Abstract
Abatacept (CTLA4–Ig) is a novel fusion protein designed to modulate the T cell co-stimulatory signal mediated through the CD28–CD80/86 pathway. Clinical trials have provided preliminary evidence of the efficacy of this compound in the treatment of rheumatoid arthritis. This review describes the molecular and biologic bases for the use of abatacept in rheumatoid arthritis and summarizes the current clinical data on its safety and effectiveness in this disease.
Introduction
Recent clinical research in rheumatoid arthritis (RA) has led to significant advances in care using inhibitors of the circulating proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin-1. However, the failure of such agents to control disease in all patients has fostered a search for other approaches to ameliorate disease activity. Although T cells are the most abundant inflammatory cells in the RA joint and exhibit phenotypic markers of activation, there is little or no evidence of efficacy of anti-CD4+ T lymphocyte strategies, even though they have resulted in prolonged depletion of peripheral CD4+ T cells [1]. In contrast to depletion, it is possible that modulation of T cell function, possibly by altering the stimulatory pathway or the Th1 : Th2 ratio, may be therapeutically beneficial. An alternate approach, in which the activity of antigen-specific T cells is controlled by targeting co-stimulatory molecules, has now been developed, and preliminary studies have shown it to be effective at controlling the clinical signs and symptoms of RA [2,3].
Molecular background
Activation of T cells requires two distinct signals. The first is an antigen-specific interaction between the T cell receptor and nominal antigen presented in the context of the MHC on the surface of an antigen-presenting cell. The second signal may be provided through a number of potential co-stimulatory molecules, of which CD28 may be the most important. Co-stimulation is especially important for the initial T cell response, and its effects are mediated by promoting proliferation and survival. Thus, therapies targeting co-stimulatory signals have the potential to target specific T cell responses, even when the actual nature of the antigen involved is unknown. Such an approach would be potentially useful in RA, in which the initial trigger for the autoimmune response remains unclear.
One of the most prominent T cell co-stimulatory signals is mediated through the CD28–CD80/86 pathway, which regulates interleukin-2 production and the expression of anti-apoptotic molecules, such as Bcl-xL [4,5]. CD28 is present on most T cells and it binds to both CD80 (B7-1) and CD86 (B7-2), which are present on antigen-presenting cells, including dendritic cells, B cells, and macrophages. These ligands are also expressed on activated T cells and are present on T cells obtained from RA joint, suggesting a self-sustaining mechanism for T cell activation [6]. Engagement with these ligands provides the second signal required for maximal T cell activation, and the absence of a co-stimulatory signal may result in anergy and apoptotic cell death. Cytotoxic T lymphocyte-associated antigen (CTLA)4 (CD152), which is upregulated on T cells following their activation, also interacts with CD80 and CD86, providing an important mechanism for regulating T cell function [7,8]. Not only does CTLA4 permit interruption of the activating CD28 pathway but it may also provide important negative signals that permit long-term tolerance. CD28/B7 interactions are critical for the generation of CD4+, CD25+, CTLA4+ T regulatory cells, and signaling through CTLA4 may promote the release of immunoregulatory cytokines such as TGFβ [5,9]. Of interest, CTLA4 is expressed on T cells in the RA joint [6], supporting the potential importance of this pathway in regulating T cell activation in RA.
The regulatory effects of interrupting CD28 interactions with CD80/86 have been harnessed in recombinant molecules (CTLA4–immunoglobulin [Ig]) that combine the extracellular domain of human CTLA4 with a portion of the Fc domain of IgG1 [10]. One of these fusion proteins, abatacept, binds CD80 more avidly than CD86. A second-generation version of this molecule (LEA29Y), with two amino acid mutations, has been developed to have increased binding avidity for CD86 [2]. This change may be important because CD86 appears to be the dominant co-stimulatory ligand in a number of experimental models, and, in treating mouse models of autoimmune disease, inhibition of CD86 was more effective than inhibition of CD80 [5]. CTLA4–Ig also interrupts signaling through cell surface CTLA4, which theoretically could affect the development of T regulatory cells and antigen specific tolerance. However, inhibition of signaling through CTLA4 has also been shown to promote Th2 development, which may be beneficial in RA [11].
