Abstract
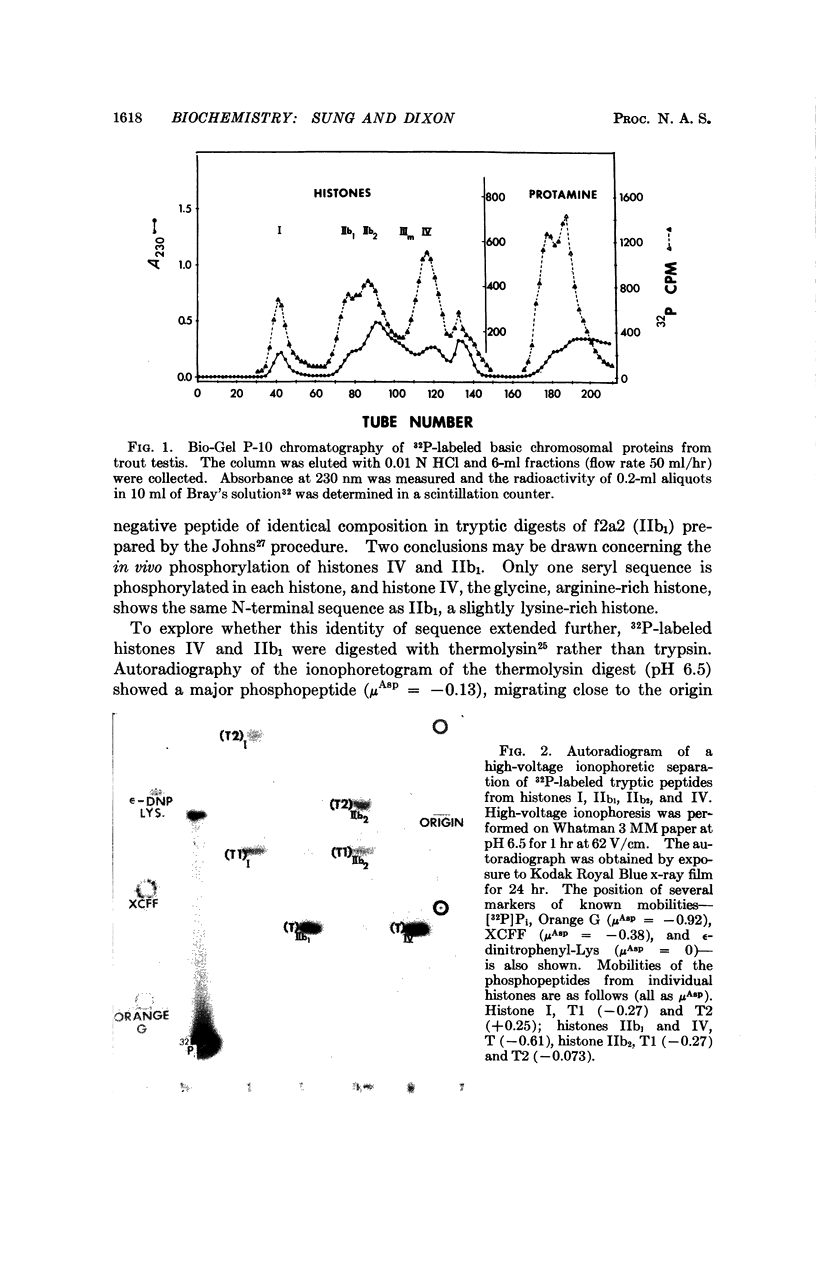
At a late stage of spermatogenesis in rainbow-trout testis, the entire complement of histones is replaced by newly synthesized protamine and histones are extensively phosphorylated and acetylated. Tryptic digestion of purified histones labeled by incubation of testicular cells with [32P]phosphate shows that phosphorylation occurs at a small number of seryl residues.
