Abstract
T cells, in particular CD4+ T cells, have been implicated in mediating many aspects of autoimmune inflammation. However, current evidence suggests that the role played by CD4+ T cells in the development of rheumatoid inflammation exceeds that of activated proinflammatory T-helper (Th)1 effector cells that drive the chronic autoimmune response. Subsets of CD4+ T cells with regulatory capacity, such as CD25+ regulatory T (Treg) cells and Th2 cells, have been identified, and recent observations suggest that in rheumatoid arthritis the function of these regulatory T cells is severely impaired. Thus, in rheumatoid arthritis, defective regulatory mechanisms might allow the breakdown of peripheral tolerance, after which the detrimental Th1-driven immune response evolves and proceeds to chronic inflammation. Here, we review the functional abnormalities and the contribution of different T cell subsets to rheumatoid inflammation.
Introduction
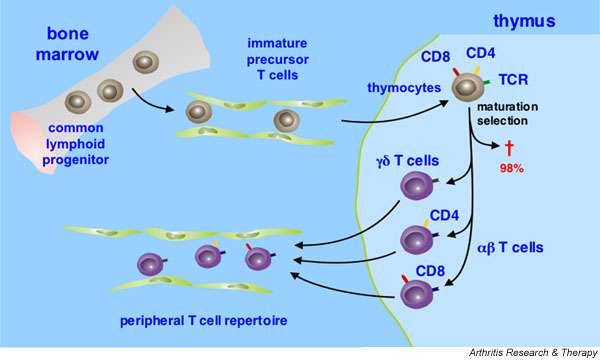
T cells derive from the common lymphoid progenitor in the bone marrow and migrate via the bloodstream into their primary lymphoid organ, the thymus, where they undergo a series of distinct maturation steps. An important role of thymic maturation is positive selection for those T cells that recognize self-MHC molecules and negative selection against those T cells that react to autoantigens [1]. As a consequence of these dual selection steps, more than 98% of thymocytes die during maturation. Those T cells that survive thymic selection leave the thymus and form the peripheral T-cell repertoire (Fig. 1).
Figure 1.
Schematic representation of T cell development. T cells originate from the common lymphoid progenitor cells in the bone marrow. They migrate as immature precursor T cells via the bloodstream into the thymus, which they populate as thymocytes. The thymocytes go through a series of maturation steps including distinct changes in the expression of cell surface receptors, such as the CD3 signaling complex (not shown) and the coreceptors CD4 and CD8, and the rearrangement of their antigen receptor (T cell receptor, TCR) genes. More than 98% of the thymocytes die during maturation by apoptosis (†), as they undergo positive selection for their TCR's compatibility with self-major histocompatibility molecules, and negative selection against those T cells that express TCRs reactive to autoantigenic peptides. In humans, the vast majority of peripheral blood T cells expresses TCRs consisting of α and β chains (αβ T cells). A small group of peripheral T cells bears an alternative TCR composed of γ and δ chains (γ/δ T cells). αβ and γδ T cells diverge early in T cell development. Whereas αβ T cells are responsible for the classical helper or cytotoxic T cell responses, the function of the γδ T cells within the immune system is largely unknown. αβ T cells that survive thymic selection lose expression of either CD4 or CD8, increase the level of expression of the TCR, and leave the thymus to form the peripheral T cell repertoire.
Peripheral T cells are characterized by the expression of an array of distinctive surface receptors [1-3]. The disulfide-linked heterodimeric T-cell receptor (TCR) confers antigen specificity to the T cell. The CD3 complex, which consists of four invariant transmembrane polypeptides (designated γδεε) mediates signaling and is also necessary for surface expression of the TCR. The TCR–CD3 complex is associated with a largely intracytoplasmic homodimer of ζ-chains that are critical for maximal signaling [4]. Finally, the co-receptors CD4 and CD8, expressions of which are mutually exclusive on mature post-thymic T cells, bind to invariant sites of the MHC class II or I molecules on antigen-presenting cells (APCs), respectively; they stabilize the MHC–peptide–TCR complex during T-cell activation, and thus they increase the sensitivity of a T cell for activation by MHC-presented antigen by approximately 100-fold [3]. The cytoplasmic domains of CD4 and CD8 are constitutively associated with the src-family tyrosine kinase p56lck, which phosphorylates particular recognition motifs within the CD3 complex (denoted immunoreceptor tyrosine-based activation motifs), thereby promoting T-cell activation.
The vast majority of human peripheral blood T cells expresses TCRs consisting of α and β chains (αβ T cells). αβ T cells mediate the classical helper or cytotoxic T cell responses. Extensive somatic DNA recombination of variable and joining region segments of the α and β TCR genes is responsible for the structural TCR diversity required for reactivity to the huge arsenal of potential antigens. TCR diversity is concentrated in the third hypervariable regions (complementarity determining region [CDR]3) of the TCR α and β chains, which form the center of the antigen-binding site of the TCR. As the αβ TCR does not bind antigen directly, T-cell activation is dependent on an interaction of the TCR with MHC molecules that present small peptide fragments that have been generated from protein antigens. MHC molecules are membrane glycoproteins that are encoded by several closely linked, highly polymorphic genes. Whereas MHC class I molecules are expressed on virtually all nucleated cells, MHC class II expression is restricted to professional APCs, such as B cells, dendritic cells and macrophages, and to activated T cells in humans. MHC class I molecules bind antigens that are generated by the particular cells themselves as well as antigens from intracellular pathogens that reside in the cytoplasm; they present their antigens to CD8+ T cells. MHC class II molecules, in contrast, present antigens derived from ingested proteins, such as extracellular bacteria or damaged self-tissue, to CD4+ T cells.
A small group of peripheral T cells bears an alternative TCR that is composed of γ and δ chains (γδ T cells). The function of the γδ T cells within the human immune system is largely unknown. γδ TCRs appear to recognize antigen directly, similar to immunoglobulins (Igs), but they do not require presentation by an MHC protein or other molecules and do not depend on antigen processing. The diversity of the γδ TCR is limited, suggesting that the ligands for the γδ TCR are conserved and invariant. γδ T cells have been shown to recognize self-peptides, such as stress-associated antigens expressed on epithelial cells, tumor lines, and primary carcinomas. Recognition of self-peptides and the production of cytokines early during an immune response indicate that γδ T cells play a role in the development of an immune response against self-tissue. Moreover, some recent evidence suggests a role for γδ T cells in the pathogenesis of rheumatoid arthritis (RA), because the frequencies of γδ T cells are elevated in the synovial infiltrates, and rheumatoid synovia with increased levels of γδ T cells presents with increased tissue inflammation as compared with RA synovia with few γδ T cells [5,6]. Despite these indications, however, the function of γδ T cells, in particular the function of synovial γδ T cells, and their contribution to rheumatoid inflammation is still as elusive as the nature of their specific antigen(s).
αβ T cells
αβ TCR expressing T cells that survive dual selection in the thymus can be divided into two subgroups that are characterized by the expression of either CD4 or CD8. CD4+ T cells primarily function as regulators of other immune cells either through secreted cytokines or by direct cell–cell contact. Consequently, CD4+ T cells are denoted T-helper (Th) cells. CD8+ T cells, on the other hand, are programmed to become cytotoxic effector cells that kill infected target cells. CD8+ T cells are therefore named cytotoxic T cells. Both CD4+ and CD8+ T cells continuously recirculate through the body from the peripheral blood to secondary lymphoid organs as they search for the presentation of their specific antigen.
T cells that emerge from the thymus belong to the naïve T cell pool that consists of T cells that have never encountered their specific antigen. Naïve T cells are long-lived and have a restricted function (e.g. CD4+naïve T cells only produce IL-2). In humans, naïve T cells are characterized phenotypically by the expression of the long isoform of CD45, namely CD45RA. Naïve T cells are normally limited to recirculate between the blood and secondary lymphoid tissues, although in some autoimmune diseases they may also accumulate in chronically inflamed tissues. Upon proper activation, naïve T cells proliferate and differentiate into specialized effector cells. Differentiation of T cells is characterized by a number of phenotypic and functional alterations, such as changes in their migratory capacities, modification to their lifespan, and secretion of effector cytokines (e.g. IL-4 and IFN-γ). Most activated naïve T cells become short-lived effector cells, but some enter the long-lived memory T cell pool. Memory T cells in humans can be characterized by the expression of the short isoform of CD45, namely CD45RO. Memory cells respond more rapidly to antigen challenge and have a diverse array of effector functions.
During T-cell stimulation, the recognition of the peptide-MHC complex by a TCR induces clustering of the TCR in concert with other cell surface receptors. Engagement of the TCR induces activation of signaling cascades that result in changes in the transcriptional program of the T cell. Naïve T cells have stringent requirements for activation and depend on a second signal, which is generally contributed by professional APCs in secondary lymphoid organs. The second signal gives an independent stimulus to the naïve T cells and is triggered by ligation of nonpolymorphic cell surface receptors. Extensive work has demonstrated that the 44 kDa glycoprotein CD28 is the major co-stimulatory molecule involved in T-cell activation [7]. CD28 co-stimulation increases the expression of lymphokine mRNAs, in particular those for IL-2 and IL-4 [8-10], and regulates the expression of Bcl-xL [11], CD152 (cytotoxic T lymphocyte-associated antigen [CTLA]4) [12], the high-affinity receptor for IL-2 (CD25) [13] and CD154 (CD40 ligand) [14], all of which contribute to successful progression of T-cell responses. In contrast to naïve T cells, memory cells do not require co-stimulation for activation. Thus, memory T cells do not depend on the interaction with professional APCs for activation, provided their specific antigen can be presented in the context of the appropriate MHC molecules by nonprofessional APCs.
CD8+ T cells in rheumatoid inflammation
The natural function of CD8+ T cells is related to protection against viral infections and tumors. CD8+ T cells perform this function by inflicting cytotoxic damage to target cells that express MHC class I molecules and the relevant antigenic peptide. Because almost all cells express MHC class I molecules, it is clear that CD8+ cells have a great potential to cause tissue damage. In addition, activated CD8+ T cells can produce very high levels of tumor necrosis factor (TNF) and IFN-γ, which may contribute directly and/or indirectly to target cell destruction in autoimmune diseases.
Some recent evidence has indicated a role for autoreactive CD8+ T cells in rheumatoid inflammation. A subgroup of CD8+ T cells, which co-express CD57, accumulates with duration of disease in the peripheral blood and the synovial fluid [15]. Of interest, those CD57+CD8+ T cells exhibit a remarkable condensation in their TCR repertoire [15-17], and unrelated RA patients carry clonally dominant CD8+ T cell β receptors with identical amino acid sequences [17]. These findings strongly suggest selection of CD57+CD8+ T cells by a common antigen, although it remains to be shown whether these CD8+ cells are selected by a self-antigen that is relevant to the pathogenesis of RA or by an environmental antigen that is independent of the disease. In this regard, CD8+ T cells specific for cytomegalovirus, Epstein-Barr virus, and influenza virus are enriched in the synovial fluid compared with peripheral blood in RA patients [18], and clonal or oligoclonal populations of CD8+ T cells dominate the responses to these viral antigens in synovial fluid from RA patients. Therefore, T cell clonality at the site of inflammation may reflect enrichment for memory T cells specific for foreign antigens rather than proliferation of autoreactive T cells specific for self-antigen.
Two independent observations indicate a role for CD8+ cells in disease progression in RA. First, synovial CD8+ T cells contain significant frequencies of IFN-γ producing effector cells that might contribute to sustained inflammation by secreting proinflammatory cytokines [19]. Second, CD8+ T cells may regulate the structural integrity and functional activity of germinal center-like structures in ectopic lymphoid follicles within the synovial membrane [20,21]. Taken together, the data suggest that activated CD8+ T cells are involved in aggravating pathologic responses in rheumatoid synovitis. Interestingly, however, studies in animals deficient for CD4 or CD8 have clearly demonstrated limited importance of CD8+ T cells in initiating and maintaining auto-immune inflammatory arthritis. Whereas B10.Q mice lacking CD4 are less susceptible to collagen-induced arthritis (CIA), but not completely resistant, the CD8 deficiency has no significant impact on the disease [22]. Moreover, in mice transgenic for the RA susceptibility gene HLA-DQ8, CD4-deficient mice were resistant to development of CIA whereas CD8-deficient mice developed disease with increased incidence and greater severity [23]. These data indicate that CD8+ T cells are not only incapable of initiating CIA but may, alternatively, have a regulatory/protective effect on auto-immune inflammation. The precise role played by CD8+ T cells as effectors and regulators of rheumatoid inflammation remains to be clarified.
CD4+ T cells in rheumatoid inflammation
It has become clear in recent years that the mechanisms resulting in the destruction of tissue and the loss of organ function during the course of an autoimmune disease are essentially the same as in protective immunity against invasive micro-organisms. Of fundamental importance in initiating, controlling, and driving these specific immune responses are activated CD4+ T cells. Once activated, CD4+ T cells differentiate into specialized effector cells and become the central regulators of specific immune responses. In RA a number of observations are consistent with the hypothesis that CD4+ T cells play a dominant role in the immuno-pathogenesis of the disease (Table 1). For example, activated CD4+ T cells can be found in the inflammatory infiltrates of the rheumatoid synovium [24]. CD4+ T cells play an important role in a variety of animal models of inflammatory arthritis, and tissue-damaging autoimmunity can be induced by transfer of CD4+ T cells from sick animals into healthy syngeneic recipients [25,26]. Moreover, appropriate T-cell directed therapies have clearly conferred clinical benefit in RA (Table 2) [27-29]. However, the most compelling finding, implying a central role for CD4+ T cells in propagating rheumatoid inflammation, remains the association of aggressive forms of the disease with particular MHC class II alleles, such as subtypes of HLA-DR4, that contain similar amino acid motifs in the CDR3 region of the DRβ chain [30,31]. Although the exact meaning of this association has not been resolved, all interpretations imply that CD4+ T cells orchestrate the local inflammation and cellular infiltration, after which a large number of subsequent inflammatory events occur.
Table 1.
Indications for a pathogenic role for CD4+ T cells in rheumatoid inflammation
| Association of rheumatoid arthritis with HLA-DR4 and HLA-DR1 subtypes (shared epitope) |
| Enrichment of activated CD4+ memory T cells in peripheral blood, synovial membrane, and synovial fluid |
| Important role in disease initiation in several animal models of inflammatory arthritis |
| Clinical efficacy of appropriate T-cell directed therapies |
Table 2.
T cell directed therapies in rheumatoid arthritis
| Intervention | Examples |
|---|---|
| Reduction in T cell number or function | Total lymphoid irradiation |
| Thoracic duct drainage | |
| Immunosuppressive drugs | Glucocorticoids |
| Methotrexate | |
| Leflunomide | |
| Cyclosporine | |
| FK 506 (tacrolimus) | |
| Rapamycin (sirolimus) | |
| Biologicals | TCR vaccination |
| mAbs to T cell surface receptors | |
| mAbs to surface receptors on cells interacting with T cells | |
| Cytokines, mAbs to cytokines | |
| Inhibitors of T cell/APC interactions |
APC, antigen-presenting cell; mAb, monoclonal antibody; TCR, T-cell receptor.
Whereas the specific antigen(s) recognized by the autoreactive CD4+ T cells is still unknown in RA, much progress has been made in defining the phenotype and function of those pathogenic CD4+ T cells. In 1986 it was discovered that repeated antigen-specific stimulation of murine CD4+ T cells in vitro results in the development of restricted and stereotyped patterns of cytokine secretion profiles in the resultant T-cell populations [32]. Based on these distinctive cytokine secretion patterns and concomitant effector functions, CD4+ T cells can be divided into at least two major subsets (Fig. 2). Th1 cells develop preferentially during infections with intracellular bacteria. Upon activation, Th1 cells secrete the proinflammatory cytokines IL-2, IFN-γ and lymphotoxin-α (LT, TNF-β). They activate macrophages to produce reactive oxygen intermediates and nitric oxide, stimulate their phagocytic functions, and enhance their ability for antigen presentation by upregulating MHC class II molecules. Moreover, Th1 cells promote the induction of complement fixing, opsonizing antibodies and of antibodies involved in antibody-dependent cell cytotoxicity (e.g. IgG1 in humans and IgG2a in mice). Consequently, Th1 cells are involved in cell-mediated immunity. Immune responses driven by Th1 cells are exemplified by the delayed-type hypersensitivity reaction [32,33]. Th2 cells predominate after infestations with gastrointestinal nematodes and helminths. They produce the anti-inflammatory cytokines IL-4 and IL-5, and provide potent help for B-cell activation and Ig class switching to IgE and subtypes of IgG that do not fix complement (e.g. IgG2 in humans and IgG1 in the mouse). Th2 cells mediate allergic immune responses and have been associated with downmodulation of macrophage activation, which is conferred largely by the anti-inflammatory effects of IL-4 [32,33].
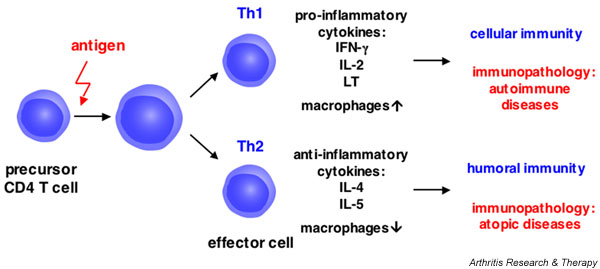
Figure 2.
Differentiation of CD4 T cells into specialized Th1 or Th2 effector cells. Upon activation with specific antigen, CD4 T cells proliferate and differentiate into either the Th1 or the Th2 subset. Th1 cells promote cellular immunity and are involved in the development of autoimmune diseases; Th2 cells mediate humoral immunity and are involved in allergic immune responses. IFN, interferon; IL, interleukin; Th, T-helper (cell).
The different functional T-cell subsets do not derive from different pre-committed lineages but rather develop from the same uncommitted precursor cell under the influence of environmental and genetic factors [34]. Differentiation of the appropriate T-cell subset is of crucial importance to the host in mounting protective immunity against exogenous micro-organisms. However, it is apparent that immune responses driven preferentially by activated T-cell subsets are also involved in the development of pathologic immune disorders. Whereas atopic diseases result from Th2-dominated responses to environmental allergens, Th1-mediated immunity is involved in the generation of several organ-specific experimental autoimmune diseases in animals, such as experimental allergic encephalomyelitis, insulin-dependent diabetes mellitus, and CIA [33]. Although dichotomizing complex diseases such as RA in terms of Th1 or Th2 patterns may be an over-simplification, evidence is accumulating that suggests that human autoimmune diseases, such as RA, might also be driven by preferentially activated Th1 cells without sufficient Th2 cell development to downregulate inflammation.
Rheumatoid inflammation is characterized by a dominant pathogenic Th1 drive
Various epidemiologic and clinical observations suggest a pathogenic Th1 drive in rheumatoid inflammation. For several decades, clinical observations have highlighted the ameliorating effect of pregnancy on the course of RA [35]. Pregnancy improves the symptoms of RA in about 75% of women, leading to significant resolution of inflammation and relief from symptoms, which enables the patients to taper or even stop the use of medications. In fact, the effect of pregnancy on RA activity is greater than the effect of some of the newer therapeutic agents. Although the mechanisms underlying this phenomenon remain unclear, a marked decrease in Th1-mediated immunity during pregnancy has been firmly established. For example, pregnant women have a higher incidence of infections than do nonpregnant females, in particular infections with intracellular pathogens. The characteristic Th1 immune reaction, delayed-type hypersensitivity, is diminished during pregnancy. Most recently, a placental derived protein (placental protein 14) could be identified that inhibited Th1 immune responses and synergized with IL-4 to promote Th2 immunity by inhibiting the downmodulation of the Th2 specific transcription factor GATA-3 [36]. Together, the data suggest that pregnancy induces a shift from Th1 to Th2 immune responses, thereby increasing anti-inflammatory cytokines, which may contribute to the gestational amelioration of RA. Interestingly, relapses of RA occur within 6 months postpartum in 90% of cases. At that time, pregnancy-associated alterations in Th subset activation can no longer be found [35], suggesting that the beneficial Th2 shift has resolved and has allowed the Th1-dominated autoimmune inflammation to recur.
Patients with RA have a decreased prevalence of allergic diseases [37]. Moreover, those patients with RA who, for example, have hay fever have less severe disease than do control patients with RA without hay fever [37]. As expected, atopic RA patients have higher levels of serum IgE and peripheral blood eosinophils, but their T cells produce less IFN-γ after maximum in vitro stimulation [37]. Because allergy is the prototype Th2 disease and activated Th2 cells are able to inhibit the generation and the function of Th1 effectors, these studies support the contention that the occurrence of a Th2-mediated immune response might be beneficial in RA by inhibiting Th1 driven immunity.
Exogenous cytokines have been used increasingly in recent years in an attempt to modulate the immune system and to initiate antitumor or antiviral cellular immune responses. These therapies, some of which are still experimental, provide opportunities to explore the effect of cytokines on T-cell function and differentiation after in vivo application and their effect on autoimmunity. IFN-α is a potent inducer of Th1 differentiation, and thus of cellular immunity in humans, and has been used in chronic viral infections and in attempts to promote antitumor immunity. However, the incidence of autoimmune diseases associated with IFN-α treatment ranges between 4% and 19%, and several authors have noted the first onset of RA or an exacerbation of pre-existing disease [38]. Like IFN-α, IL-12 is a strong inducer of Th1 cell development in humans. In an attempt to enhance antitumor cellular cytotoxicity, IL-12 has been employed in an experimental treatment for different forms of cancer. When IL-12 was given to a woman with metastatic cervical cancer, a severe exacerbation in her RA was noted [39]. Together, these in vivo effects of cytokines that induce Th1 immune responses strongly emphasize the role played by differentiated Th1 effectors in the pathogenesis of RA.
Apart from these clinical observations, various experimental approaches have also emphasized the dominance of activated Th1 effector cells in rheumatoid inflammation. For example, the vast majority of T cell clones from the human rheumatoid synovial membrane functionally represent the Th1 subset, producing large amounts of IFN-γ but no IL-4 upon challenge with their specific antigens [40,41]. In the majority of synovial biopsies, IFN-γ – as assessed by different techniques -prevails, whereas IL-4 is rarely found [42,43]. Importantly, synovial fluid and synovial tissue derived T cells express activation markers on their surface, indicating that these IFN-γ expressing cells are actively engaged in driving synovial inflammation. The frequency of IFN-γ producing CD4+ T cells is significantly increased in the synovial fluid as compared with the peripheral blood [44], resulting in a markedly elevated Th1/Th2 ratio in the synovial fluid that correlates with disease activity [45]. Likewise, drastically reduced synthesis of IL-4 and IL-10 mRNA by synovial fluid mononuclear cells from RA patients correlates with disease activity [46]. Finally, when synovial fluid T cells were cultured in the presence of IL-4, they were remarkably stable and resistant to Th2 inducing priming conditions [44]. Together, these data strongly suggest that CD4+ T cells from the inflamed rheumatoid synovium represent activated Th1 cells, secreting IFN-γ, which, in turn, orchestrates synovial inflammation.
Activated CD4+ T cells expressing elevated mRNA levels for IL-2 and/or for IFN-γ can also be detected in the peripheral blood of patients with active RA [47]. Most interestingly, when re-entry of circulating T cells into sites of inflammation in vivo was blocked by administration of a mAb to intercellular adhesion molecule (ICAM)-1 (CD54), a significant increase in IFN-γ mRNA levels in the peripheral blood occurred that might reflect a redistribution of activated Th1 cells from sites of inflammation into the peripheral circulation [47]. Moreover, the frequencies of IFN-γ secreting peripheral blood T cells in patients with new onset synovitis (duration <1 year) correlate well with disease activity, emphasizing the role of Th1 cells in the initiation of the disease [48].
Together, the data strongly imply that Th1 cells and their cytokines are not only present in RA but significantly contribute to the perpetuation of chronic inflammation.
CD4+ T cells as regulators of autoimmune inflammation
In recent years it has become apparent that CD4+ T cells do not only propagate specific immune responses as effector cells; subsets of CD4+ T cells have also been identified that are able to inhibit the initiation of immune reactions and even downregulate established immune responses. These CD4+ T cells are termed regulatory T cells and have, because of their role in the immunopathogenesis of autoimmune diseases and their potential use in therapeutic applications, become the focus of intensive research. Several T cells with regulatory capacity have been described. Tr1 cells have immune regulatory activities both in vitro and in vivo and produce large amounts of IL-10 [49,50]. The small subset of Th3 cells predominantly secretes the immunomodulatory cytokine transforming growth factor-β and develops in vivo after immunization through an oral or other mucosal route [51]. Th3 cells have been detected in patients with multiple sclerosis after oral administration of myelin basic protein [52]. Like Tr1 cells, Th3 cells can downregulate Th1 inflammation, and it is speculated that both subsets play a role in maintaining peripheral tolerance [53]. However, the precise function of those cells in immune homeostasis and, moreover, in autoimmune inflammation has not been conclusively addressed.
One particularly interesting CD4+ T-cell subset with regulatory capacity is defined by the constitutive expression of the α chain of the IL-2 receptor, CD25. CD25+ regulatory CD4+ T cells (Treg cells) were isolated first in mice, in which it was shown that transfer of CD4+ T cells that were depleted of the CD25-expressing T-cell fraction into athymic syngeneic Balb/c mice resulted in the development of various organ-specific autoimmune diseases, such as thyroiditis, gastritis, colitis, and insulin-dependent autoimmune diabetes [54]. Furthermore, co-transfer of CD4+CD25+ T cells with the pathogenic CD4+CD25- T cells prevented the development of experimentally induced autoimmune diseases [55,56]. These data imply that CD25+ Treg cells are able to regulate actively the responsiveness of autoreactive T cells that have escaped central tolerance. Subsequently, Treg cells were also detected in humans [57-63]. Treg cells are part of the physiologic peripheral T-cell repertoire and constitute between 5% and 15% of CD4+ T cells in the peripheral blood of healthy individuals. Treg cells are anergic (i.e. they do not proliferate in response to mitogenic stimulation) [64]. Of importance, CD25+CD4+ T cells, in contrast to their CD25- counterparts, are able to inhibit activation-induced proliferation of autologous responder T cells in a contact-dependent and cytokine-independent manner [58]. Both anergy and inhibition of proliferation can be prevented by the addition of exogenous IL-2 [65]. Apart from their constitutive expression of CD25, Treg cells are characterized phenotypically by surface expression of CTLA4 [66] and glucocorticoid induced TNF receptor family related protein [67], as well as by the expression of the transcription factor Foxp3 [68]. The importance of Foxp3 for the regulatory function of Treg cells has been demonstrated by transfection of CD25-CD4+ T cells with a plasmid encoding Foxp3, which conferred a regulatory capacity to the transfected T cells [68]. A comprehensive review on the function of Treg cells in immune homeostasis is beyond the scope of this article; excellent summaries have been published elsewhere [68,69]. Together, the accumulated evidence indicates that Treg cells may play an important role in maintaining peripheral tolerance and preventing the evolution of autoimmune inflammation.
In a series of recent reports, Treg cells were identified and analyzed in different rheumatic diseases, but their role is incompletely understood. Whereas controversy exists with regard to the frequency of Treg cells in the peripheral circulation of patients with rheumatoid inflammation [49,52,70], it appears clear that elevated numbers of Treg cells are present in the inflamed synovial tissue of patients with RA as compared with the peripheral circulation [50,70]. When examined in conventional in vitro assays, synovial Treg cells are able to suppress the proliferation of autologous CD4+CD25- responder T cells of synovial and peripheral origin [50,52,70]. Of interest, synovial Treg cells exhibit an increased suppressive capacity as compared with blood Treg cells in RA [70].
Several mechanisms may contribute to the apparent paradox of local inflammation despite increased frequencies of Treg cells with enhanced suppressor activity in vitro. First, as synovial Treg cells exhibit functional differences before and after anti-TNF-treatment, it has been suggested that TNF inhibits Treg cells and prevents their regulatory function in vivo [49]. Similarly, other constituents of the synovial environment, such as IL-2, and other mediators of inflammation, such as IL-7, or APCs that are able to engage co-stimulatory ligands on the synovial Treg cells, abrogate the function of Treg cells [65,71]. Second, synovial responder T cells express decreased susceptibility to the regulatory effect of Treg cells as compared with peripheral blood responder T cells, thereby 'compensating' for the enhanced regulatory capacity of the synovial Treg cells [70]. Finally, although suppression by Treg cells is probably not antigen specific but may involve neighboring T cells in a 'bystander' manner [51], Treg cells require activation through their TCR to deliver their regulatory function. Thus, if the specific antigen for the synovial Treg cells is not presented either in the secondary lymphoid organs or in the inflamed synovia, or, alternatively, if Treg cells in RA express an altered threshold for antigen-specific activation, then synovial Treg cells, albeit present, will not become activated and therefore will fail to inhibit ongoing inflammation.
A final important CD4+ T-cell subset with regulatory capacity for Th1 inflammation is the Th2 subset that antagonizes the generation of Th1 cells and their effector functions largely via its signature cytokine IL-4. For instance, the generation of Th1 cells can be effectively blocked by high concentrations of IL-4, even in the presence of IL-12 [72]. At the level of effector functions, IL-4 antagonizes much of the proinflammatory effect of IFN-γ and inhibits the proliferation of Th1 cells. Consequently, IL-4 has been used in vivo as a treatment for experimental autoimmune diseases in animals and in patients with psoriasis [73]. In animal models, IL-4 is the most successful means by which to ameliorate autoimmune disorders that are caused by activated Th1 cells. For example, IL-4 improves experimental allergic encephalomyelitis, delays the onset and diminishes clinical symptoms of CIA, and prevents joint damage and bone erosion in this experimental autoimmune disease [74]. In vitro, IL-4 suppresses metalloproteinase production and stimulates production of tissue inhibitor of metalloproteinases-1 in human mononuclear phagocytes and cartilage explants, indicating a protective effect of IL-4 toward extracellular matrix degradation. Furthermore, IL-4 inhibits bone resorption through an effect on both osteoclast activity and survival [75] and reduces the spontaneous secretion of proinflammatory cytokines and Ig in ex vivo cultured pieces from the rheumatoid synovial membrane [76]. Finally, IL-4 downregulates the surface expression of CD5 on B cells and inhibits spontaneous Ig and IgM rheumatoid factor production in patients with RA [77]. Together, the Th2 cytokine IL-4 has potent immunomodulatory functions that affect different cellular targets and is capable of ameliorating signs and symptoms of chronic arthritis.
As discussed above, rheumatoid inflammation is characterized by the predominance of IFN-γ and the absence of IL-4. However, these data do not yet permit a conclusion to be drawn regarding whether Th1 cells are the initiators of rheumatoid inflammation or rather appear as a consequence of it. In other words, the observations described above do not identify the mechanisms underlying the dominant pathogenic Th1 drive in RA. In order to address this issue, studies were carried out to assess the functional capability of T cells in RA patients with regard to their plasticity to differentiate into Th1 and Th2 effector cells. In these studies, it became obvious that isolated memory CD4+ T cells from the majority of patients with very early treatment-naïve RA (disease duration < 6 months and no previous treatment with disease modifying antirheumatic drugs [DMARDs] or corticosteroids) manifest a profound inability to mount Th2 responses [78]. Thus, at the onset of the disease, those patients cannot generate immunoregulatory Th2 cells that might downregulate ongoing Th1-mediated inflammation. Failure to downregulate activated Th1 cells at disease initiation might thereby allow Th1 inflammation to persist and evolve into chronic inflammation, characterized by the continuous activation of T cells, macrophages, fibroblasts, and osteoclasts and, subsequently, the destruction of tissue. Because this functional abnormality of CD4+ T cells in RA is evident at the time of initial clinical symptoms of arthritis [78], the data strongly suggest that the failure of CD4+ T cells in RA to generate Th2 effectors is the basis that allows Th1 dominated chronic immunity to develop, and is not merely its consequence.
T-cell directed therapies
Based on the concept that activated T cells are the key mediators of chronic autoimmune inflammation, various T-cell directed therapeutic interventions have been introduced for the treatment of RA. Comprehensive reviews have discussed the concepts and the clinical efficacy of T-cell directed therapy in RA [79-82]. Here, we review those approaches that target the pathogenetically important alterations in CD4+ T cell functions as outlined above.
Because RA is driven by proinflammatory Th1 cells with impaired differentiation of immunoregulatory Th2 cells, a shift in the balance of Th1/Th2 effector cells toward anti-inflammatory Th2 cells would be expected to be clinically beneficial. The concept of modulating the Th1/Th2 balance as a treatment for chronic autoimmunity has been successfully applied in a number of animal models of autoimmune diseases [83,84]. It is therefore of interest that several recent studies have indicated that DMARDs appear to be able to modulate the Th1/Th2 balance. For example, leflunomide, a potent nontoxic inhibitor of the rate-limiting enzyme of the de novo synthesis of pyrimidines, dihydro-orotate dehydrogenase [85], selectively decreases the activation of proinflammatory Th1 cells while promoting Th2 cell differentiation from naïve precursors [86]. Sulfasalazine potently inhibits the production of IL-12 in a dose-dependent manner in mouse macrophages stimulated with lipopolysaccharide. Importantly, pretreatment of macrophages with sulfasalazine either in vitro or in vivo reduces their ability to induce the Th1 cytokine IFN-γ and increases the ability to induce the Th2 cytokine IL-4 in antigen-primed CD4+ T cells [87]. Methotrexate significantly decreases the production of IFN-γ and IL-2 by in vitro stimulated peripheral blood mononuclear cells while increasing the concentration of IL-4 and IL-10 [88]. Likewise, clinical efficacy of cyclosporine is associated with decreased serum levels of IFN-γ, IL-2 and IL-12, and with significant increases in IL-10 [89]. Bucillamine decreases the frequency of IFN-γ producing CD4+ T cells generated after a priming culture of mononuclear cells from the peripheral blood [90]. Finally, reports have suggested that glucocorticoids inhibit cytokine expression indirectly through promotion of a Th2 cytokine secretion profile, presumably by their action on monocyte activation [91]. Together, the data suggest that the anti-inflammatory effect of a number of current treatment modalities in RA is characterized by an inhibition of Th1 cell activation and effector cell generation, and by favoring Th2 differentiation, thereby shifting the Th1/Th2 balance toward Th2.
In an attempt to target only those cells that perpetuate the chronic inflammation specifically, with minimal effects on other aspects of the immune or inflammatory systems, therapeutic tools ('biologicals') with defined targets and effector functions have been designed and tested in clinical applications. Because CD4+ T cells are central in initiating and perpetuating the chronic autoimmune response in rheumatic diseases, many biologicals are aimed at interfering with T-cell activation and/or migration.
A major advance in our understanding of T-cell activation has been the identification of the critical co-stimulatory molecules on T cells, such as CD28, lymphocyte function-associated antigen (LFA)-1, CD2, CD4, CD30, CD44, and CD154 (CD40L), and their interacting ligands on APCs or B cells. Although these molecules act through different mechanisms, some delivering co-stimulatory biochemical signals to the T cell and some enhancing adhesion to target tissues, they all have the ability to augment the T-cell proliferative responses to antigenic stimuli. Biologicals designed to interfere with co-stimulation via inhibiting engagement of co-stimulatory ligands have been used in several animal models of inflammatory arthritis and in treatment trials in RA. In experimental autoimmune diseases in animals, mAbs to CD4 have been used to prevent the induction of the disease [92,93]. Of relevance to human disease, mAbs to CD4 were also able to inhibit further progression when given after the initial inflammation had already become manifest [93,94], although, with one notable exception [95], controlled human trials have largely failed to demonstrate favorable results to date [79]. Interaction of CD2 with its ligand, CD58 has been blocked by application of a soluble fully human recombinant fusion protein comprising the first extracellular domain of CD58 and the hinge, CH2 and CH3 sequences of human IgG1 (LFA-3-IgG1; alefacept). Alefacept has been employed in patients with psoriasis, with substantial clinical response [96].
Inhibition of CD28-mediated co-stimulatory signals is a potent means of immunosuppression that can be achieved by blocking either CD28 or CD80 and CD86. Currently, humanized anti-B7 mAbs are in phase II clinical trials for solid organ transplantation, graft versus host disease, and mild to severe plaque psoriasis. An alternative approach to block CD28 co-stimulation is by coating CD80 and CD86 with a soluble Ig fusion protein of the extracellular domain of CTLA4 (CD152). CTLA4 is a homolog to CD28 and is expressed by activated T cells. It can bind both CD80 and CD86 with higher affinity than CD28. Because CD152 has a high affinity for CD80 and CD86, soluble forms of CTLA4 inhibit the interaction of CD28 with its ligands. In clinical trials, CTLA4-Ig (CTLA4-IgG1; abatacept) had favorable effects in patients with psoriasis vulgaris [97] and in patients with RA [98,99].
The adhesion receptor/counter-receptor pair, LFA-1 (CD11α/CD18) and ICAM-1, is critical for transendothelial migration of T cells and their subsequent activation [100]. Therefore, mAbs to LFA-1 and ICAM-1 have been employed in auto-immune diseases in an attempt to block migration of T cells into sites of inflammation and their subsequent stimulation by locally expressed antigenic peptides in vivo [47,101]. Significant clinical benefit was achieved with a mAb to ICAM-1 in patients with active RA [101]. It is of interest that clinical benefit was restricted to those patients who showed a marked increase in the levels of Th1 cytokine producing T cells in their circulation immediately after administration of the mAb [47]. Thus, it can be reasoned that, in responding patients, the circulatory pattern of activated Th1 cells was altered by inhibiting their migration into the inflamed synovium. These data emphasize the pathogenic Th1 drive in those patients who respond to therapy.
Together, T-cell directed therapy in RA is based on the concept that CD4+ T cells initiate and continuously drive systemic rheumatoid inflammation. T-cell directed DMARDs and some of the recently employed mAbs have been successful in ameliorating signs and symptoms of the diseases, and some also seem able to slow disease progression. Thus, although sustained clinical improvement has not been achieved with a short course of biologicals, the idea that targeting CD4+ T cells as the controllers of rheumatoid inflammation will interrupt chronic autoimmune inflammation and subsequent tissue destruction has received strong support.
Conclusion
Together, strong evidence indicates a critical role for CD4+ T cells in the pathogenesis of rheumatoid inflammation. However, their role appears to exceed that of activated proinflammatory Th1 effector cells, which promote many aspects of synovial inflammation. Rather, it has become apparent that, in RA, CD4+ T cell subsets with regulatory capacity, such as Th2 cells and CD25+ Treg cells, are functionally impaired, thus allowing Th1 driven immunity to evolve and progress into chronic inflammation. Interference with the activation and generation of Th1 cells and with the activity of their secreted cytokines might therefore be beneficial in the treatment of RA. Those therapies might include biologicals that target CD4+ effector T cells but also novel approaches that induce and/or enhance the function of regulatory T cells in vivo.
Abbreviations
APC = antigen-presenting cell; CDR = complementarity determining region; CIA = collagen-induced arthritis; CTLA = cytotoxic T lymphocyte-associated antigen; DMARD = disease-modifying antirheumatic drug; ICAM = intercellular adhesion molecule; IFN = interferon; IL = interleukin; Ig = immunoglobulin; LFA = lymphocyte function-associated antigen; MHC = major histocompatibility complex; mAb = monoclonal antibody; RA = rheumatoid arthritis; TCR = T-cell receptor; Th = T-helper (cell); Treg = regulatory T (cell); TNF = tumor necrosis factor.
Competing interests
The author(s) declare that they have no competing interests.
Acknowledgements
This work was supported in part by the Deutsche Forschungsgemeinschaft (Schu 786/2–3 and 2–4) and by the Interdisciplinary Center for Clinical Research (IZKF) at the University Hospital of the University of Erlangen-Nuremberg (Projects B27 and B3).
References
- Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- Spits H. Development of alphabeta T cells in the human thymus. Nat Rev Immunol. 2002;2:760–772. doi: 10.1038/nri913. [DOI] [PubMed] [Google Scholar]
- Weiss A. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell. 1993;73:209–212. doi: 10.1016/0092-8674(93)90221-b. [DOI] [PubMed] [Google Scholar]
- Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Jacobs MR, Haynes BF. Increase in TCR gamma delta T lymphocytes in synovia from rheumatoid arthritis patients with active synovitis. J Clin Immunol. 1992;12:130–138. doi: 10.1007/BF00918143. [DOI] [PubMed] [Google Scholar]
- Olive C, Gatenby PA, Serjeantson SW. Evidence for oligoclonality of T cell receptor delta chain transcripts expressed in rheumatoid arthritis patients. Eur J Immunol. 1992;22:2587–2593. doi: 10.1002/eji.1830221018. [DOI] [PubMed] [Google Scholar]
- June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Lindsten T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of inter-leukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Li-Weber M, Giasi M, Krammer PH. Involvement of Jun and Rel proteins in up-regulation of interleukin-4 gene activity by the T cell accessory molecule CD28. J Biol Chem. 1998;273:32460–32466. doi: 10.1074/jbc.273.49.32460. [DOI] [PubMed] [Google Scholar]
- Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Alegre ML, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- Cerdan C, Martin Y, Courcoul M, Brailly H, Mawas C, Birg F, Olive D. Prolonged IL-2 receptor alpha/CD25 expression after T cell activation via the adhesion molecules CD2 and CD28. Demonstration of combined transcriptional and post-transcriptional regulation. J Immunol. 1992;149:2255–2261. [PubMed] [Google Scholar]
- Yin D, Zhang L, Wang R, Radvanyi L, Haudenschild C, Fang Q, Kehry MR, Shi Y. Ligation of CD28 In vivo induces CD40 ligand expression and promotes B cell survival. J Immunol. 1999;163:4328–4334. [PubMed] [Google Scholar]
- Arai K, Yamamura S, Seki S, Hanyu T, Takahashi HE, Abo T. Increase of CD57+ T cells in knee joints and adjacent bone marrow of rheumatoid arthritis (RA) patients: implication for an anti-inflammatory role. Clin Exp Immunol. 1998;111:345–352. doi: 10.1046/j.1365-2249.1998.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EC, Lawson TM, Vedhara K, Moss PA, Lehner PJ, Borysiewicz LK. CD8high+ (CD57+) T cells in patients with rheumatoid arthritis. Arthritis Rheum. 1997;40:237–248. doi: 10.1002/art.1780400208. [DOI] [PubMed] [Google Scholar]
- Hingorani R, Monteiro J, Furie R, Chartash E, Navarrete C, Pergolizzi R, Gregersen PK. Oligoclonality of V beta 3 TCR chains in the CD8+ T cell population of rheumatoid arthritis patients. J Immunol. 1996;156:852–858. [PubMed] [Google Scholar]
- Fazou C, Yang H, McMichael AJ, Callan MF. Epitope specificity of clonally expanded populations of CD8+ T cells found within the joints of patients with inflammatory arthritis. Arthritis Rheum. 2001;44:2038–2045. doi: 10.1002/1529-0131(200109)44:9<2038::AID-ART353>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Berner B, Akca D, Jung T, Muller GA, Reuss-Borst MA. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol. 2000;27:1128–1135. [PubMed] [Google Scholar]
- Kang YM, Zhang X, Wagner UG, Yang H, Beckenbaugh RD, Kurtin PJ, Goronzy JJ, Weyand CM. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195:1325–1336. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner UG, Kurtin PJ, Wahner A, Brackertz M, Berry DJ, Goronzy JJ, Weyand CM. The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis. J Immunol. 1998;161:6390–6397. [PubMed] [Google Scholar]
- Ehinger M, Vestberg M, Johansson AC, Johannesson M, Svens-son A, Holmdahl R. Influence of CD4 or CD8 deficiency on collagen-induced arthritis. Immunology. 2001;103:291–300. doi: 10.1046/j.1365-2567.2001.01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja V, Taneja N, Paisansinsup T, Behrens M, Griffiths M, Luthra H, David CS. CD4 and CD8 T cells in susceptibility/protection to collagen-induced arthritis in HLA-DQ8-transgenic mice: implications for rheumatoid arthritis. J Immunol. 2002;168:5867–5875. doi: 10.4049/jimmunol.168.11.5867. [DOI] [PubMed] [Google Scholar]
- Van Boxel JA, Paget SA. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975;293:517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Webber C, Poole AR. The induction of arthritis in mice by the cartilage proteoglycan aggrecan: roles of CD4+ and CD8+ T cells. Cell Immunol. 1992;144:347–357. doi: 10.1016/0008-8749(92)90250-s. [DOI] [PubMed] [Google Scholar]
- Breedveld FC, Dynesius-Trentham R, de Sousa M, Trentham DE. Collagen arthritis in the rat is initiated by CD4+ T cells and can be amplified by iron. Cell Immunol. 1989;121:1–12. doi: 10.1016/0008-8749(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Panayi GS, Tugwell P. The use of cyclosporin A in rheumatoid arthritis: conclusions of an international review. Br J Rheumatol. 1994;33:967–969. doi: 10.1093/rheumatology/33.10.967. [DOI] [PubMed] [Google Scholar]
- Paulus HE, Machleder HI, Levine S, Yu DT, MacDonald NS. Lymphocyte involvement in rheumatoid arthritis. Studies during thoracic duct drainage. Arthritis Rheum. 1977;20:1249–1262. doi: 10.1002/art.1780200614. [DOI] [PubMed] [Google Scholar]
- Strober S, Tanay A, Field E, Hoppe RT, Calin A, Engleman EG, Kotzin B, Brown BW, Kaplan HS. Efficacy of total lymphoid irradiation in intractable rheumatoid arthritis. A double-blind, randomized trial. Ann Intern Med. 1985;102:441–449. doi: 10.7326/0003-4819-102-4-441. [DOI] [PubMed] [Google Scholar]
- Calin A, Elswood J, Klouda PT. Destructive arthritis, rheumatoid factor, and HLA-DR4. Susceptibility versus severity, a case-control study. Arthritis Rheum. 1989;32:1221–1225. doi: 10.1002/anr.1780321006. [DOI] [PubMed] [Google Scholar]
- Winchester R. The molecular basis of susceptibility to rheumatoid arthritis. Adv Immunol. 1994;56:389–466. doi: 10.1016/s0065-2776(08)60456-3. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Rocken M, Saurat JH, Hauser C. A common precursor for CD4+ T cells producing IL-2 or IL-4. J Immunol. 1992;148:1031–1036. [PubMed] [Google Scholar]
- Da Silva JA, Spector TD. The role of pregnancy in the course and aetiology of rheumatoid arthritis. Clin Rheumatol. 1992;11:189–194. doi: 10.1007/BF02207955. [DOI] [PubMed] [Google Scholar]
- Mishan-Eisenberg G, Borovsky Z, Weber MC, Gazit R, Tykocinski ML, Rachmilewitz J. Differential regulation of Th1/Th2 cytokine responses by placental protein 14. J Immunol. 2004;173:5524–5530. doi: 10.4049/jimmunol.173.9.5524. [DOI] [PubMed] [Google Scholar]
- Verhoef CM, van Roon JA, Vianen ME, Bruijnzeel-Koomen CA, Lafeber FP, Bijlsma JW. Mutual antagonism of rheumatoid arthritis and hay fever; a role for type 1/type 2 T cell balance. Ann Rheum Dis. 1998;57:275–280. doi: 10.1136/ard.57.5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou Y, Isenberg DA. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 2000;43:1431–1442. doi: 10.1002/1529-0131(200007)43:7<1431::AID-ANR3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Peeva E, Fishman AD, Goddard G, Wadler S, Barland P. Rheumatoid arthritis exacerbation caused by exogenous interleukin-12. Arthritis Rheum. 2000;43:461–463. doi: 10.1002/1529-0131(200002)43:2<461::AID-ANR29>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Miltenburg AM, van Laar JM, de Kuiper R, Daha MR, Breedveld FC. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992;35:603–610. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Quayle AJ, Chomarat P, Miossec P, Kjeldsen-Kragh J, Forre O, Natvig JB. Rheumatoid inflammatory T-cell clones express mostly Th1 but also Th2 and mixed (Th0) cytokine patterns. Scand J Immunol. 1993;38:75–82. doi: 10.1111/j.1365-3083.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- Kusaba M, Honda J, Fukuda T, Oizumi K. Analysis of type 1 and type 2 T cells in synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 1998;25:1466–1471. [PubMed] [Google Scholar]
- Canete JD, Martinez SE, Farres J, Sanmarti R, Blay M, Gomez A, Salvador G, Munoz-Gomez J. Differential Th1/Th2 cytokine patterns in chronic arthritis: interferon gamma is highly expressed in synovium of rheumatoid arthritis compared with seronegative spondyloarthropathies. Ann Rheum Dis. 2000;59:263–268. doi: 10.1136/ard.59.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LS, Cush JJ, Schulze-Koops H, Lipsky PE. Rheumatoid synovial CD4+ T cells exhibit a reduced capacity to differentiate into IL-4-producing T-helper-2 effector cells. Arthritis Res. 2001;3:54–64. doi: 10.1186/ar140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff WL, Prins AP, Niers TM, Dijkmans BA, van Lier RA. Quantitation of interferon gamma- and interleukin-4-producing T cells in synovial fluid and peripheral blood of arthritis patients. Rheumatology. 1999;38:214–220. doi: 10.1093/rheumatology/38.3.214. [DOI] [PubMed] [Google Scholar]
- Miyata M, Ohira H, Sasajima T, Suzuki S, Ito M, Sato Y, Kasukawa R. Significance of low mRNA levels of interleukin-4 and -10 in mononuclear cells of the synovial fluid of patients with rheumatoid arthritis. Clin Rheumatol. 2000;19:365–370. doi: 10.1007/s100670070029. [DOI] [PubMed] [Google Scholar]
- Schulze-Koops H, Lipsky PE, Kavanaugh AF, Davis LS. Elevated Th1- or Th0-like cytokine mRNA in peripheral circulation of patients with rheumatoid arthritis: modulation by treatment with anti-ICAM-1 correlates with clinical benefit. J Immunol. 1995;155:5029–5037. [PubMed] [Google Scholar]
- Kanik KS, Hagiwara E, Yarboro CH, Schumacher HR, Wilder RL, Klinman DM. Distinct patterns of cytokine secretion characterize new onset synovitis versus chronic rheumatoid arthritis. J Rheumatol. 1998;25:16–22. [PubMed] [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–23. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001;3:947–954. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335–R346. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- Taams LS, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol. 2001;31:1122–1131. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Roncarolo MG. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Stephens LA, Mottet C, Mason D, Powrie F. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymo-cytes. J Exp Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glu-cocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003;81:331–371. doi: 10.1016/s0065-2776(03)81008-8. [DOI] [PubMed] [Google Scholar]
- Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4+CD25+ regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- van Amelsfort JM, Noordegraaf M, Bijlsma JWJ, Taams LS, Lafeber FPJG. Influence of the inflammatory milieu on the suppressive function of CD4+CD25+ T cells in rheumatoid arthritis. Arthritis Rheum. 2004;50:S526. [Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria -induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K, Thomas P, Breit S, Dugas M, Mailhammer R, Van Eden W, Van Der Zee R, Biedermann T, Prinz J, Mack M. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9:40–46. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- Horsfall AC, Butler DM, Marinova L, