Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) are highly effective in treating the pain and inflammation associated with osteoarthritis and rheumatoid arthritis, but it is well recognized that these agents are associated with substantial gastrointestinal toxicity. Treatment guidelines suggest that patients with one or more risk factors for NSAID associated ulcers should be prescribed preventive treatment. However, well over 80% of such patients may not receive an appropriate therapeutic intervention. Multiple strategies are available to reduce the risk for NSAID associated gastrointestinal complications. First, risk may be reduced by using non-NSAID analgesics. Second, use of the minimum effective dose of the NSAID may reduce risk. Third, co-therapy with a proton pump inhibitor or misoprostol may be desirable in at-risk patients. Use of cyclo-oxygenase-2 inhibitors may also reduce the risk for gastrointestinal events, although this benefit is eliminated in patients who receive aspirin, and cyclo-oxygenase-2 inhibitors may increase cardiovascular adverse events. The optimal management of NSAID related gastrointestinal complications must be based on the individual patient's risk factors for gastrointestinal and cardiovascular disease, as well as on the efficacy and tolerability of both the NSAID and accompanying gastroprotective agent.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most prescribed classes of medications worldwide, with over 111 million prescriptions written between September 1999 and August 2000 [1]. In addition, more than 30 billion over-the-counter (OTC) NSAID tablets are purchased annually. NSAID use is common in all age groups, with most frequent use among the elderly, of whom nearly 70% take NSAIDs at least weekly.
The NSAIDs are highly effective in treating the pain and inflammation associated with osteoarthritis (OA) and rheumatoid arthritis (RA), but it is well recognized that these agents are associated with an increased risk for upper gastrointestinal toxicity, ranging from dyspepsia to gastroduodenal ulcers and bleeding. Although only a small proportion of patients who use these agents develops gastrointestinal complications, the widespread use of these agents magnifies the frequency into a large absolute number of clinical gastrointestinal events. Individually and together, NSAID related adverse events have an important impact on medical outcomes, patient quality of life, and health care costs.
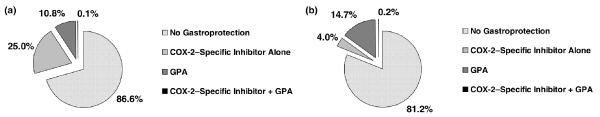
Treatment guidelines suggest that patients with one or more risk factors for NSAID associated upper gastrointestinal tract ulcer complications should be prescribed preventive treatment. Despite these recommendations, gastroprotective strategies (e.g. acid suppressive drugs, misoprostol, or selective cyclo-oxygenase [COX]-2 inhibitors) appear to be under-utilized in patients who receive NSAIDs. A retrospective observational cohort study conducted in The Netherlands [2], using data from early 1996 to mid-2002, found that only 7.9% of NSAID users during this time period received a preventive therapy. Of these, 6.6% received gastroprotective agents, and an additional 1.3% received COX-2 inhibitors. A greater percentage of patients with one or two risk factors for upper gastrointestinal injury received gastroprotective drugs, but well over 80% of these patients were provided with no preventive strategy (Fig. 1). A large treatment gap persists, despite an increase in the overall prevalence of use of gastroprotective strategies from 5.1% in 1996 to 15.9% in 2002 [2].
Figure 1.
Underutilization of preventive strategies in patients receiving NSAIDs. (a) Patients with one risk factor for upper gastrointestinal ulcer complications. (b) Patients with two or more risk factors for upper gastrointestinal ulcer complications. Percentages total more than 100% because of rounding. COX, cyclo-oxygenase; GPA, gastroprotective agent; NSAID, nonsteroidal anti-inflammatory drug. Reproduced with permission from Oxford University Press [2].