Preclinical studies
CTLA4 shares significant sequence homology between the human and murine versions of the molecule, so that fusion compounds containing this ligand can be effectively studied in murine models of a variety of human diseases. CTLA4–Ig has been studied in preclinical transplant models as well as in models of systemic lupus erythematosus, experimental allergic encephalitis (a murine model of multiple sclerosis), and collagen induced arthritis [12-16]. In the B/W murine model of lupus, treatment with CTLA4–Ig delayed the onset of renal disease and prolonged survival when treatment was given before the development of nephritis [12]. Combination therapy with CTLA4–Ig and cyclophosphamide improved proteinuria and prolonged survival when it was given to mice that already had advanced renal disease [17]. In that experiment, the combination of CTLA4–Ig and cyclophosphamide was shown to be more effective than either therapy given alone.
In the collagen induced arthritis model, infusion of a human CTLA4–murine IgG2a Fc was shown to limit both clinical and histopathologic manifestations of joint disease when it was given before the animals were immunized with collagen [16]. In these experiments, rats were immunized with a single intradermal injection of bovine type II collagen. Clinical evidence of hindpaw disease first appears in these rats at day 10 after immunization, with complete ankylosis of the hindpaws by days 16–18 in 100% of animals. Intraperitoneal injections of 1 mg/kg of the CTLA4–Ig fusion protein, begun 1 day before collagen immunization and continued every other day through to day 10, completely abrogated clinical and histological signs of arthritis. These observations suggest that CD28–CD80/86 interactions are important in the initiation of the arthritis. Supporting these observations, CD28 deficient mice were resistant to the induction of collagen induced arthritis [18]. Interestingly, up to 20% of T cells in the RA joint may be CD28 negative [6], and the frequency of peripheral blood CD4+CD28- T cells in patients with RA corresponds with extra-articular manifestations [19].
Pilot studies
The CTLA4–Ig fusion protein abatacept has been specifically studied in human RA. In a dose ranging pilot study of patients with active RA [2], both abatacept and LEA29Y produced dose dependent reductions in the clinical manifestations of disease. A total of 214 patients were entered into this three arm study. A total of 90 received abatacept at doses of 0.5 mg/kg, 2 mg/kg, and 10 mg/kg; 92 received LEA29Y at the same doses; and 32 received placebo infusions. Infusions were given at baseline, week 2, week 4, and week 8.
The study population in this trial was predominantly female (75%), Caucasian (91%), and receiving corticosteroids at an allowed dose of 10 mg/day or less (90%). Their mean disease duration was only 3.2 years but they had very active disease, with a mean of 30.3 tender and 21.9 swollen joints at randomization. The mean baseline level of C-reactive protein was 4.0 mg/dl. Most patients (79%) had been treated with methotrexate, which was discontinued per protocol at least 4 weeks before the first dose of study drug.
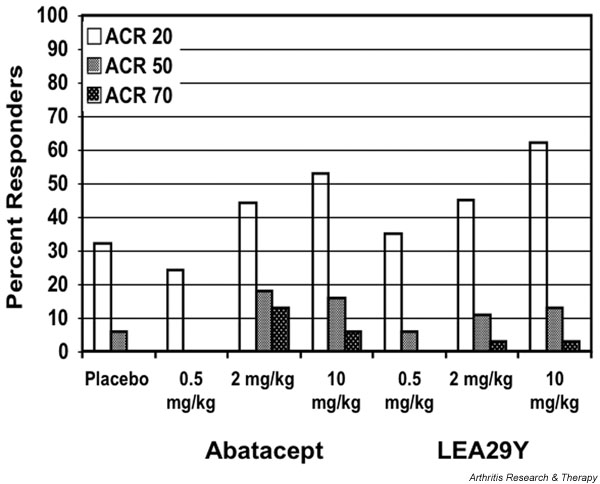
There was a dose dependent response to both compounds in this study (Fig. 1). For abatacept, 44% of the patients in the 2 mg/kg group and 53% in the 10 mg/kg group achieved an American College of Rheumatology (ACR) 20 response at 12 weeks (the primary outcome of the study) as compared with 32% in the placebo arm. Response was seen across all of the components in the ACR core set, with the two highest dose treatment groups for each compound exhibiting a consistently higher percentage improvement than placebo. The 10 mg/kg dose of abatacept was generally more effective than 2 mg/kg.
