Abstract
The majority of animal studies examining the recovery of function following spinal cord injury use the BBB Open-Field Locomotor Scale as a primary outcome measure. However, it is now well known that rehabilitation strategies can bring about significant improvements in hindlimb function in some animal models. Thus, improvements in walking following spinal cord injury in rats may be influenced by differences in activity levels and housing conditions during the first few weeks post-injury. Swimming is a natural form of locomotion that animals are not normally exposed to in the laboratory setting. We hypothesized that deficits in, and functional recovery of, swimming would accurately represent the locomotor capability of the nervous system in the absence of any retraining effects. To test this hypothesis, we have compared the recovery of walking and swimming in rats following a range of standardized spinal cord injuries and two different retraining strategies. In order to assess swimming, we developed a rating system we call the Louisville Swimming Scale (LSS) that evaluates three characteristics of swimming that are highly altered by spinal cord injury— namely, hindlimb movement, forelimb dependency, and body position. The data indicate that the LSS is a sensitive and reliable method of determining swimming ability and the improvement in hindlimb function after standardized contusion injury of the thoracic spinal cord. Furthermore, the data suggests that when used in conjunction with the BBB Open-field Locomotor Scale, the LSS assesses locomotor capabilities that are not influenced by a retraining effect.
Keywords: swimming, functional recovery, contusion injury, outcome measure
INTRODUCTION
Numerous studies have demonstrated the importance of both limb-loading (propriospinal) and cutaneous feedback during the rehabilitation process following spinal cord injury (Barbeau et al., 1987; Harkema et al., 1997; Abel et al., 2002; Maegele et al., 2002; Bouyer and Rossignol, 2003). Currently, the most widely used rehabilitation method involving these types of feedback is body weight-supported treadmill training, which uses a combination of manual limb manipulation and treadmill contact/movement to retrain spared circuitry below the level of injury in a progressive fashion (Harkema et al., 1997; Fouad et al., 2000). However, very few studies have reported significant improvement in hindlimb function during overground locomotion following treadmill training in adult rats with contusive spinal cord injuries (Multon et al., 2003), and, as previously proposed by Fouad and colleagues (2000), we hypothesize that this is due to a retraining process that occurs as the rats move about in their cages. If this hypothesis is correct, terminal outcome measures based on overground walking could be influenced by a number of factors associated with the housing and handling of the rats, including bedding, cage size, overall health, and activity levels, such as overground activity associated with behavioral assessments.
One of the perceived weaknesses of body weight-supported treadmill training in both the clinical situation and in animal models is the relatively small number of step cycles that is achieved during each training session (Pepin et al., 2003; Fouad et al., 2000). Our laboratory has developed a rehabilitation strategy based on the work of Muir and Steeves (1995) that capitalizes on the natural swimming ability of rats (Smith et al., 2006). This approach greatly increases the step-cycle number and rate attained during each training session by not requiring the limbs to provide any weight support and by providing supplemental cutaneous feedback that is normally absent during swimming (Muir and Steeves, 1995; Smith et al., 2006). We have used swim training to demonstrate task specificity during retraining by showing that animals retrained to swim show improved swimming capabilities while showing no change in the recovery of overground locomotion (Smith et al., 2006). While several laboratories employ swimming as an assessment tool to evaluate locomotor recovery after injury (de Leon et al., 1994; Saunders et al., 1998; Hutchinson et al., 2004), a standardized evaluation method, such as that used for over-ground locomotion (Basso et al., 1995), does not currently exist. Thus, there is need for a standardized assessment tool to evaluate swimming.
We have found that normal rats swim using their hindlimbs only to provide forward motion. Their fore-limbs are tucked under their chins and are used only occasionally for steering. They exhibit rapid, alternating hindlimb strokes and a body position that is almost parallel to the water surface with head, neck, and approximately 30% of the dorsal surface of their backs above the surface. The body shows very little rotation along the long axis, which we describe as trunk instability (TI), and the tail is at or just below the surface, which we describe as body angle (BA). Most animals swim rapidly from point-to-point and seldom display exploratory behavior that is typical of open-field locomotion. Following a thoracic spinal cord injury, the majority of rats are variably dependent on their forelimbs for forward motion. They display a variable tail-down BA and variable rotation along the long axis of the body (TI). Hindlimb movements, when they occur, often show out-of-phase movements (lack of alternation) or simultaneous kicking (hopping). Each of these key characteristics—forelimb dependency, body position (angle and rotation), and hindlimb movement and alternation—appears to be dependent on injury severity and, to a lesser degree, duration of time post-injury.
The purpose of the current study was two-fold. First, we sought to evaluate the sensitivity and reliability of a novel swimming scale, the Louisville Swimming Scale (LSS), which was designed to complement the current outcome measure used for over ground locomotion, the BBB Open-Field Locomotor Scale (Basso et al., 1995). Second, we intended to compare the recovery of overground walking using the BBB Scale with the recovery of swimming using the LSS in swim-trained and untrained animals following spinal cord injuries of varying severity. Our findings show that the LSS is a sensitive and reliable predictor of swimming ability and spared white matter following mild-to-moderately severe thoracic spinal cord contusion injuries in the rat. Our findings also support the hypothesis that the recovery of walking and swimming are influenced by task-specific rehabilitation strategies.
MATERIALS AND METHOD
Pool Design
The swimming pool is a Plexiglas chamber that is 60″ long, 7″ wide, and 12″ deep. There is an adjustable Plexiglas ramp at one end of the pool, covered with a 5-mm-thick, soft neoprene computer mouse pad that provides exceptionally good traction, allowing even injured animals to exit the pool. The pool was filled to a depth of 8″ with warm tap water (27–30°C) for each swimming session and was thoroughly cleaned daily.
Pretraining and Testing
One week prior to surgery, animals were exposed to the swimming pool for several minutes per day for 5 consecutive days. During this time, the research personnel responsible for swimming and testing handled the animals on a daily basis and fed them sweetened cereal at the completion of each swimming session. Rats are natural swimmers and were quickly acclimatized to the task. By the third exposure to the water, none of the animals showed any signs of stress (escape behaviors, vocalization, disorientation); they simply swam the length of the pool to the ramp to crawl out of the water or to wait to be transferred back to the far end of the pool. In practice, it takes approximately 3–4 sec of swimming and 4–5 sec of transfer time per lap. In this way, upwards of 30 laps can be completed in each 4-min session. Baseline BBB and LSS scores were obtained for all the animals on the fifth day of pretraining prior to injury.
Spinal Cord Injury—NYU Contusion
Sixty-nine female Sprague-Dawley rats, ranging in weight from 160–200 g, were used for these experiments. All procedures involving experimental animals were performed according to the guidelines of the University of Louisville Institutional Animal Care and Use Committee (1996). Each animal was anesthetized with sodium pentobarbital solution (50 mg/kg i.p.) and was given prophylactic antibiotics (gentomycin sulfate, 15 mg/kg s.c.). Body temperatures were continuously monitored during surgery and were maintained between 36 and 37°C until the animal recovered from the anesthetic. A dorsal midline incision was made over the midthoracic spinal cord. A single level laminectomy was performed at the T9 vertebra. The spine was immobilized using clamps applied to the T8 and T10 spinous processes. The NYU Impactor (W. Young, Rutgers University, NJ) was used to produce either mild (6.25 g-cm), moderate (12.5 g-cm), moderately severe (25 g-cm), or severe (50 g-cm) weight drop contusion injuries. After injury, the wounds were closed in layers and a topical antibiotic ointment was applied to the incision. The animals were placed in recovery cages on heating pads until they recovered from the anesthesia. Injured animals were housed individually and received daily postoperative care, including manual bladder expression until adequate spontaneous voiding occurred. Five animals served as controls and received laminectomies without contusion injuries.