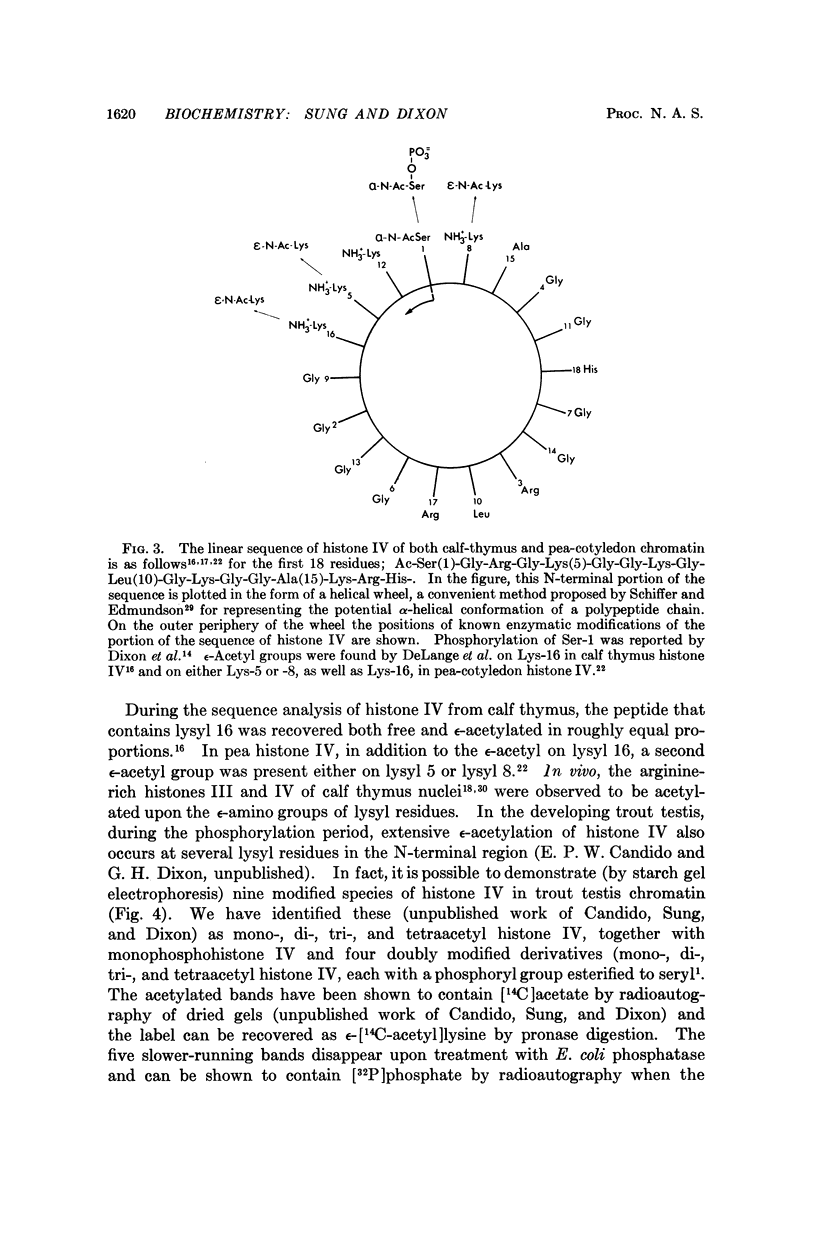
Histone I (lysine-rich) is phosphorylated in the sequence Lys-Ser(PO4)-Pro-Lys, which is located in the lysine-rich C-terminal region of the molecule. Histones IIb1 (slightly lysine-rich) and IV (glycine, arginine-rich) give rise to the same phosphopeptide, Ac-Ser(PO4)-Gly-Arg, which comprises the amino terminus of each histone. Thermolysin digests of phosphohistones IIb1 and IV also released a phosphopeptide with composition corresponding to the first six residues of histone IV: Ac-Ser(PO4)-Gly-Arg-Gly-Lys-Gly. An α-helical model of the N-terminal region of histone IV shows that this region is a possible DNA-binding site. Phosphorylation at serine 1 together with ε-amino acetylation at lysines 5, 8, 12, and 16 (observed in histone IV from trout testis) could profoundly modify ionic interactions and lead to an „unzipping” of histone IV from DNA
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALFERT M. Chemical differentiation of nuclear proteins during spermatogenesis in the salmon. J Biophys Biochem Cytol. 1956 Mar 25;2(2):109–114. doi: 10.1083/jcb.2.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLFREY V. G., FAULKNER R., MIRSKY A. E. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 May;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Cole R. D. Bisection of a lysine-rich histone by N-bromosuccinimide. J Biol Chem. 1969 Oct 10;244(19):5291–5294. [PubMed] [Google Scholar]
- Comb D. G., Sarkar N., Pinzino C. J. The methylation of lysine residues in protein. J Biol Chem. 1966 Apr 25;241(8):1857–1862. [PubMed] [Google Scholar]
- DeLange R. J., Fambrough D. M., Smith E. L., Bonner J. Calf and pea histone IV. 3. Complete amino acid sequence of pea seedling histone IV; comparison with the homologous calf thymus histone. J Biol Chem. 1969 Oct 25;244(20):5669–5679. [PubMed] [Google Scholar]
- DeLange R. J., Fambrough D. M., Smith E. L., Bonner J. Calf and pea histone IV. II. The complete amino acid sequence of calf thymus histone IV; presence of epsilon-N-acetyllysine. J Biol Chem. 1969 Jan 25;244(2):319–334. [PubMed] [Google Scholar]
- Fambrough D., Bonner J. Selective dissociation of pea bud nucleohistone. Biochim Biophys Acta. 1968 Apr 9;154(3):601–602. doi: 10.1016/0005-2795(68)90025-1. [DOI] [PubMed] [Google Scholar]
- Frenster J. H. A model of specific de-repression within interphase chromatin. Nature. 1965 Jun 19;206(990):1269–1270. doi: 10.1038/2061269a0. [DOI] [PubMed] [Google Scholar]
- Gershey E. L., Vidali G., Allfrey V. G. Chemical studies of histone acetylation. The occurrence of epsilon-N-acetyllysine in the f2a1 histone. J Biol Chem. 1968 Oct 10;243(19):5018–5022. [PubMed] [Google Scholar]
- Ingles C. J., Dixon G. H. Phosphorylation of protamine during spermatogenesis in trout testis. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1011–1018. doi: 10.1073/pnas.58.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles C. J., Trevithick J. R., Smith M., Dixon G. H. Biosynthesis of protamine during spermatogenesis in salmonoid fish. Biochem Biophys Res Commun. 1966 Mar 22;22(6):627–634. doi: 10.1016/0006-291x(66)90192-6. [DOI] [PubMed] [Google Scholar]