Risk factors for NSAID related gastrointestinal complications
In view of the recent controversies surrounding the cardiovascular effects of COX-2 selective agents, the number of patients who receive traditional NSAIDs is likely to increase substantially. Consequently, the number at risk for NSAID related gastrointestinal complications is also expected to increase. Accurate identification of those who are at high risk for NSAID related gastrointestinal toxicity is therefore essential. (These issues are taken up in greater detail elsewhere in this supplement [3].)
A number of factors have been identified that increase the risk for NSAID associated upper gastrointestinal complications, including ulcers [4]. Use of multiple NSAIDs (including OTC NSAIDs and aspirin) and high dosages of medication increase risk. Interestingly, the greatest relative risk for gastrointestinal complications exists during the first month of treatment. Other important risk factors include prior ulcer complications, advanced age, and concomitant corticosteroid or anticoagulant use. The severity of RA may appear to increase risk independently for adverse gastrointestinal events. In contrast, dyspepsia and other upper gastrointestinal symptoms do not predict the development of upper gastrointestinal events [1].
The role of Helicobacter pylori infection in NSAID associated gastrointestinal disease remains somewhat controversial. However, a recent meta-analysis [5] indicated that both H pylori infection and NSAID use are independent risk factors for gastrointestinal complications. It is clear that eradication of the infection, although indicated in all patients with a history of ulcer disease, is insufficient to reduce the NSAID associated risk for ulcer and its associated complications.
Pharmacotherapeutic strategies for prevention and treatment of NSAID related ulcers
Several strategies are available to reduce the risk for NSAID associated gastrointestinal adverse events. First, risk may be reduced by the use of non-NSAID analgesics such as acetaminophen, but this strategy is unlikely to be sufficient in all patients or in those with more severe disease. Second, the use of the minimum effective dose of NSAID may reduce the risk for complications. Third, co-therapy with gastroprotective agents may be necessary in patients at high risk for complications. Although these agents reduce the risk for gastrointestinal events, each is associated with its own spectrum of side effects. In addition, increased medication burden (cost and compliance issues) must be considered, particularly in elderly patients who are likely to be receiving multiple medications for concomitant conditions. Recommendations for the treatment of NSAID related dyspepsia and mucosal injury are summarized in Table 1[6].
Table 1.
Recommendations for the treatment of NSAID related dyspepsia and mucosal injury
| Clinical situation | Recommendation |
|---|---|
| Dyspepsia | Empirical treatment with H2 receptor antagonist or PPI; individualize therapy |
| Helicobacter pylori infection | Treatment to eradicate infection in patients with a history of peptic ulcer; PPI therapy |
| Active gastroduodenal ulcer | |
| NSAID discontinued | Treatment with H2 receptor antagonist or a PPI |
| NSAID continued | Treatment with a PPI |
| Prophylactic therapy | Treatment with a PPI or misoprostol or a COX-2 (preferential) or selective NSAID |
COX, cyclo-oxygenase; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton pump inhibitor. Adapted, with permission, from [6]. Copyright © 1999 Massachusetts Medical Society. All rights reserved.
Finally, the use of COX-2 inhibitors may reduce the risk for gastrointestinal events. However, this benefit is eliminated in patients who receive aspirin, and COX-2 inhibitors may also be associated with increased risk for adverse cardiovascular events.
Misoprostol
The efficacy of misoprostol, a synthetic prostaglandin E1 analog, as prophylaxis for NSAID related gastric ulcers has been demonstrated in several trials. In one study [7] 638 patients who were taking a traditional NSAID but did not have a gastric or duodenal ulcer at screening were randomly assigned to 12 weeks of treatment with misoprostol 200 μg or placebo four times daily. Endoscopy was performed at baseline and after 4, 8, and 12 weeks. In the intent-to-treat population, 1.9% of those receiving misoprostol and 7.7% of those in the placebo arm developed a gastric ulcer.
In a second study [8] 8843 ulcer free patients receiving continuous therapy with NSAIDs were randomly assigned to therapy with misoprostol 200 μg/day or placebo four times daily. The primary outcome measure was the development of serious upper gastrointestinal complications, as detected by clinical symptoms or findings. Over the course of the 6-month study, the incidence of serious upper gastrointestinal complications was 40% lower among patients who received misoprostol than among those who received placebo. More patients in the misoprostol group (42%) than in the placebo group (36%) withdrew from therapy, primarily because of gastrointestinal side effects (e.g. diarrhea, abdominal pain, and flatulence).
Although these data indicate that misoprostol is effective in the prevention of ulcers, poor compliance because of the relatively high rate of adverse events and the need for frequent dosing are important concerns. One study [9] found that lower doses of misoprostol are better tolerated. However, the drug had to be taken at least twice daily to provide effective prophylaxis against NSAID related ulcers as quantified by endoscopy. Outcomes data are not available for such lower dosages.
Misoprostol may also be effective in the treatment of patients with established NSAID associated ulcers, but comparative studies suggest that proton pump inhibitors (PPIs) are substantially more effective in these patients [10].
The potential for poor compliance with misoprostol may, in part, be reduced by fixed dose combinations of misoprostol with an NSAID. Arthrotec® (Pfizer, Inc., New York, USA), a combination agent consisting of diclofenac 50 mg or 75 mg plus misoprostol 200 μg, was evaluated in a clinical study that enrolled 572 patients with symptomatic OA of the knee or hip and a history of gastric or duodenal ulcers or 10 or more erosions [11]. In this study, endoscopically diagnosed ulcers were significantly less frequent in patients receiving the combination agent than in those receiving diclofenac alone. Outcomes data are not available for this combination.
Sucralfate
Sucralfate, a basic aluminum salt of sucrose octasulfate, forms an ulcer adherent complex at duodenal ulcer sites, protecting the ulcer and promoting healing. It may also inhibit pepsin activity in gastric fluid. Sucralfate has been shown to be effective in the treatment of NSAID associated duodenal ulcers, particularly when the NSAID is stopped, but it is not effective in the treatment or prevention of NSAID related gastric ulcers [6]. Its use is not recommended because of the availability of far superior therapeutic options.
H2 receptor antagonists
H2 receptor antagonists modulate gastric pH through thecompetitive inhibition of the action of histamine at H2 receptor sites on the gastric parietal cell. The efficacy of the H2 antagonist famotidine at high dose (double the usual dose) for preventing ulcers in patients receiving long-term therapy with NSAIDs was examined in a double blind, parallel group, randomized study conducted in patients with RA or OA [12]. A total of 285 patients were randomly assigned to therapy with famotidine (20 mg or 40 mg twice daily) or placebo, and followed for 24 weeks for the incidence of gastric or duodenal ulceration. Gastric ulcers were observed in 13% and 8% of patients receiving the 20 mg and 40 mg dosages of famotidine, respectively, and in 20% of patients in the placebo group (P = 0.03 for famotidine 40 mg versus placebo). The percentages of patients with gastroduodenal ulcers were significantly lower in the famotidine 20 mg group (4%) and the 40 mg group (2%) compared with the placebo group (13%).
Although this agent has been shown to be effective in preventing ulcers in patients taking NSAIDs in this single study, H2 receptor antagonists are not recommended for routine treatment of asymptomatic patients for a variety of reasons, including their potential to mask dyspeptic symptoms associated with mucosal injury [6]. Furthermore, data suggest that the H2 receptor antagonists are less effective in healing gastroduodenal ulcers than are PPIs, whether or not NSAIDs are continued, and are inferior in preventing ulcer recurrence [13,14].
Proton pump inhibitors
Unlike the H2 receptor antagonists, PPIs act by binding irreversibly to resident proton pumps (H+/K+-ATPase), thus inhibiting the final common pathway for acid secretion. PPIs are administered as prodrugs that are activated in the acidic environment of the parietal cell secretory canaliculus [15]. Once converted to their active form, PPIs bind to cysteine residues in the proton pump and inhibit acid secretion into the canalicular lumen [16].
Ulcer healing and prevention of relapse
Several large scale clinical trials have compared the efficacies of PPIs and H2 receptor antagonists in patients with established ulcers. In one trial [13] 541 patients with an ulcer or more than 10 erosions were randomly assigned to receive omeprazole 20 mg/day or 40 mg/day, or ranitidine 150 mg twice daily. Healing of gastric lesions was observed in significantly more patients who received omeprazole (80%) than in those who received ranitidine (61%; P < 0.001). Ulcer healing was substantially more rapid with the PPI than with the H2 receptor antagonist.
In another trial [14] 353 patients with confirmed gastric ulcers were randomly assigned to therapy with lansoprazole 15 mg/day or 30 mg/day, or ranitidine 150 mg twice daily. After 8 weeks of therapy, ulcer healing rates in the lansoprazole group ranged from 69% to 73%, as compared with 53% for ranitidine.
The efficacies of PPIs and misoprostol have also been compared in several studies conducted in patients with established ulcers and erosions. In a double blind study conducted by Hawkey and colleagues [10] 935 patients who had developed ulcers or more than 10 erosions in the stomach or duodenum, or both, were randomly assigned to 4–8 weeks of therapy with omeprazole (20 mg/day or 40 mg/day) or 200 μg misoprostol four times daily. Patients in whom treatment was successful were re-randomized to treatment with 20 mg/day omeprazole, 200 μg misoprostol twice daily, or placebo for an additional 6-month maintenance phase. Patients were assessed clinically and endoscopically during both phases. Treatment success, defined as the absence of ulcers or the presence of fewer than five erosions at each site and no more than mild dyspepsia, was observed in 76% and 75% of patients receiving omeprazole 20 mg/day and 40 mg/day, respectively, and in 71% of those given misoprostol. The rate of gastric ulcer healing was significantly higher in patients receiving the 20 mg dosage of omeprazole (87%) than in those receiving misoprostol (73%); the rate of duodenal ulcer healing was higher in the omeprazole 20 mg and 40 mg groups (93% and 89%, respectively) than in the misoprostol group (77%). The percentage of patients with moderate or severe dyspepsia declined to a significantly greater extent in the omeprazole 40 mg group than in the misoprostol group. During the maintenance phase, significantly fewer patients in the omeprazole 20 mg/day group than in the misoprostol group suffered a relapse (27% of patients in the placebo group).
A prospective, double blind, active and placebo controlled study conducted by Graham and colleagues [17] compared the efficacy of lansoprazole and misoprostol in the prevention of ulcer recurrence among patients with a history of endoscopically detectable gastric ulcers who were free from H pylori infection at baseline. A total of 537 patients were randomized to 12 weeks of therapy with 30 mg/day lansoprazole, 15 mg/day lansoprazole, misoprostol 200 μg four times daily, or placebo. The primary end-point was ulcer status as determined by endoscopy. Evaluable patients in the lansoprazole groups remained free from gastric ulcer significantly longer than those who received placebo but for a shorter time than those who received misoprostol. After 12 weeks of therapy, 93% of patients in the misoprostol group were free from gastric ulcers, compared with 80% and 82% in the lansoprazole 15 mg and 30 mg groups. Significantly more patients in the misoprostol groups reported treatment related adverse events and withdrew from the study. When withdrawal from the study was considered as a treatment failure, lansoprazole and misoprostol were clinically equivalent.
Outcome studies with proton pump inhibitors
The efficacy of PPIs in the prevention of recurrences of ulcer complications from long-term, low-dose aspirin use was examined in a trial conducted in 123 patients in Hong Kong [18]. All patients initially received antibiotic treatment for H pylori plus 30 mg lansoprazole twice daily, followed by treatment with famotidine 20 mg twice daily for 5 weeks. Patients with healed ulcers and in whom H pylori infection had been eradicated were randomized to 12 months of therapy with aspirin 100 mg/day plus lansoprazole 30 mg/day or to aspirin plus placebo. Recurrence of ulcer complications was observed in 1.6% of patients in the lansoprazole group (one episode of gastrointestinal bleed) and in 14.8% of those in the placebo group (nine gastrointestinal bleeds). After controlling for confounding covariates, patients who received placebo were at a 9.6-fold increased risk for recurrent ulcers.
Similarly, a study conducted by Chan and colleagues [19] demonstrated that PPIs are superior in preventing recurrent ulcer complications in patients receiving aspirin or other NSAIDs who were also infected with H pylori. In this study, 400 patients with a history of upper gastrointestinal bleeding were treated with omeprazole 20 mg/day until ulcer healing, and then assigned to treatment with omeprazole 20 mg/day or to 1 week of H pylori eradication therapy. Patients previously receiving aspirin were treated with aspirin 80 mg/day; those receiving NSAIDs took naproxen 500 mg twice daily. The risk for recurrent bleeding was 1.9% in aspirin patients who received eradication therapy and 0.9% in those who received omeprazole; among patients receiving other NSAIDs the corresponding risks were 18.8% with eradication therapy and 4.4% with omeprazole.
COX-2 inhibitors
The reduction in incidence of gastrointestinal complications in patients receiving COX-2 inhibitors is reviewed elsewhere in this supplement [3]. Given recent concerns regarding the cardiovascular safety of this class of medications, and additional data showing that the incidence of gastrointestinal complications is not reduced in patients who receive concomitant aspirin, the risks and benefits of COX-2 inhibitors must be carefully balanced when considering therapy with these agents. In light of these concerns, in July 2005 the US Food and Drug Administration recommended labeling changes for OTC and prescription NSAIDs, including COX-2 selective NSAIDs, that include a boxed warning highlighting the potential not only for increased risk for cardiovascular events but also for life-threatening gastrointestinal bleeding associated with their use [20].
Existing arthritis treatment guidelines, published before the COX-2 cardiovascular safety controversy, recommend that patients at risk for ulcer disease who require treatment for arthritis receive either COX-2 selective agents or a nonselective NSAID in combination with a PPI [21-23]. The relative efficacies of these strategies were compared in a randomized, controlled study conducted in 287 patients who had used NSAIDs for arthritis and had a history of ulcer bleeding [24]. Patients were randomized to 6 months of therapy with celecoxib 200 mg twice daily plus daily placebo or 75 mg diclofenac twice daily plus 20 mg/day omeprazole, and followed for recurrent ulcer bleeding. The primary endpoint occurred in seven patients who received celecoxib and nine patients who received diclofenac plus omeprazole; the corresponding probabilities of recurrent bleeding during the study were 4.9% and 6.4% (Fig. 2). Adverse events, including hypertension, peripheral edema, and renal failure, occurred in 24.3% and 30.8% of patients receiving celecoxib and diclofenac plus omeprazole, respectively. Later analysis of endoscopic data from this study [25] showed that neither regimen prevented ulcer recurrence.
Figure 2.
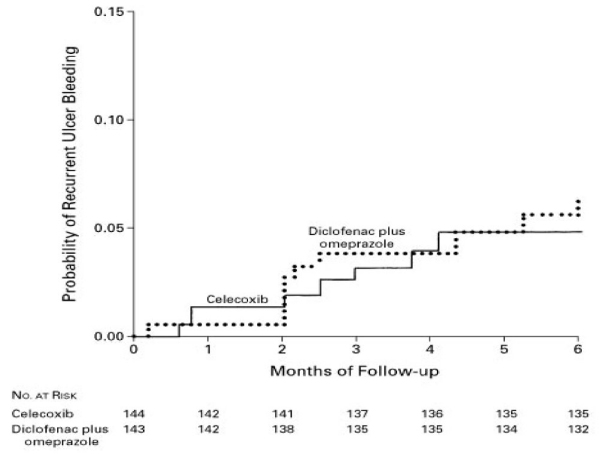
Cumulative probability of recurrent ulcer bleeding in patients receiving celecoxib or diclofenac plus omeprazole. Reproduced with permission from [24]. Copyright © 2002 Massachusetts Medical Society. All rights reserved.
It should be noted, however, that additional long-term outcomes studies will be necessary to quantify better the benefits of PPI co-therapy for chronic NSAID users compared with the selective COX-2 inhibitors with regard to the risk for bleeding throughout the gastrointestinal tract, because PPI co-therapy affects only injury in the acid vulnerable proximal gastrointestinal tract.
Guidelines for anti-inflammatory therapy and ulcer prophylaxis in the post-rofecoxib/valdecoxib age
The decision by Merck to voluntarily withdraw rofecoxib from the market (followed recently by the removal of valdecoxib [Bextra®] by Pfizer) has had a profound impact on clinician and patient choices regarding NSAIDs, COX-2 inhibitors, and prophylactic therapy to prevent ulcers. Recently, a guide to NSAID therapy in the post-rofecoxib/valdecoxib age was proposed in the American Journal of Managed Care (Table 2) [26]. It should be noted that these guidelines have not been endorsed by a major medical society, but in light of recent evidence they represent a reasonable approach to selecting therapy for patients who require NSAIDs.
Table 2.
The clinicians' guide to NSAID therapy in the post-rofecoxib/valdecoxib age
| Risk | No/low NSAID gastrointestinal risk | NSAID gastrointestinal risk |
|---|---|---|
| No cardiovascular risk (no aspirin) | Traditional NSAID | Traditional NSAID + PPI |
| or | ||
| Coxib + PPI1 (if necessary) | ||
| Consider non-NSAID therapy | ||
| Cardiovascular risk (consider aspirin) | Traditional NSAID2 plus PPI if gastrointestinal risk warrants gastroprotection | A gastroprotective agent must be added if a traditional NSAID2 is prescribed |
| Consider non-NSAID therapy | Consider non-NSAID therapy |
1The US Food and Drug Administration has determined that over-the-counter and prescription nonsteroidal anti-inflammatory drugs (NSAIDs; including cyclo-oxygenase [COX]-2 selective agents), when used as monotherapy, may be linked to potentially life-threatening gastrointestinal bleeding [20]. 2Ibuprofen should be used cautiously in individuals taking aspirin. PPI, proton pump inhibitor. Reproduced with permission from [25]. Copyright © 2004 Am Med Pubg Assn.
For patients without cardiovascular risk factors who require aspirin prophylaxis and who are at low risk for gastrointestinal events, monotherapy with a traditional NSAID remains the initial approach to anti-inflammatory therapy. Data suggest that strategies consisting of COX-2 inhibitors or NSAIDs plus a PPI are equally effective in reducing the risk for ulcer bleeding. Therefore, patients who do not require aspirin and who are at relatively high risk for NSAID related gastrointestinal events may be prescribed a COX-2 inhibitor or an NSAID with PPI co-therapy. In treating those with cardiovascular risk that requires aspirin prophylaxis, COX-2 inhibitors should generally be avoided; these patients should receive a traditional NSAID plus a PPI if the absolute risk of a gastrointestinal event warrants gastroprotection.
Conclusion
The management of NSAID related ulcer disease must be individualized according to the patient's risk factors for gastrointestinal complications and cardiovascular disease, as well as the efficacy and tolerability of both the NSAID and gastroprotective co-therapy.
As discussed elsewhere in this supplement [3], the concomitant use of aspirin, particularly in an older population at generally higher cardiovascular risk, is an important factor to consider. Although it is clear that COX-2 inhibitors reduce the risk for gastrointestinal events, use of concomitant aspirin negates the gastrointestinal benefits of COX-2 inhibitors. Moreover, use of certain NSAIDs such as ibuprofen may interfere with the antiplatelet effect of aspirin. In general, a strategy that carefully balances treatment benefits, potential for gastrointestinal complications, and cardiovascular risk is warranted in all patients who receive NSAIDs.
Finally, given the widespread use of OTC NSAIDs, careful attention should be paid to educating patients about the gastrointestinal and cardiovascular risks associated with NSAIDs and the potential for undesirable interactions among these medications.
Abbreviations
COX = cyclo-oxygenase; NSAID = nonsteroidal anti-inflammatory drug; OA = osteoarthritis; OTC = over-the-counter; PPI = proton pump inhibitor; RA = rheumatoid arthritis.
Competing interests
JMS has received a grant/research support from AstraZeneca and is a consultant to AstraZeneca, Merck, Nitromed, McNeil, Novartis, TAP, Pfizer, The GI Company and Pozen. JMS is on the Speaker's Bureau for AstraZeneca, TAP, Boehringer Ingelheim and Santarus but is not a shareholder in any of the above. AMF is a consultant for Merck, Astra Zeneca Pharmaceuticals, Merck-Medco, Proctor & Gamble, Glaxo Smith-Kline, Pfizer, Eli Lilly and Company, TAP Pharmaceuticals, Aventis Pharmaceuticals and Amgen.
References
- Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120:594–606. doi: 10.1053/gast.2001.21907. [DOI] [PubMed] [Google Scholar]
- Sturkenboom MCJM, Burke TA, Dieleman JP, Tangelder MJD, Lee F, Goldstein JL. Underutilization of preventive strategies in patients receiving NSAIDs. Rheumatology. 2003. pp. iii23–iii31. [DOI] [PubMed]
- Peura DA, Goldkind L. Balancing the gastrointestinal benefits and risks of nonselective nonsteroidal anti-inflammatory drugs. Arthritis Research & Therapy. 2005;7(suppl 4):S7–S13. doi: 10.1186/ar1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiman JM. NSAIDs, gastrointestinal injury, and cytoprotection. Gastroenterol Clin North Am. 1996;25:279–298. doi: 10.1016/S0889-8553(05)70247-8. [DOI] [PubMed] [Google Scholar]
- Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- Graham DY, White RH, Moreland LW, Schubert TT, Katz R, Jaszewski R, Tindall E, Triadafilopoulos G, Stromatt SC, Teoh LS. Duodenal and gastric ulcer prevention with misoprostol in arthritis patients taking NSAIDs. Misoprostol Study Group. Ann Intern Med. 1993;119:257–262. doi: 10.7326/0003-4819-119-4-199308150-00001. [DOI] [PubMed] [Google Scholar]
- Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, Geis GS. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:241–249. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]
- Raskin JB, White RH, Jackson JE, Weaver AL, Tindall EA, Lies RB, Stanton DS. Misoprostol dosage in the prevention of non-steroidal anti-inflammatory drug-induced gastric and duodenal ulcers: a comparison of three regimens. Ann Intern Med. 1995;123:344–350. doi: 10.7326/0003-4819-123-5-199509010-00004. [DOI] [PubMed] [Google Scholar]
- Hawkey CJ, Karrasch JA, Szczepanski L, Walker DG, Barkun A, Swannell AJ, Yeomans ND. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group. N Engl J Med. 1998;338:727–734. doi: 10.1056/NEJM199803123381105. [DOI] [PubMed] [Google Scholar]
- Bocanegra TS, Weaver AL, Tindall EA, Sikes DH, Ball JA, Wallemark CB, Geis GS, Fort JG. Diclofenac/misoprostol compared with diclofenac in the treatment of osteoarthritis of the knee or hip: a randomized, placebo controlled trial. Arthrotec Osteoarthritis Study Group. J Rheumatol. 1998;25:1602–1611. [PubMed] [Google Scholar]
- Taha AS, Hudson N, Hawkey CJ, Swannell AJ, Trye PN, Cottrell J, Mann SG, Simon TJ, Sturrock RD, Russell RI. Famotidine for the prevention of gastric and duodenal ulcers caused by non-steroidal antiinflammatory drugs. N Engl J Med. 1996;334:1435–1439. doi: 10.1056/NEJM199605303342204. [DOI] [PubMed] [Google Scholar]