Swim Training With or Without Cutaneous Feedback
We based this approach on a report by Muir and colleagues (1995) who compared hindlimb function in spinal-injured chicks exposed to either treadmill walking or swimming. They utilized buoyant inverted centrifuge tubes suspended from the bottom of the swimming pool to provide phasic cutaneous input to the feet of spinal cord-injured chicks. For the present study, 1.5 mL centrifuge tubes were suspended from two perforated metal plates by 30 lb-test fishing line. The water depth was adjusted to ensure that the tubes consistently made contact with the paws and hindlimbs of the rats as they swam across the pool (Smith et al., 2006). Depending on their body angle, the tubes occasionally made contact with the tail and lower abdomen of the poorer swimmers. Animals were trained and tested either with or without cutaneous feedback, depending on the study design (see Experimental Design below).
Shallow-Water Walking
Rats were placed in a pool of similar dimensions to that used for swimming, with a Duplo floor to prevent slipping. The water level was maintained at 1.5″, which enabled the animal to walk across the pool with ~40% body-weight support, based on water displacement measurements. After each completed length, the rats were picked up and placed at the opposite end of the pool, and the process was repeated for 4 min. At the end of every length, the rats were rewarded with a small piece of sweetened cereal. Untrained rats in the study were handled daily and given the same number of cereal pieces as the trained animals.
Experimental Design
Five different training paradigms were used during the development of the LSS (Table 1). Study 1 involved moderately injured rats (12.5 g-cm NYU at T9) and a rehabilitation paradigm employing both swimming and shallow water walking, or no training. Study 2 used rats with moderately severe injuries (25 g-cm NYU at T9) that were divided into either swimming or nonswimming groups. Study 3 also used rats with moderately severe injuries divided into two groups, animals that engaged in both swimming and shallow-water walking and animals that did not train. Study 4 used mildly injured rats (6.25 g-cm NYU at T9) that did not train. Study 5 was identical to study 1 except the rats received severe injuries (50 g-cm NYU).
Table 1.
Training and Testing Conditions
| Study | No. | Severity (g-cm) | Group(s) | Testing conditions |
|---|---|---|---|---|
| 1 | 6 | 12.5 | S & SWW | (+), (−) Feedback |
| 5 | 12.5 | NS | (−) Feedback | |
| 2 | 8 | 25 | S | (−) Feedback |
| 8 | 25 | S | (+) Feedback | |
| 7 | 25 | NS | (−) Feedback | |
| 3 | 5 | 25 | S & SWW | (+), (−) Feedback |
| 6 | 25 | NS | (−) Feedback | |
| 4 | 8 | 6.25 | NS | (−) Feedback |
| 5 | 6 | 50 | S & SWW | (+), (−) Feedback |
| 5 | 50 | NS | (−) Feedback |
Different combinations of training and testing conditions used for the development of the LSS scale are shown.
S, swim; NS, non-swim; SWW, shallow-water walking.
Training Period and Intensive Swimming
For studies 1, 3, and 5, the rats in the training groups were reintroduced to the pool 10 days after injury. During the first week of training, levels were increased by one session per day from 1 × 4 min session on day 1 to 4 × 4 min sessions per day on day 4. Training was conducted with supplementary cutaneous feedback. For the second week of training, the rats swam a 1 × 4 min session with cutaneous feedback, followed by a single 4-min session of shallow-water walking per day. For the subsequent weeks, the rats completed 3 × 4 min swim sessions with cutaneous feedback, followed by 3 × 4 min sessions of shallow-water walking per day for 4 days a week. The animals in the untrained group remained in the training room throughout the sessions, were handled daily, and received sweetened cereal pieces, but did not swim. For study 2, the animals were reintroduced to the swimming pool 2 weeks post-injury and divided into two groups, swimming or nonswimming. The swimming (trained) group received 6 × 4 min swimming sessions daily, with cutaneous feedback. Each animal had at least 20 min rest between training sessions. The remaining animals were not trained. For all studies, the animals were trained (or remained in their cages) for 4 days each week (Monday to Thursday) and were assessed using the BBB Scale and LSS on the fifth day (Friday). Animals were assessed during a single 4-min session (walking in the open field or swimming laps) each Friday.
Behavioral Outcome Measures
The BBB Open-Field Locomotor Scale (Basso et al., 1995) involves placing the animal in an open field (36″ in diameter) and evaluating hindlimb function for 4 min. The BBB evaluation was performed weekly by the same two observers. The low end of the BBB scale (0–7) is characterized by individual hindlimb joint movements, whereas the intermediate (8–13) and high (14–21) parts of the scale are characterized by weight support, coordination, and paw position. Animals were assessed preoperatively and at weekly intervals thereafter. Scores were analyzed with repeated measures ANOVA followed by Student Newman-Keuls post-hoc t tests when appropriate.
The focus of this study was the LSS, an 18-point scale (0–17) with three ranges—0–5, 6–11, and 12–17 (Fig. 1). It was designed to evaluate swimming performance based on the three primary components of swimming described earlier, forelimb dependency, hindlimb activity and alternation, and body position. While the categories shown in Figure 1 were developed using the basic structure of the BBB Scale (Basso et al., 1995) as a rough guide, a strong emphasis was placed on the swimming patterns of normal and spinal cord injured rats.
FIG. 1.
LSS scoring sheet with scale categories. One sheet is used for each animal at each time point. The score of each item is indicated on the sheet and the total is noted. HL, hindlimb; FL, forelimb; Alt, alternation; Dep, dependency; TR, trunk; N/S, none or seldom; Inst, instability.
Swimming performance was assessed using the LSS prior to injury, at 2 weeks post-injury, and at weekly intervals thereafter. For each assessment, the animal swam one 4-min session and were scored by two independent observers who were blinded to the injury type or training group and by a third person who handled the animals and videotaped 1 min of each swim session with a Basler high-speed (60 Hz) digital camera or the entire session with a Sony Mini-DV digital camcorder. During the assessments, the rats were placed at one end of the pool and picked up and transferred when they reached the opposite end with the exit ramp. The majority of the animals readily swam the entire length without stopping. If a rat remained stationary, the animal handler would gently tap on the wall of the pool in order to stimulate the rat to resume swimming. If the rat failed to respond to this stimulus, the animal handler would pick the rat up and placed it at the far end of the pool, opposite the ramp. For study 2, rats in the cutaneous feedback group were evaluated with the supplementary cutaneous feedback (centrifuge tubes) in the pool, and the animals in the standard swimming group and the nonswimmers were tested without the tubes. For the other studies, all rats, regardless of their training status, were evaluated both with and without cutaneous feedback. The water depth and temperature were kept constant. LSS scores were analyzed with repeated measures ANOVA, followed by Student Newman-Keuls post-hoc t tests when appropriate.
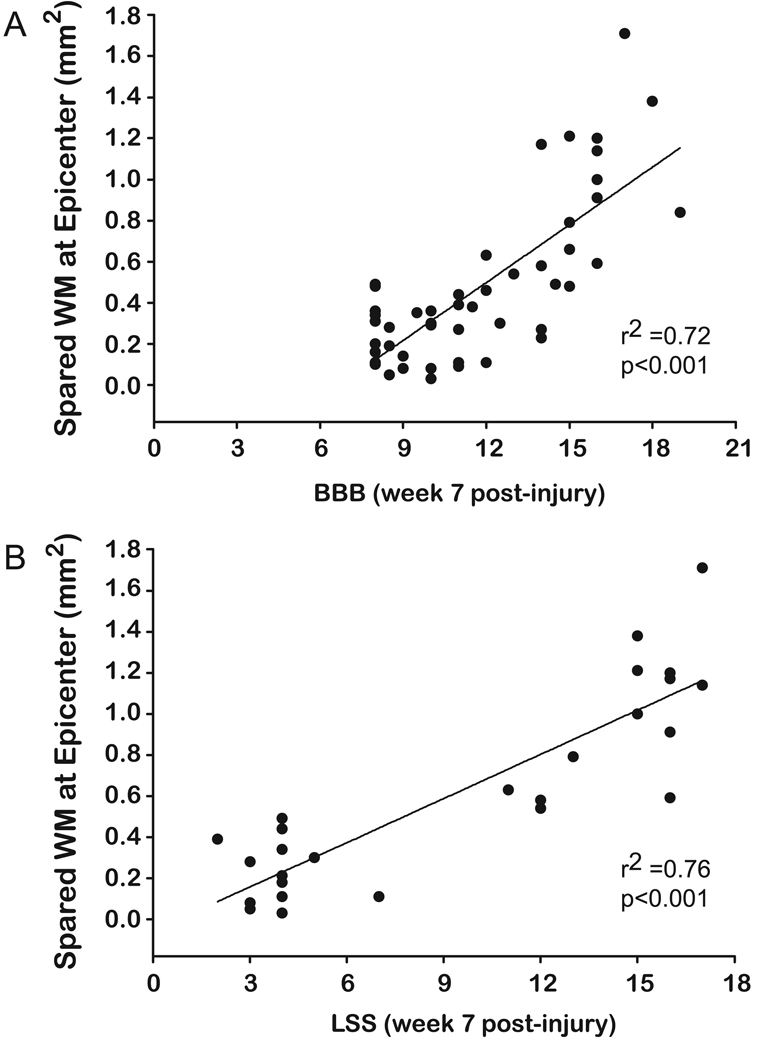
To examine the relationship between the BBB and LSS scores, scatter plots were constructed and the lines of best fit were plotted for the total data set and also for the individual groups. Pearson correlation coefficients were calculated for each line of best fit, with the level of significance set at 95%. Correlation coefficients were calculated for each of the four swimming conditions at weeks 4 and 7. Pearson correlation coefficients were calculated to examine the relationship between the BBB or LSS scores and the amount of white matter (WM) spared at the epicenter.
Histologic Outcomes
At the completion of each study, the rats were euthanized with excess anesthetic (ketamine/xylazine, 80 mg/kg and 10 mg/kg i.p., respectively) and perfused transcardially with calcium free tyrodes and 4% paraformaldehyde. The spinal cords were removed and post-fixed in the same fixative overnight, followed by cry-oprotection in 30% sucrose. Spinal cord tissue was cut at 30 μ and mounted onto five sets of glass slides. One set of slides was stained with cresyl violet, as previously described (Hadi et al., 2000; Magnuson et al., 2005). Every second section (every tenth section overall) was photographed with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI) attached to a Macintosh G4 computer. The sections were then traced using a Wacom Intuos (Vancouver, WA) drawing tablet with Appleworks 6.0 and saved as bit-map files. The bit-map images were opened in NIH image, and the areas of white matter, gray matter, and cavity were calculated for each section. The injury epicenter was determined as the section from each spinal cord with the least spared white matter. The area measurements were transferred to an Excel spreadsheet and graphed. The spared white matter at the injury epicenter was calculated as a percent of the white matter area taken from uninjured animals (Takami et al., 2002). Differences in spared white matter between the groups (trained vs. untrained) and injury levels (6.25, 12.5, and 25 g-cm) were assessed using two-way ANOVA. Finding no significant differences between the groups, one-way ANOVA was used to assess differences in spared white matter among injury severities for each group. Tukey HSD post-hoc t tests were used when appropriate.
Histologic analysis revealed that one rat had much greater spared white matter at the injury epicenter compared to that of the others in its group (25 g-cm injury). The amount of sparing in this rat was more than 2.5 times the SD greater than the mean; it was thus considered an outlier and excluded from the study.
Scale Reliability
The internal consistency of the five different components of the swimming scale (forelimb dependency, hindlimb movement, hindlimb alternation, trunk instability, and body angle) was examined using the reliability analysis covariance model to determine the reliability coefficient, Cronbach’s alpha. Cronbach’s alpha estimates the reliability of the scale based on the average inter-item correlations in the scale (i.e., the ratings of the items that make up the scale, which are summed to yield a total score). The consistency and flexibility of the scale were examined by testing the reliability of the scale during assessment of swimming ability on two (postop, post-training) swim testing time points (weeks 4 and 7) under four combinations of swim training and testing conditions (swim training or no training and swim testing with or without cutaneous feedback). The standardized Cronbach’s alpha reliability coefficient is reported here.
The relationships between injury severity and swim score were examined using Spearman rank correlations. The line of best fit was determined for the outcome measure of swimming ability for each of the experimental groups.
RESULTS
Category Definitions
With the LSS, animals that score in the 0–5 range are poor swimmers that rely heavily on their forelimbs (FL) for forward motion and have little or no hindlimb (HL) movement. Animals in this range also exhibit frequent-to-consistent, moderate or severe trunk instability while swimming. Animals that score in the 6–11 range are intermediate swimmers with occasional to frequent hindlimb movement but with some retained dependency on their forelimbs for forward motion, occasional-to-consistent mild or occasional-to-frequent moderate trunk instability (TI) and a moderate (21–45° tail down) body angle (BA) during forward motion (Fig. 1). Animals that score 12 or higher have consistent hindlimb movement, little-to-no forelimb dependency, little (seldom mild)-to-no trunk instability, and frequent-to-consistent alternating hindlimb movement.
Scale Reliability and Consistency
No swim training—swim testing without cutaneous feedback
Swimming ability was assessed in rats with 6.25, 12.5, 25, and 50 g-cm contusion (NYU impactor) injuries at T9 that received no swim training and were assessed without supplemental cutaneous feedback. Reliability coefficients for the swimming scale were very high at both post-injury testing times (week 4: 0.92 and week 7: 0.91) (Table 2A). All reliability analysis results for week 7 post-injury were very similar to those of week 4; therefore the results presented are for week 4.
Table 2.
Reliability Analysis
| A. Scale consistency: Cronbach’s alpha* | |||
|---|---|---|---|
| Time point | Score | Time point | Score |
| Wk 4 | 0.92 | Wk 7 | 0.91 |
| B. Interitem correlations | ||||
|---|---|---|---|---|
| Wk4 | HL | FL | Alt | TI |
| HL | ||||
| FL | 0.86 | |||
| Alt | 0.84 | 0.87 | ||
| TI | 0.67 | 0.78 | 0.73 | |
| BA | 0.41 | 0.63 | 0.51 | 0.69 |
| C. Item to scale total score correlations | ||
|---|---|---|
| Wk 4 | When excluded | Corrected |
| HL | 0.78 | 0.83 |
| FL | 0.85 | 0.92 |
| Alt | 0.83 | 0.88 |
| TI | 0.59 | 0.80 |
| BA | 0.36 | 0.61 |
Reliability analysis coefficients for the Louisville Swim Scale assessed in untrained groups in rats with NYU Impactor injuries at four severity levels (6.25, 12.5, 25, and 50 g-cm). HL, hind limb; FL, forelimb; Alt, alternation; TI, trunk instability.
(A) Scale consistency assessed by Cronbach’s alpha for all untrained NYU injured animals at weeks 4 and 7.
(B) Inter-item correlations among the scored characteristics for week 4.
(C) Item-scale correlations representing the contribution of each item in the scale to assess swimming ability at week 4.
Standardized.
Item-item correlations
Intercorrelations among the items (scored characteristics) showed that most of the items were highly related to each other and consistently measured swimming ability (Table 2B). The intercorrelations among the items was highest for HL movement, FL dependency, and hindlimb alternation (Alt). Intercorrelations of TI with the other items varied from low-to-moderate, and BA did not correlate highly with the other items. These results reflect the difference in the nature of the BA and TI items in that, in addition to assessing swimming, each also measures another characteristic, the body position of the animal in relation to swimming. Overall, the average inter-item correlation (i.e., all items) was moderate (0.69).
Item-scale correlations
The contribution of each item in the scale to assess swimming ability can be extrapolated by examining the change in the scale’s reliability when an item is excluded from the analysis (Table 2C). Reliability coefficients decreased when HL, FL, Alt, and TI each were excluded (from 0.92 to 0.87), showing that they provide a substantial contribution to the scale. Although coefficients increased slightly for BA (amount of change, 0.01–0.03), the corrected correlations for each item in the scale showed that they contribute highly (HL: 0.83, FL: 0.92, Alt: 0.88 and TI: 0.80) or moderately (BA: 0.61) to the scale’s reliability.
Lines of best fit
As previously noted, swimming scores decreased as injury severity increased. The relationship between swimming ability and injury groups was examined using nonparametric Spearman rank correlation analyses, which revealed a strong, negative correlation (rs = −0.81, rs2 = 0.66, p < 0.001). Low LSS scores (3–8) were observed in the more severe (moderately severe [25 g-cm] and severe [50 g-cm]) injury groups, and swim scores were substantially higher (9–17) in the less severely injury groups (mild-to-moderate [12.5 g-cm] and mild [6.25 g-cm]). As clearly illustrated by the scatter plot in Figure 2, a curvilinear function (r2 = −0.79, p < 0.001) best describes the consistent decline in swimming ability with increasing injury severity in untrained animals that leveled off following the moderately severe injury. There was little or no change in the swimming scores of the 50 g-cm group compared to those of the 25 g-cm injury group (i.e., a flat line: r2 = 0.23) in contrast to the steep decline in scores from the 6.25 and 12.5 to the 25 g-cm (r2 = −0.89) injury groups (Fig. 2). The swimming scores of the 50 g-cm injury group were comparable to those of the 25 g-cm group in these untrained animals. The 25 g-cm group also illustrated the highest variability in swimming ability. The same trend in function was also observed in the relationship between injury severity and open-field locomotor scores for the untrained animals shown in Figure 2 (r2 = −0.89) (Fig. 3).
FIG. 2.
Scatter plot representing the relationship between injury severity and LSS score at 4 weeks post-injury for untrained animals only. Assessments were done without supplemental cutaneous feedback. Animals included were the untrained control animals from studies 1 to 5 in Table 1 (NS). Similar scores at each time point are shown as markers placed side-by-side. The line of best fit is shown with a curvilinear function (r2, p < 0.001). The inset graph demonstrates the lines of best fit using two linear functions—r2 of 0.89 for 6.25, 12.5 and 25 g-cm injuries and r2 of 0.23 for 25 and 50 g-cm injuries.
FIG. 3.
Scatter plot representing the relationship between injury severity and open-field locomotor scores (BBB) for untrained animals at 4 weeks post-injury. Animals included were the untrained control animals from studies 1 to 5 in Table 1 (NS). Similar scores at each time point are shown as markers placed side-by-side. The line of best fit is shown with a curvilinear function (r2 = 0.85, p < 0.001).
Relationship Between LSS and BBB Scores
There was a strong relationship between swimming and walking ability in the no-swim (untrained) group (Fig. 4). Pearson correlation coefficients at both post-injury testing times were very high (week 4: r2 = 0.81, p < 0.001 and week 7: r2 = 0.88, p < 0.001, n = 30), showing that, as swimming scores increased, BBB scores correspondingly increased. The correlation between the LSS and BBB scores in the no-swim group, excluding the 6.25 g-cm injury group, decreased slightly (week 4: r2 = 0.66, week 7: r2 = 0.85, both p < 0.001, n = 22). Strong correlations were observed also for each of the other three swimming conditions (p < 0.001) (Table 3).
FIG. 4.
Scatter plot representing the relationship between the LSS and BBB scores for untrained animals at 4 weeks post-injury. Assessments were done without supplemental cutaneous feedback. Similar scores at each time point are shown as markers placed side-by-side. The line of best fit is shown with a linear function (r2= 0.81; p < 0.001). Correlations for the training groups are listed in Table 3.
Table 3.
Pearson Correlation Coefficients for BBB and LSS
|
Conditions of training and testing (n) |
Pearson correlation (r2) |
|
|---|---|---|
| Wk 4 | Wk 7 | |
| Untrained | ||
| Tested without feedback (30) | 0.81 | 0.88 |
| Excluding 6.25 g-cm injury (22) | 0.66 | 0.85 |
| Trained with feedback | ||
| Tested with feedback (25) | 0.77 | 0.69 |
| Trained with feedback | ||
| Tested without feedback (17) | 0.75 | 0.79 |
| Untrained | ||
| Tested with feedback (15) | 0.81 | 0.94 |
Pearson correlation coefficients for BBB and LSS scores for the untrained groups with and without the mild (6.25) injury group at weeks 4 and 7 post-injury. Also shown are results for the trained animals tested both with and without cutaneous feedback and the untrained animals tested with feedback. All, p < 0.001.
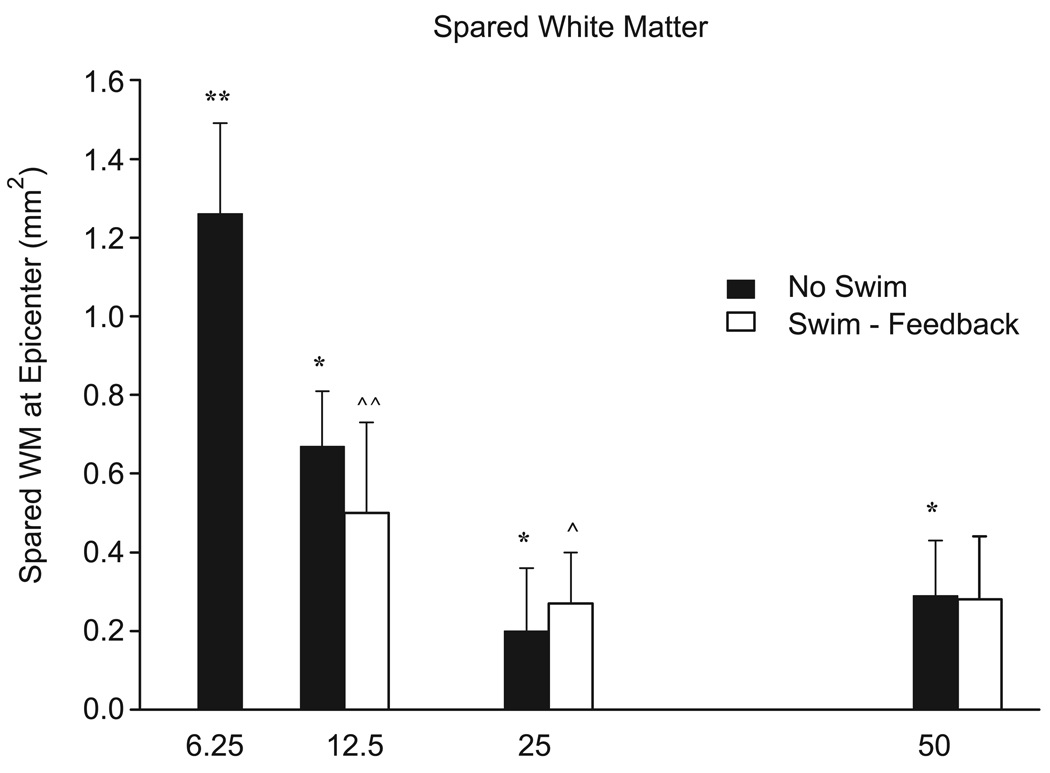
The widest range of LSS scores were observed in the moderately severe injury group. This was not the case for the BBB scores, however, as the mildly injury group displayed the highest variability in BBB scores. When we compared behavioral scores (LSS and BBB) with white matter sparing, we found good correlations with assessments made at 7 weeks post-injury for the 12.5, 25, and 50 g-cm injured groups. The correlation with the degree of WM spared varied slightly depending on the swimming group. Correlation coefficients between WM sparing and both LSS and BBB scores were significant in the no-swim (untrained) group (LSS: r2 = 0.64, p < 0.005; BBB: r2 = 0.43, p = 0.001). As expected, the best correlations for LSS and BBB scores versus white matter spared were observed when all injury severities were included (6.25, 12.5, 25, and 50 g-cm; [LSS: r2 = 0.76, p < 0.001, n = 26; BBB: r2 = 0.73, p < 0.001, n = 26; Fig. 5). There was no significant difference in WM sparing between swimming groups following a one-way ANOVA (Fig. 5). As expected, significant differences in WM sparing were observed between all the injury groups (no swim, p < 0.001; swim, p < 0.05) (Fig. 6).