Figure 1.
Percentage of patients meeting American College of Rheumatology (ACR) 20, ACR 50, and ACR 70 response criteria after 85 days of therapy with abatacept, LEA29Y, or placebo. Adapted with permission from Moreland and coworkers [2].
Of the patients included in the study, 19% withdrew before the 12 week visit. The most common reason for discontinuation, worsening arthritis, led to the withdrawal of 32% of the placebo group, 13% of the patients treated with abatacept, and 4% of the patients treated with LEA29Y. Adverse events were similar in the active drug and placebo groups. Peri-infusional adverse events occurred in about 30% of those in each of the three treatment groups. Four patients receiving abatacept withdrew because of adverse events: two because of worsening arthritis (one each in the 0.5 mg/kg and 2 mg/kg groups), one because of an anxiety attack, and one (in the 0.5 mg/kg group) because of breast cancer discovered at week 8. One patient treated with LEA29Y withdrew because of a viral upper respiratory tract infection. Fifteen serious adverse events occurring in 12 patients were judged by the investigators not to be due to the study drugs, including a septic elbow in one patient 88 days after the last infusion of abatacept 2 mg/kg and 40 days after the joint was injected with corticosteroids. No drug-specific antibodies against either compound were detected.
In a second pilot study [20], 121 patients with active RA taking etanercept 25 mg subcutaneously twice weekly were randomly assigned to receive either monthly infusions of abatacept 2 mg/kg or placebo. The lower dose was chosen because of safety concerns regarding co-administration of the two biologic response modifiers. The patients included in this trial had longstanding disease (mean 13 years) and active synovitis despite etanercept (mean of 29 tender and 20 swollen joints). ACR 20, 50, and 70 responses occurred in 48%, 26%, and 11% in the abatacept group and in 28%, 19%, and 0% in the placebo group, respectively. In this study the differences between active drug and placebo were statistically significant (P < 0.05) only for ACR 20 and ACR 70 responses. Adverse events were similar between the two treatment groups. Again, no anti-abatacept antibodies were detected.
Phase II trials
Following the pilot studies that demonstrated efficacy of abatacept in treating the signs and symptoms of RA, a large, multicenter trial was reported in 2003 that confirmed these results [3]. In this phase II trial, 339 patients with active RA, despite methotrexate treatment, were randomly assigned to receive infusions of abatacept 2 mg/kg, abatacept 10 mg/kg, or placebo monthly for 6 months. Unlike the dose ranging trial [2], in which participants received abatacept as monotherapy, patients included in this trial were all taking methotrexate, at a mean dose of 15–15.8 mg weekly. Their mean age was 55.0 years, 68% were women, and 87% were Caucasian. They had longstanding disease (means of 8.9–9.7 years in the three treatment groups). Despite this, they had relatively mild functional impairment, with a mean Modified Stanford Health Assessment Questionnaire score of 1.0 in all groups.
Dosing of active drug or placebo was done in a blinded manner at baseline, 2 weeks, 4 weeks, and then monthly through to 6 months. There were more discontinuations in the placebo group, primarily because of lack of efficacy (n = 29); the difference from the number of discontinuations due to lack of efficacy in each of the two abatacept groups (13 and 12) was statistically significant (P < 0.05).
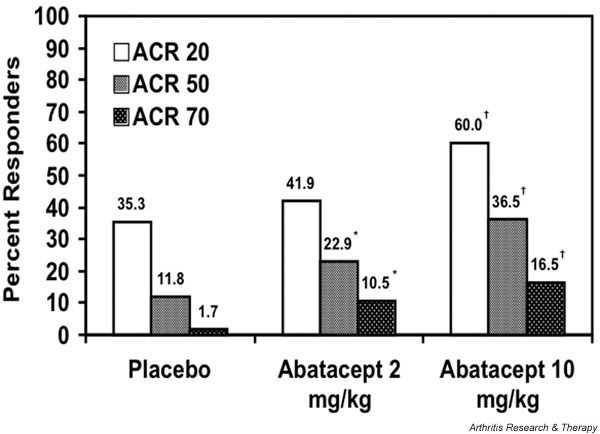
The primary end-point for the study was ACR 20 response at 6 months. Patients who discontinued therapy before 6 months had their last efficacy observation carried forward. The percentage of patients achieving this level of response was 60.0% in the abatacept 10 mg/kg group versus 35.3% in the placebo group (P < 0.001). Higher levels of response (ACR 50 and ACR 70) were also achieved more frequently in the 10 mg/kg group (Fig. 2). The response in the abatacept 2 mg/kg treatment group was only statistically better than placebo at the ACR 50 and 70 response levels (Fig. 2). A secondary analysis, in which patients who discontinued were considered nonresponders, yielded similar results, with 57.4% of the abatacept 10 mg/kg group responding at an ACR 20 level versus 31.1% in the placebo group (P < 0.001). Again, ACR 20 responses in the abatacept 2 mg/kg and placebo groups were not statistically different.
Figure 2.
Percentage of patients meeting American College of Rheumatology (ACR) 20, ACR 50, and ACR 70 response criteria after 6 months of therapy with abatacept or placebo. From data presented by Kremer and coworkers [3]. *P ≤ 0.05 versus placebo; †P ≤ 0.001 versus placebo.
Improvement in the individual components of the ACR response criteria was also generally greater in the 10 mg/kg treatment group than in the 2 mg/kg group. Mean changes in tender joint count, swollen joint count, pain, physical function, patient and physician's global assessment, and C-reactive protein were all significantly greater with abatacept 10 mg/kg than in the placebo group. Finally, improvement in all 36-item Short Form subscales and summary scores in the abatacept 10 mg/kg group were clinically and statistically significant when compared with baseline scores.
In the safety analysis for this trial, no deaths, malignancies, or opportunistic infections were reported for any of the abatacept treated patients during the 6 months of therapy. There were three serious adverse events in the 10 mg/kg abatacept group, 12 in the 2 mg/kg group, and 12 in the placebo group. None of the serious adverse events in the high dose group was judged to be related to the study drug. The only reported serious infection occurred in a patient in the abatacept 2 mg/kg group, who was hospitalized because of cellulitis.
Most patients included in the study had pre-existing antibodies to abatacept, which, although not stated in the article, might have been due to rheumatoid factor reacting with the Fc portion of abatacept. No patients developed abatacept specific antibodies during the trial. One patient in each of the two active treatment groups developed CTLA4 specific antibodies.
Patients in the phase II trial continued on blinded therapy for an additional 6 months and were then permitted to continue on open label therapy with abatacept. Data presented in abstract form only [21,22] demonstrate that response to therapy was maintained during the second 6 months of blinded therapy, as measured by both ACR response and improvement in 36-item Short Form parameters. Seventy-five of the patients originally treated with methotrexate and abatacept 10 mg/kg continued on therapy for 2 years. Response to therapy was sustained with continued treatment. ACR 50 response was achieved by 56% of those patients who remained on therapy at 1 year and by 54.7% of those who remained on therapy at 2 years [23]; 48% of patients who remained on therapy achieved remission by Disease Activity Score criteria at 1 year that was sustained through 2 years [24]. There were no significant differences in serious adverse events or serious infections between active drug and placebo during the second year of blinded therapy [25].
Phase III trials
Preliminary data from two large phase III studies of abatacept in RA have recently been reported, at least in abstract form. In the first, 652 patients with inadequate response to methotrexate were randomly assigned to receive either placebo or a fixed dose of abatacept approximating 10 mg/kg while remaining on background methotrexate therapy [26]. The patients had a mean disease duration of approximately 8.6 years. At 1 year the percentages achieving ACR 20, ACR 50, and ACR 70 responses with active drug were 73.1%, 48.3%, and 28.8%, respectively; the corresponding percentages for the placebo group were 39.7%, 18.2%, and 6.1%. Radiographic evaluation in this trial showed significant reductions in progression of erosions, joint space narrowing, and total Sharp score. Serious infections were similar between the two treatment groups.
In the second phase III study [27], abatacept therapy was evaluated in RA patients with an inadequate response to TNF antagonist therapy. In this 24 week study, 391 patients who had failed to respond adequately to at least 3 months of TNF antagonist therapy were randomly assigned to receive either placebo or the same fixed dose of abatacept, approximating 10 mg/kg [27]. TNF antagonist therapy was discontinued at the time of enrollment, if this had not been done previously. The patients in this trial had longstanding (mean disease duration 11.4–12.2 years) and very active (Disease Activity Score 6.9) disease, with significant functional limitations (Health Assessment Questionairre score 1.8). ACR 20, 50, and 70 responses in this trial were 50.4%, 20.3%, and 3.8%, respectively; the corresponding placebo responses were 19.5%, 3.8%, and 1.5%. The incidence of adverse events, including serious infections, was similar between the two treatment groups.
