only a fraction of transplanted cells enter the brain of rats by intravenous delivery. In a similar ischemic stroke study,1,23 only an estimated 3.1% of the total intravenously injected neural progenitor cells were found to be recruited around the damaged brain.
A small portion of Y chromosome positive cells were stained with GFAP and MAP2 antibody, and the colocalization suggests that a certain number of transplanted MSCs have developed into cells that exhibit either neuronal or astrocyte phenotype. However, the percentage of these cells expressing either neuronal or astrocyte characteristic markers is rather low when compared to the total number of Y chromosome positively stained cells. This small fraction of BMSCs expressing either neuronal or astrocyte markers are unlikely to ameliorate the neurological dysfunction, which we observed through both mNSS and corner turn test. Immunohistochemistry studies at 2 weeks following injury, using other neuronal markers, demonstrated significantly increased immature neuronal formation, migrating neuroblasts and synaptogenesis formation in the 1.0 × 106 BMSCs dose group. This suggests that the primary function of the transplanted BMSCs in vivo may be to stimulate endogenous neurorestorative mechanisms.
In our previous studies using MSCs for the treatment of stroke and TBI, we have demonstrated that functional recovery begins within days after treatment and even persists for a year after stroke or for months after TBI.10–12,15,18,22 Thus, the 14-day improved functional response and reduced tissue loss observed in the short time frame after ICH is consistent with the long-term recovery evident under conditions in which the treatment does not alter lesion volume, such as after stroke and TBI.
In the present study, although we did not emphasize the neuroprotective effects of MSC treatment, the improvement in neurobehavioral function and the reduction of tissue loss might be partially due to the neuroprotective effects of MSC-mediated growth factor production.4,14 The MSC-mediated neuroprotection might be related to stem cell-mediated immunomodulation. Undifferentiated transfused cells can express immunorelevant receptors, which in turn are able to restrict the inflammatory environments and neutralize inflammatory cytokines, free radicals and excitotoxins which are known to be released immediately following an acute brain injury event.2,11,13 Additionally, transfused MSCs might increase the survival and function of endogenous neuronal progenitors and insulted neurons by releasing neurotrophic growth factors and angiogenic factors.4,24 It has been shown in a collagenase-induced ICH animal model that intravenous transplantation of stem cells can reduce initial neurologic deterioration and exhibit anti-inflammatory and anti-apoptotic properties with attenuating brain edema.9
In this study, we have primarily focused on the possible neurogenesis enhanced by the transfused MSCs and the possible transdifferentiation of MSCs into cells with parenchymal markers. Based on our work in stroke and TBI as well as the present data, it is reasonable that the cells induce improved functional recovery partially attributed to brain remodeling, as is evident in the neurogenesis data provided here.
Conclusions
The underlying mechanisms behind the contribution of MSCs to neurological functional recovery have been well documented in ischemic stroke and traumatic brain injury animal models.10,12,15,17 These growth factors and angiogenic factors are believed not only to counteract host tissue damage or cell death but also to promote endogenous neurogenesis and migration. Future documentation of these growth factors in the ICH animal models will substantiate the underlying mechanisms of how transplanted MSCs contribute to the neurological functional recovery after ICH.
Acknowledgments and Funding
Special thanks to Susan MacPhee-Gray for editorial assistance. This work was supported by Internal (Departmental) Funds and National Institutes of Health grant NSO58581.
References
- 1.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, Mayberg M, Morgenstern L, Ogilvy CS, Vespa P, Zuccarello M. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116:e391–e413. doi: 10.1161/CIRCULATIONAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 5.Cheung RT. Update on medical and surgical management of intracerebral hemorrhage. Rev Recent Clin Trials. 2007;2:174–181. doi: 10.2174/157488707781662751. [DOI] [PubMed] [Google Scholar]
- 6.Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 7.Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res. 2000;871:57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- 8.Harvey RL, Chopp M. The therapeutic effects of cellular therapy for functional recovery after brain injury. Phys Med Rehabil Clin N Am. 2003;14:S143–S151. doi: 10.1016/s1047-9651(02)00058-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Lindvall O, Kokaia Z. Recovery and rehabilitation in stroke: stem cells. Stroke. 2004;35:2691–2694. doi: 10.1161/01.STR.0000143323.84008.f4. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18:813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- 16.MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, Colbourne F. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- 17.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. discussion 702–693. [DOI] [PubMed] [Google Scholar]
- 18.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57:1026–1031. doi: 10.1227/01.neu.0000181369.76323.50. discussion 1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seyfried D, Ding J, Han Y, Li Y, Chen J, Chopp M. Effects of intravenous administration of human bone marrow stromal cells after intracerebral hemorrhage in rats. J Neurosurg. 2006;104:313–318. doi: 10.3171/jns.2006.104.2.313. [DOI] [PubMed] [Google Scholar]
- 20.Seyfried D, Han Y, Lu D, Chen J, Bydon A, Chopp M. Improvement in neurological outcome after administration of atorvastatin following experimental intracerebral hemorrhage in rats. J Neurosurg. 2004;101:104–107. doi: 10.3171/jns.2004.101.1.0104. [DOI] [PubMed] [Google Scholar]
- 21.Seyfried DM, Yang DM, Savant-Bhonsale S. Mannitol enhances delivery of marrow stromal cells to the brain after experimental intracerebral hemorrhage. Brain Res. 2008;1224:12–19. doi: 10.1016/j.brainres.2008.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, et al. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38:2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- 23.Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 24.Shintani A, Nakao N, Kakishita K, Itakura T. Protection of dopamine neurons by bone marrow stromal cells. Brain Res. 2007;1186:48–55. doi: 10.1016/j.brainres.2007.09.086. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]