FIG. 5.
Scatter plots representing the relationship between spared white matter at the injury epicenter and the terminal BBB score for all animals (A) and the terminal LSS score for untrained animals only (B), assessed without supplemental cutaneous feedback. The line of best fit is shown with a linear function for the BBB (r2 = 0.72, p < 0.001) and LSS scores (r2 = 0.76, p < 0.001).
FIG. 6.
The mean spared white matter (WM) at the injury epicenter (± SD) for each injury and swim group. There was no significant difference in WM sparing between the swimming groups but significant differences were observed between all injury groups. *no-swim (untrained) group, p < 0.001; ^, no-swim (untrained) group, p < 0.05.
Effects of Cutaneous Feedback in Swim Training and Testing Conditions
To assess the effects of cutaneous feedback during swim training and/or swim assessments (LSS scoring), rats were trained and/or tested with and without the presence of cutaneous stimulation to the hindpaws in the water leading to four different training/testing conditions (trained or untrained, tested with or without feedback). Reliability analyses were performed on LSS scores of three injury severity groups (NYU impactor, 12.5, 25 and 50 g-cm) for each of the four swim training/testing conditions. The groups with cutaneous feedback did not include rats with the 6.25 g-cm injury, which therefore resulted in a smaller range of swimming scores and smaller sample sizes compared to the no-training group.
The consistency of the swimming scale was relatively high at both weeks 4 and 7 post-injury testing times (range 0.79–0.88), with an average reliability coefficient of 0.84. Scale reliability results were similar at both weeks 4 and 7. As observed with the no-swim data, intercorrelations of the items were high among HL, FL, and Alt (range, 0.79–0.96), low-to-moderate for TI (>0.29–0.74), and BA illustrated low intercorrelations (0.13–0.45, 1 = 0.64) with the other items. However, the average of all of the inter-item correlations was lower (average = 0.51) than that observed in the untrained group (0.70). Examination of the relationship of the items to the scale closely resembled those of the untrained animals (correlations predominantly between 0.7 and 0.9). All Spearman rank correlation coefficients were moderately high (rs2 range, 0.55–0.77, p ≤ 0.001), illustrating that LSS scores declined as injury severity increased. As observed in the untrained animals, the swimming scores in the 50 g-cm group were comparable to those of the 25 g-cm injury group.
One difference between the results for the untrained and cutaneous feedback trained/tested groups was the line of best fit for each condition. With the scores for the 6.25 g-cm group removed, the lines of best fit for the cutaneous feedback trained/tested groups exhibited a less-steep decline overall. Due to this narrower range of injury severity levels, a linear function fit the data best rather than the curvilinear function found for the untrained group, although results were virtually identical for the linear and curvilinear (quadratic) functions (r2 = 0.54 and 0.54, 0.69 and 0.71, 0.63 and 0.63, respectively; all, p < 0.001). Thus, the wider range of injury severity levels in the untrained dataset yielded a better representation of the changes in swimming ability (LSS scores) based on injury severity (i.e., the initial steepness of the decline before leveling off when severity was increased).
An interesting pattern in the correlations of TI and BA was observed. As previously stated, the BA and TI items are related to the assessment of body position during swimming. Interestingly, a pattern in the inter-item correlations of TI and BA showed moderately high correlations of TI with the other items when training did not include cutaneous feedback (regardless of testing condition) and low inter-item correlations for BA when testing was conducted with cutaneous feedback (regardless of testing conditions). These results suggest that TI may be assessing swimming characteristics that are similar to other items being assessed when cutaneous feedback is not provided during training, rather than body position. Overall, BA had the least in common with the other items in its assessment of swimming characteristics compared to the other items, especially when testing included cutaneous feedback. Thus, body angles differed when the rats had no cutaneous feedback, suggesting that rats adopted a different swimming strategy when cutaneous feedback was present. The BA pattern indicates that the shared characteristics of swimming ability that are assessed by the other items are reduced even further in the BA item when cutaneous feedback is present during testing, perhaps indicating that its assessment may be related more to body position under these conditions.
Comparison of Swimming and Walking in Trained Animals
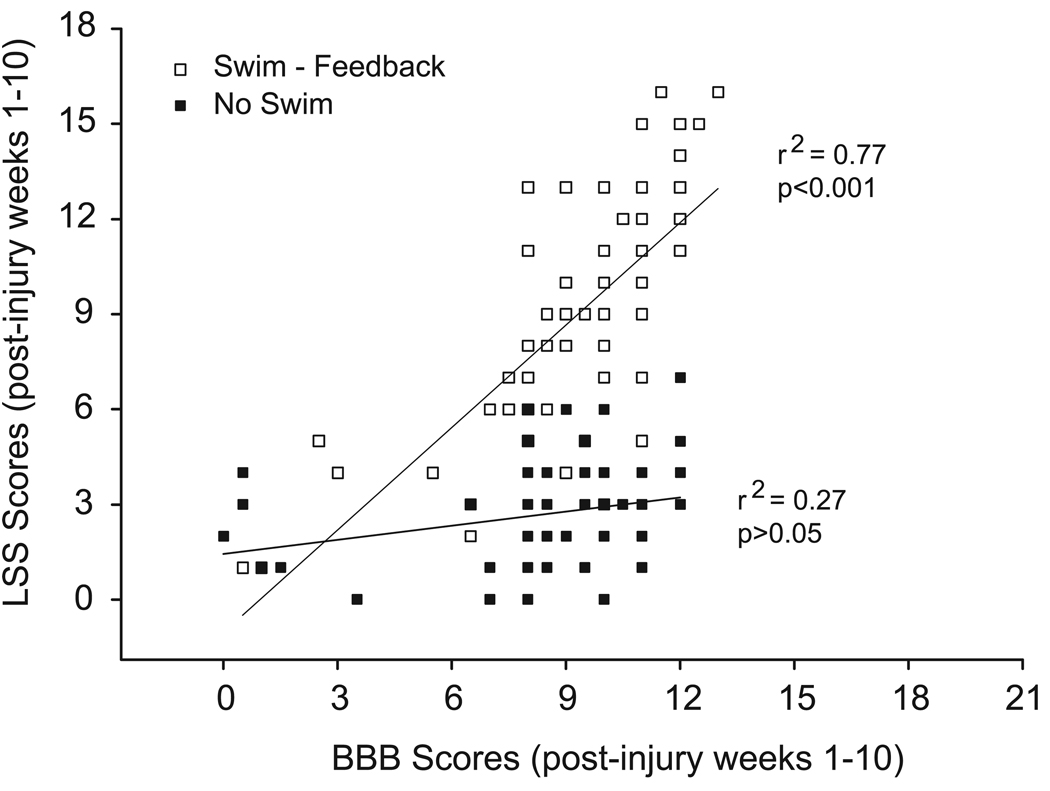
Recently, we showed that swim training, with or without supplemental cutaneous feedback, could positively influence the recovery of swimming as assessed by the LSS Scale, without influencing the normal course of recovery of walking as assessed by the BBB Scale (Smith et al., 2006). Here we compare the pattern of recovery of swimming in animals that received swim training with supplemental cutaneous feedback to the normal pattern of recovery of walking following a moderately severe 25 g-cm contusion injury at T9. In Figure 9, we plotted the weekly LSS and BBB scores for animals that received swim training with cutaneous feedback (swim-feedback) and those that received no swim training (no swim). We found that the LSS and BBB scores show significant correlation for the swim-trained animals (r2 = 0.77; p < 0.001) but show poor correlation for the untrained group (r2 = 0.27; p > 0.05), suggesting that the patterns of recovery of LSS and BBB scores over time are very similar when the animals are receiving swim training with supplemental cutaneous feedback. It is of interest to note that the slope of the line of best fit for the trained group is close to 1 (1.1) and is 0.15 for the untrained group, indicating that the LSS and BBB scores show almost parallel improvement in trained animals and a large functional separation between swimming and walking in the untrained group.
FIG. 9.
LSS and BBB scores for 12.5 and 25 g-cm injured animals that received swim training with cutaneous feedback (S, (+) Feedback) or remained untrained (NS, (−) Feedback; study 2 from Table 1). The LSS and BBB scores for each time point are plotted; a line of best fit is shown. The correlation coefficients are shown (r2). The slope of the fitted line for the trained animals is 1.1 and for the untrained group is 0.15.
DISCUSSION
Our findings show that the LSS is a sensitive and reliable assessment of swimming ability and predictor of white matter sparing after thoracic contusion injuries. This scale was developed to assess locomotor (swimming) performance following various thoracic spinal cord injuries.
Using the LSS
Similar to the BBB Scale, the LSS requires that animals are handled (“gentled”) and acclimated to the testing apparatus prior to injury. We found that 4 days of exposure to the pool (1 × 4 min session per day) was sufficient to alleviate overt signs of stress during swimming; and all the animals appeared to be completely comfortable in the water by the fifth day of pretraining during which the baseline LSS assessment was made. Similar to the BBB Scale, it did not appear that additional training was necessary post-injury to render successfully completed LSS assessments. The majority of animals showed no additional signs of stress when assessed at 2 weeks post-injury.
Unlike the BBB Scale in which certain scoring categories may be ignored based on an animal’s phase of recovery at a particular post-injury time point, the LSS is an additive scale with five categories that must be scored individually and summed to produce a final score. In common with the BBB Scale (Basso et al., 1995), each category includes frequency of occurrence (none, seldom, occasional, frequent, and consistent) (Fig. 1). Scorers must also decide on the predominate characteristic of a behavior, for example, by determining whether the predominate body angle is mild, moderate, or severe. In our experience, the predominate characteristic is usually expressed during the middle 2 min of the 4 min scoring session. In all cases, scorers are trained to score conservatively by assigning the lower score when in doubt. Each animal is scored using the sheet shown in Figure 1, which enables examiners to make notes and to ensure that each category is accurately assessed during the testing period, referring to digital video of the session when necessary. Also in common with the BBB Scale, the LSS was designed to be performed by two examiners, and each animal is observed and scored for 4 min. We found that it was advantageous to have a third person who handles the animals in order to allow the two examiners to stay focused during the testing period. Scorers were trained by observing normal and injured animals in the swimming pool, discussing scoring principles with experienced scorers, and viewing and discussing digital videos made during actual testing. When possible, we included a novice scorer in several actual scoring sessions as part of their training.
LSS Scoring Categories
The hindlimb movement and forelimb dependency categories of the LSS each have a range of 0 to 4 points (Fig. 1). For the hindlimb movement category, any movement is counted. For example, if an animal moves only the left hindlimb more than 95% of the time but fails to move the right hindlimb, the hindlimb score would still be a 4, indicating consistent hindlimb movement. The lack of movement in the right himdlimb would be reflected as a deficit in the hindlimb alternation category since only one hindlimb exhibited movement.
The forelimb dependency category assesses the level of dependency on the forelimbs for forward motion, both in terms of time (proportion of time the animal depends solely on its forelimbs) and effort (how much forward motion is provided by the forelimbs). Scores range from 0 for complete dependency to 4 for no use of the forelimbs to provide forward motion (Fig. 1). Forelimb movements associated with steering or pushing off the side of the pool are not considered since uninjured animals will occasionally use their forelimbs in this manner. The forelimb swimming stroke is accomplished by the paw reaching forward and then moving ventrally and caudally along the long axis of the body. The forelimb movement associated with steering is easily distinguished from the swimming stroke because the paw is moved laterally with the toes extended. In general, animals that show consistent hindlimb kicking with alternation but still exhibit some forelimb swimming strokes (<50% of swimming time) will score a 2 in this category.
The hindlimb alternation category ranges from 0 to 3 points and reflects the proportion of time an animal shows alternating hindlimb movements while swimming (Fig. 1). A normal rat will move its hindlimbs in a strict right-left alternating fashion while swimming. In contrast, injured animals have difficulty maintaining this pattern and show some combination of no hindlimb movement at all, independent kicking frequencies for the right and left hindlimbs, in-phase simultaneous kicking (i.e., hopping), or strict right-left alternation. Any movements other than strict alternation are scored in the hindlimb movement category.
The TI category is the most complicated category to score because examiners must determine both the degree of rotation (none, mild, moderate, or severe) and the frequency of rotation (none, seldom, occasional, frequent, or consistent). In practice, an uninjured animal with normal trunk position will not show more than 20° of rotation about the long axis of its body while swimming (Fig. 7). During assessment, this means that the depth of each paw during the stroke (extension) phase will be similar, not varying by more than 1 cm or so. An animal with moderate trunk instability will be rotationally unstable, typically resulting in 21–45° of rotation about the long axis. If the animal is rotated away from the scorer, some proportion of the far-side hindlimb and paw will be hidden and parts of the lower back will be visible to the scorer. Once the animal reaches an angle of ~20° of rotation, the far paw will extend only to the level of the near-side ankle. When the rotation exceeds ~45°, the far-side hindlimb and paw will be completely hidden from view and the lower back will be completely visible to the scorer. If the animal is rotated toward the scorer the far-side hindlimb and paw will extend deeper into the pool than the near-side paw. Once the animal reaches an angle of ~20°, the near-side paw will extend only to the level of the far side ankle during each stroke. When the rotation exceeds ~45°, the far-side hindlimb remains completely below the near-side paw throughout the stroke cycle and much of the lower abdomen of the animal is visible to the scorer. When an approximate angle of 20° is exceeded, the animal is scored as having moderate trunk instability. The animal is assigned a score of severe trunk instability when the predominate trunk rotation exceeds 45°. The score for this section is designed so that an animal will exhibit at least one feature for the assigned score. For example, an animal that has its trunk rotated 21–45° (enough so that the two limbs are obviously at different depths and/or the abdomen or back is visible to the scorer) would score a 1 on the trunk instability category 80% of the time. This same animal may also display occasional periods of severe rotation in which the rotation is extreme enough to force the animal to swim at least partly on its back.
FIG. 7.

Photographs of various categories of trunk instability (TI) observed after a thoracic spinal cord injury. (Al-4) Series of stroke cycles in a normal rat without TI. (Bl) Rat with occasional mild TI that is barely detectable. This same rat also experienced an episode of moderate TI in which the body rotated a little more (B2–4). (Cl–4) Rat exhibits moderate TI but experiences the rotation more frequently than the rat in B. (CI and 2) Episode of moderate rotation. (Dl–4) Rat experiencing an episode of severe TI. In this example, the animal rotates to a degree that forces it to stop swimming for a brief period of time in order to regain a more stable body position. Notice that the hindlimbs extend to one side (Dl) and the animal must use its forelimbs to help change the trunk position. (D4) The animal just after a better trunk position has been achieved. (E) Rat experiencing an episode of TI so severe that swimming is prevented (E4). (El–3) Same animal attempting to swim a lap. The animal begins by swimming on its back but as it attempts to correct its body position, the hindlimbs drop, preventing the animal from swimming any further.
The final category of the LSS is the body angle (head-to-tail angle relative to the water surface). Animals with normal-to-mild body angles will be positioned so that their head-to-tail angle will be no more than 20° relative to the surface of the water (Fig. 8). They will have some proportion of their back at or above the surface, the tail will be parallel to the surface, and the depth of each hind-paw during the stroke (extension) phase will not exceed 9 cm (3.5″) that can be easily recognized by placing a landmark on the side of the pool. Animals with a moderate body angle show a 21–45° tail-down tilt that is easily recognized because their entire back will be submerged, their tail will be angled towards the bottom of the pool, and the depth of each hindpaw during the stroke phase increases to between 9 and 14 cm (3.5–5.5″). Animals with severe body angle will have a head-to-tail angle greater than 46°, their tails will often touch the bottom, and their hindpaws will extend to a depth of 14 cm (5.5″) or more. These landmark depths can be adjusted for animals of different sizes, as required. In scoring this section, the examiners look for the predominate body angle over the 4-min period. Many animals will begin the session with a mild body angle but will adopt either a moderate or severe angle for the majority of the session. It is important to note that animals with a severe body angle cannot be scored for trunk instability because of their steep, tail-down swimming posture. These animals automatically receive a score of 0 for trunk instability, in addition to a 0 for body angle (Fig. 7E4). It was observed that animals with moderately severe and severe injuries often exhibit severe body angles for the first few weeks after injury but establish swimming skills sufficient to maintain a mild or moderate body angle even without developing frequent or consistent hindlimb movement.
FIG. 8.
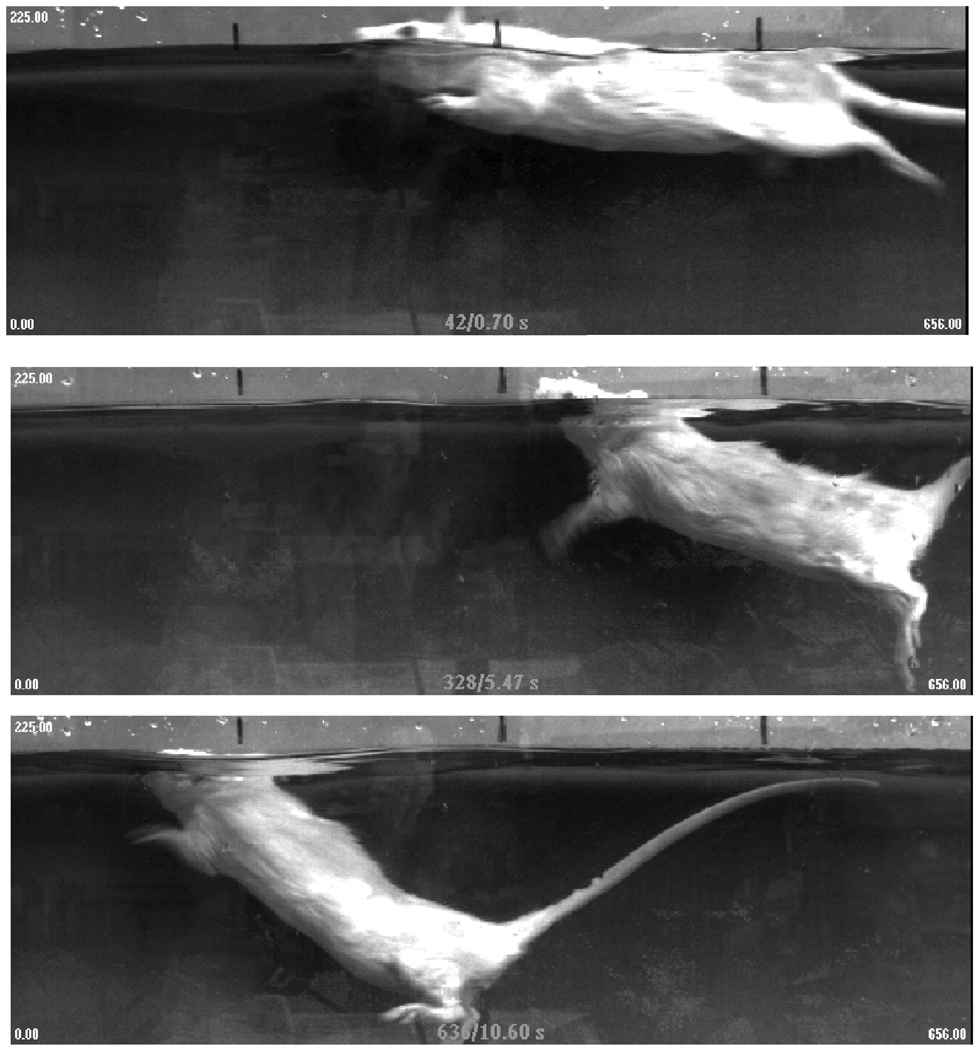
Photographs of the three categories of body angles observed after spinal cord injury. (A) Animal with normal/mild body position. Notice that the rostral portion of the back of the animal is out of the water and the trunk is parallel to the water surface. (B) Rat with moderate body angle. Animals in this category swim with their entire back under water and have a head-to-tail body angle of 21–45° from the surface of the water. The paws reach a depth of 9–14 cm. (C) The animal shown in the bottom panel is experiencing severe body angle. The body of the animal is more than 45° from the surface of the water, the head is pointing towards the ceiling, and the feet are more than 14 cm deep.
Reliability and Consistency of Scale Selection
To assess the reliability and consistency of the LSS, we examined the relationships between the scale and the two other primary variables, injury severity and spared white matter. We found strong correlations between the LSS and injury severity (Fig. 2), with the exception of the severely injured group (see below). We also found strong correlations between the LSS and white matter spared at the epicenter (Fig. 4). Most importantly, we found a high degree of reliability in the relationship between the different items scored in the LSS (inter-item reliability) as determined by the reliability coefficient (Cronbach’s alpha) (Table 2A).
Scoring Severely Injured Animals
In the untrained groups, we observed that the more severely injured animals exhibited lower LSS scores. An exception to this finding was the 50 g-cm NYU injury group. Animals in this group had LSS scores that were comparable to the animals in the untrained, 25 g-cm injury category. Since the animals were not trained, only a few in either injury group developed any hindlimb movement, which improved the LSS score. Likewise, some of the severely injured animals adopted a strategy to improve their trunk stability and body angle by spreading their hindlimbs apart and swimming using only their forelimbs. Thus, these animals will be more rotationally stable than some 25 g-cm injured animals that exhibit a more severe body angle and trunk instability when attempting to use their hindlimbs. This is a weakness of the LSS that may limit its use to studies using injuries that are less severe than 50 g-cm.
Temporal Profile of Functional Recovery
We found that the relationship between the LSS and BBB scores, over time, is strongly correlated for animals that received swim training with supplemental cutaneous feedback following moderately severe contusion injuries at T9 (Fig. 9). However, when these scores are plotted for a control group of animals that remained untrained, the LSS and BBB scores are not correlated. This finding suggests that the patterns of recovery of swimming and walking are similar for animals that receive swim training and distinct for animals that remain untrained. Thus, it appears that moderately severe thoracic injuries in rats induce long-lasting deficits in one of the normal modes of locomotion, that is, swimming, that can be reversed by a task-specific rehabilitation strategy (Smith et al., 2006). Importantly, this finding also argues, as suggested previously by Fouad et al. (2000), that the normal course of recovery of walking following incomplete thoracic contusion injuries is significantly influenced by retraining achieved as the animal moves about in its cage, is handled, and performs various assessment tasks, all potential sources of variability.
Subjective and Objective Assessments of Locomotion
The BBB Scales and LSS are based on real-time observations made by trained evaluators and, by definition, are subjective. In contrast, there are a number of well-characterized objective assessments of locomotion, each carrying specific advantages and disadvantages. Grid walking and other assessments that rely on hindlimb paw placement (ladder walking) are based on tasks that require high levels of sensorimotor integration to accomplish (Behrmann et al., 1992; Metz et al., 2000; Bolton et al., 2006) and may help identify sensory or sensorimotor deficits not easily assessed by the BBB Scale or LSS. However, these and other novel assessments (rope walking, narrow beam) have limited usefulness in assessing moderately severe or severely injured animals because they are not capable of performing the tasks at a measurable level. Kinematic assessment has been used successfully to assess the hindlimb activity during overground walking of rats and mice following spinal cord injury (Thota et al., 2001; Metz et al., 2000; Leblond et al., 2003). This assessment tool has the huge advantages of being objective and useful for any injury severity, including complete transection. It is, however, expensive and labor intensive to employ. Several years ago, Fouad and colleagues showed that treadmill training following a thoracic dorsal over-hemisection injury did not result in improved outcome measures, and they used a full battery of tests, including BBB, gridwalking, footprint analysis, and two-dimensional kinematics (Fouad et al., 2000).
In summary, the Louisville Swim Scale is a novel tool developed to aid in the overall behavioral assessment of rats following experimental spinal cord injury. It is sufficiently sensitive to distinguish between 6.25, 12.5, and 25 g-cm thoracic injuries delivered by the NYU Impactor. It shows strong inter-item reliability and a significant correlation with spared white matter. It provides potentially important additional information when used in conjunction with the BBB Open-Field Locomotor Scale because it assesses a locomotion behavior that injured animals are not normally exposed to. Importantly, we demonstrate that the LSS can distinguish between two groups of animals with similar BBB scores that received swim training with cutaneous feedback from those that remained untrained.
ACKNOWLEDGMENTS
The authors wish to acknowledge the excellent technical assistance of Christine Nunn, Kim Fentress, and Aaron Puckett. This work was supported by a grant from the Kentucky Spinal Cord and Head Injury Research Trust to DSKM and by NIH/NCRR P20-RR15576. DSKM is supported, in part, by Norton Health Care, Louisville, Kentucky.
REFERENCES
- Abel R, Schablowski M, Rupp R, Gerner HJ. Gait analysis on the treadmill—monitoring exercise in the treatment of paraplegia. Spinal Cord. 2002;40:17–22. doi: 10.1038/sj.sc.3101239. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Wainberg M, Finch L. Description and application of a system for locomotor rehabilitation. Med. Biol. Eng. Comput. 1987;25:341–344. doi: 10.1007/BF02447435. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Behrmann DL, Bresnahan JC, Beattie MS, Shah BR. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: Behavioral and histologic analysis. J. Neurotrauma. 1992;9:197–217. doi: 10.1089/neu.1992.9.197. [DOI] [PubMed] [Google Scholar]
- Bolton DA, Tse AD, Ballerman M, Misiaszek JE, Fouad K. Task specific adaptations in rat locomotion: Runway versus horizontal ladder. Behav. Brain Res. 2006;168:272–279. doi: 10.1016/j.bbr.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J. Neurophysiol. 2003;90:3640–3653. doi: 10.1152/jn.00497.2003. [DOI] [PubMed] [Google Scholar]
- De Leon R, Hodgson JA, Roy RR, Edgerton VR. Extensor- and flexor-like modulation within motor pools of the rat hindlimb during treadmill locomotion and swimming. Brain Res. 1994;654:241–250. doi: 10.1016/0006-8993(94)90485-5. [DOI] [PubMed] [Google Scholar]
- Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav. Brain Res. 2000;115:107–113. doi: 10.1016/s0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Hadi B, Zhang YP, Burke DA, Shields CB, Magnuson DS. Lasting paraplegia caused by loss of lumbar spinal cord interneurons in rats: No direct correlation with motor neuron loss. J. Neurosurg. 2000;93:266–275. doi: 10.3171/spi.2000.93.2.0266. [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Leblond H, L’Esperance M, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. J. Neurosci. 2003;36:11411–11419. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegele M, Muller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J. Neurotrauma. 2002;19:1217–1229. doi: 10.1089/08977150260338010. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Lovett MR, Coffee C, et al. Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J. Neurotrauma. 2005;22:529–543. doi: 10.1089/neu.2005.22.529. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp. Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;833:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- Muir GD, Steeves JD. Phasic cutaneous input facilitates locomotor recovery after incomplete spinal injury in the chick. J. Neurophysiol. 1995;74:358–368. doi: 10.1152/jn.1995.74.1.358. [DOI] [PubMed] [Google Scholar]
- Multon S, Franzen R, Poirrier AL, Scholtes F, Schoenen J. The effect of treadmill training on motor recovery after a partial spinal cord compression-injury in the adult rat. J. Neurotrauma. 2003;20:699–706. doi: 10.1089/089771503767869935. [DOI] [PubMed] [Google Scholar]
- Pepin A, Norman KE, Barbeau H. Treadmill walking in incomplete spinal-cord-injured subjects: 1. Adaptation to changes in speed. Spinal Cord. 2003;41:257–270. doi: 10.1038/sj.sc.3101452. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Kitchener P, Knott GW, Nicholls JG, Potter A, Smith TJ. Development of walking, swimming and neuronal connections after complete spinal cord transection in the neonatal opossum, Monodelphis domestica. J. Neurosci. 1998;18:339–355. doi: 10.1523/JNEUROSCI.18-01-00339.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RR, Shum-Siu A, Baltzley R, Bunger M, Baldini AD, Burke DA, Magnuson DSK. The Effects of swimming on functional recovery after incomplete spinal cord injury in rats. J. Neurotrauma. 2006;23:908–919. doi: 10.1089/neu.2006.23.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami T, Oudega M, Bethea JR, Wood PM, Kleitman N, Bunge MB. Methylprednisolone and interleukin-10 reduce gray matter damage in the contused Fischer rat thoracic spinal cord but do not improve functional outcome. J. Neurotrauma. 2002;19:653–666. doi: 10.1089/089771502753754118. [DOI] [PubMed] [Google Scholar]
- Thota A, Carlson S, Jung R. Recovery of locomotor function after treadmill training of incomplete spinal cord injured rats. Biomed. Sci. Instrum. 2001;37:63–67. [PubMed] [Google Scholar]