Conclusion
Abatacept, the fusion protein combining the extracellular portion of human CTLA4 and IgG1 Fc, has clearly been shown to be effective at controlling the signs and symptoms of RA, particularly at a dose of 10 mg/kg given monthly. The 2 mg/kg dose has not been shown to be as consistently effective, even when given in combination with methotrexate or etanercept. Trials to date have not demonstrated an increase in adverse events, including infections, compared with placebo. Further data will be required to confirm the long-term safety of this therapy as a component of combination regimens for the treatment of RA. The data discussed in this review provide the first evidence of the effectiveness of a co-stimulatory modulator – abatacept – in RA, and as such this agent represents a potentially effective new approach to the management of this disease.
Abbreviations
ACR = American College of Rheumatology; CTLA = cytotoxic T lymphocyte-associated antigen; Ig = immunoglobulin; RA = rheumatoid arthritis; Th = T-helper (cell); TNF = tumor necrosis factor.
Competing interests
EMR has received research support and consulting fees from Bristol-Myers Squibb.
References
- Keystone EC. Abandoned therapies and unpublished trials in rheumatoid arthritis. Curr Opin Rheumatol. 2003;15:253–258. doi: 10.1097/00002281-200305000-00012. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Alten R, Van den Bosch F, Appelboom T, Leon M, Emery P, Cohen S, Luggen M, Shergy W, Nuamah I. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum. 2002;46:1470–1479. doi: 10.1002/art.10294. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. [PubMed] [Google Scholar]
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- Verwilghen J, Lovis R, De Boer M, Linsley PS, Haines GK, Koch AE, Pope RM. Expression of functional B7 and CTLA4 on rheumatoid synovial T cells. J Immunol. 1994;153:1378–1385. [PubMed] [Google Scholar]
- Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting Edge: CD28 controls peripheral homeostasis of CD4+, CD25+ Regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas TL, Bluestone JA. CTLA-4 regulates tolerance induction and T cell differentiation in vivo. J Immunol. 1998;160:3855–3860. [PubMed] [Google Scholar]
- Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- Perrin PJ, Scott D, Quigley L, Albert PS, Feder O, Gray GS, Abe R, June CH, Racke MK. Role of B7:CD28/CTLA-4 in the induction of chronic relapsing experimental allergic encephalomyelitis. J Immunol. 1995;154:1481–1490. [PubMed] [Google Scholar]
- Cross AH, Girard TJ, Giacoletto KS, Evans RJ, Keeling RM, Lin RF, Trotter JL, Karr RW. Long-term inhibition of murine experimental autoimmune encephalomyelitis using CTLA-4-Fc supports a key role for CD28 costimulation. J Clin Invest. 1995;95:2783–2789. doi: 10.1172/JCI117982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvin AM, Dal Canto MC, Rhee L, Salomon B, Sharpe A, Bluestone JA, Miller SD. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J Immunol. 2000;164:136–143. doi: 10.4049/jimmunol.164.1.136. [DOI] [PubMed] [Google Scholar]
- Knoerzer DB, Karr RW, Schwartz BD, Mengle-Gaw LJ. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. J Clin Invest. 1995;96:987–993. doi: 10.1172/JCI118146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikh DI, Wofsy D. Cutting edge: reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide. J Immunol. 2001;166:2913–2916. doi: 10.4049/jimmunol.166.5.2913. [DOI] [PubMed] [Google Scholar]
- Tada Y, Nagasawa K, Ho A, Morito F, Ushiyama O, Suzuki N, Ohta H, Mak TW. CD28-deficient mice are highly resistant to collagen-induced arthritis. J Immunol. 1999;162:203–208. [PubMed] [Google Scholar]
- Pawlik A, Ostanek L, Brzosko I, Brzosko M, Masiuk M, Machalinski B, Gawronska-Szklarz B. The expansion of CD4+ CD28- T cells in patients with rheumatoid arthritis. Arthritis Res Ther. 2003;5:R210–R213. doi: 10.1186/ar766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt M, Schiff M, Goldman M, Kremer J, Breazna A, Becker JC. A pilot, multi-center, randomized, double-blind, placebo controlled [study] of a co-stimulation blocker CTLA4Ig (2 mg/kg) given monthly in combination with etanercept in active rheumatoid arthritis [abstract] Arthritis Rheum. 2002. p. S204.
- Emery P, Russell A, Kremer J, Williams GR, Li T, Nuamah I, Becker JC, Weisman MH. Improvement in health-related quality of life with treatment of CTLA4Ig (BMS-188667), a selective co-stimulation modulator, over one year in patients with active rheumatoid arthritis using methotrexate [abstract] Arthritis Rheum. 2003. p. S4.
- Emery P, Westhovens R, Moreland L, Nuamah I, Aranda R, Becker J, Kremer J. CTLA4Ig (BMS-188667) in a phase IIb, multi-center, randomized, double-blind, placebo controlled study in rheumatoid arthritis patients receiving methotrexate showed correlation between the clinical response and key biomarkers [abstract] Presented at: European League Against Rheumatism Annual Meeting, Lisbon, Portugal. 2003. http://mcic3.textor.com/cgi-bin/mc/printabs.pl?APP=eular2003SCIE-abstract&TEMPLATE=&keyf=0896&showHide=show&client=
- Kremer J, Shergy W, Tindall E, Aranda R, Nuamah I, Zhou Y, Becker JC. Sustained clinical efficacy demonstrated by the selective co-stimulation modulator abatacept (CTLA4Ig) in combination with methotrexate at 2 years in rheumatoid arthritis patients with an inadequate response to methotrexate [abstract] Arthritis Rheum. 2004. p. S182.
- Dougados M, Weswthovens R, St Clair EW, Aranda R, Nuamah I, Zhou Y, Vratsanos G, Becker JC. Sustained remission and major clinical response at 2 years shown with abatacept (CTLA4Ig) in combination with methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate [abstract] Arthritis Rheum. 2004. p. S185.
- Moreland L, Weisman M, Alten R, Aranda R, Zhou Y, Wu K, Nuamah I, Becker JC. Abatacept (CTLA4Ig) in combination with methotrexate for the treatment of rheumatoid arthritis: favorable safety and tolerability profile sustained over 2 years [abstract] Arthritis Rheum. 2004. p. S563.
- Kremer J, Westhovens R, Moreland L, Emery P, Russell A, Ge Z, Aranda R, Becker JC. Efficacy and safety of the selective co-stimulation modulator abatacept with methotrexate for treating rheumatoid arthritis: 1-year clinical and radiographic results from the phase III AIM (Abatacept in INadequate responders to Methotrexate) trial [abstract] Presented at: American College of Rheumatology Annual Scientific Meeting, San Antonio, TX. 2004. http://www.abstractsonline.com/viewer/viewAbstract.asp?CKey={85C06A69-418B-47A1-BEF0-F919C8C4F640}&MKey={6A95EDC3-27BB-49C8-9CE4-3C06FC50D63E}&AKey={AA45DD66-F113-4CDD-8E62-01A05F613C0D}&SKey={8FF4FA39-962F-41CF-B617-5E132EB117E5}
- Genovese MC, Luggen M, Schiff M, Sherrer Y, Nuamah I, Aranda R, Becker JC, Dougados M. Efficacy and safety of abatacept (CTLA4Ig), a selective co-stimulation modulator, in rheumatoid arthritis patients not responding adequately to anti-TNF-a[ED:alpha] therapy: results of the phase III ATTAIN (Abatacept Trial in Treatment of Anti-TNF Inadequate responders) trial [abstract] Presented at: American College of Rheumatology Annual Scientific Meeting, San Antonio, TX. 2004. http://www.abstractsonline.com/viewer/viewAbstract.asp?CKey={460C7526-77D4-4A49-AD2D-440A2515EE22}&MKey={6A95EDC3-27BB-49C8-9CE4-3C06FC50D63E}&AKey={AA45DD66-F113-4CDD-8E62-01A05F613C0D}&SKey={100FBD25-6760-48B9-A4F5-EEEE63E1DAAA}