- Yeomans ND, Tulassay Z, Juhasz L, Racz I, Howard JM, van Rensburg CJ, Swannell AJ, Hawkey CJ. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid Suppression Trial: Ranitidine versus Omeprazole for NSAID-associated Ulcer Treatment (ASTRONAUT) Study Group. N Engl J Med. 1998;338:719–726. doi: 10.1056/NEJM199803123381104. [DOI] [PubMed] [Google Scholar]
- Agrawal NM, Campbell DR, Safdi MA, Lukasik NL, Huang B, Haber MM. Superiority of lansoprazole vs ranitidine in healing nonsteroidal anti-inflammatory drug-associated gastric ulcers: results of a double-blind, randomized, multicenter study. NSAID-Associated Gastric Ulcer Study Group. Arch Intern Med. 2000;160:1455–1461. doi: 10.1001/archinte.160.10.1455. [DOI] [PubMed] [Google Scholar]
- Modlin IM, Sachs G. In: Acid Related Diseases: Biology and Treatment. Modlin IM, Sachs G, editor. Konstanz, Germany: Schnetztor-Verlag GmbH D-Konstanz; 1998. Inhibition of the gastric acid pump; pp. 126–145. [Google Scholar]
- Huber R, Kohl B, Sachs G, Senn-Bilfinger J, Simon WA, Sturm E. Review article: the continuing development of proton pump inhibitors with particular reference to pantoprazole. Aliment Pharmacol Ther. 1995;9:363–378. doi: 10.1111/j.1365-2036.1995.tb00394.x. [DOI] [PubMed] [Google Scholar]
- Graham DY, Agrawal NM, Campbell DR, Haber MM, Collis C, Lukasik NL, Huang B. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med. 2002;162:169–175. doi: 10.1001/archinte.162.2.169. [DOI] [PubMed] [Google Scholar]
- Lai KC, Lam SK, Chu KM, Wong BC, Hui WM, Hu WH, Lau GK, Wong WM, Yuen MF, Chan AO. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346:2033–2038. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- Chan FK, Chung SC, Suen BY, Lee YT, Leung WK, Leung VK, Wu JC, Lau JY, Hui Y, Lai MS. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N Engl J Med. 2001;344:967–973. doi: 10.1056/NEJM200103293441304. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration, Center for Drug Evaluation and Research. COX-2 selective (includes Bextra, Celebrex, and Vioxx) and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) http://www.fda.gov/cder/drug/infopage/COX2/default.htm
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- Dubois RW, Melmed GY, Henning JM, Laine L. Guidelines for the appropriate use of non-steroidal anti-inflammatory drugs, cyclo-oxygenase-2-specific inhibitors and proton pump inhibitors in patients requiring chronic anti-inflammatory therapy. Aliment Pharmacol Ther. 2004;19:197–208. doi: 10.1111/j.0269-2813.2004.01834.x. [DOI] [PubMed] [Google Scholar]
- Chan FK, Hung LC, Suen BY, Wu JC, Lee KC, Leung VK, Hui AJ, To KF, Leung WK, Wong VW. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med. 2002;347:2104–2110. doi: 10.1056/NEJMoa021907. [DOI] [PubMed] [Google Scholar]
- Chan FK, Hung LC, Suen BY, Wong VW, Hui AJ, Wu JC, Leung WK, Lee YT, To KF, Chung SC. Celecoxib versus diclofenac plus omeprazole in high-risk arthritis patients: results of a randomized double-blind trial. Gastroenterology. 2004;127:1038–1043. doi: 10.1053/j.gastro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Fendrick AM. COX-2 inhibitor use after Vioxx: careful balance or end of the rope? Am J Manag Care. 2004;10:740–741. [PubMed] [Google Scholar]