- Jergil B., Dixon G. H. Protamine kinase from rainbow trout testis. Partial purification and characterization. J Biol Chem. 1970 Jan 25;245(2):425–434. [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966 May;55(5):1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marushige K., Dixon G. H. Developmental changes in chromosomal composition and template activity during spermatogenesis in trout testis. Dev Biol. 1969 Apr;19(4):397–414. doi: 10.1016/0012-1606(69)90050-5. [DOI] [PubMed] [Google Scholar]
- Marushige K., Ling V., Dixon G. H. Phosphorylation of chromosomal basic proteins in maturing trout testis. J Biol Chem. 1969 Nov 10;244(21):5953–5958. [PubMed] [Google Scholar]
- Matsubara H., Singer A., Sasaki R., Jukes T. H. Observations on the specificity of a thermostable bacterial protease "thermolysin". Biochem Biophys Res Commun. 1965 Nov 8;21(3):242–247. doi: 10.1016/0006-291x(65)90278-0. [DOI] [PubMed] [Google Scholar]
- Murray K. The acid extraction of histones from calf thymus deoxyribonucleoprotein. J Mol Biol. 1966 Feb;15(2):409–419. doi: 10.1016/s0022-2836(66)80116-x. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Quagliarotti G., Jordan J., Taylor C. W., Starbuck W. C., Busch H. Structural analysis of the glycine-rich, arginine-rich histone. 3. Sequence of the amino-terminal half of the molecule containing the modified lysine residues and the total sequence. J Biol Chem. 1969 Aug 25;244(16):4387–4392. [PubMed] [Google Scholar]
- Ohlenbusch H. H., Olivera B. M., Tuan D., Davidson N. Selective dissociation of histones from calf thymus nucleoprotein. J Mol Biol. 1967 Apr 28;25(2):299–315. doi: 10.1016/0022-2836(67)90143-x. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Protein methylase I. Purification and properties of the enzyme. J Biol Chem. 1968 May 10;243(9):2108–2114. [PubMed] [Google Scholar]
- Phillips D. M. N-Terminal acetyl-peptides from two calf thymus histones. Biochem J. 1968 Mar;107(2):135–138. doi: 10.1042/bj1070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen R. H., Cole R. D. Chromosomal proteins. Annu Rev Biochem. 1969;38:951–990. doi: 10.1146/annurev.bi.38.070169.004511. [DOI] [PubMed] [Google Scholar]
- Sung M., Smithies O. Differential elution of histones from gel-trapped nuclei. Biopolymers. 1969;7(1):39–58. doi: 10.1002/bip.1969.360070105. [DOI] [PubMed] [Google Scholar]
- Vidali G., Gershey E. L., Allfrey V. G. Chemical studies of histone acetylation. The distribution of epsilon-N-acetyllysine in calf thymus histones. J Biol Chem. 1968 Dec 25;243(24):6361–6366. [PubMed] [Google Scholar]
- WAYMOUTH C. A serum-free nutrient solution sustaining rapid and continuous proliferation of strain L (Earle) mouse cells. J Natl Cancer Inst. 1956 Sep;17(3):315–327. [PubMed] [Google Scholar]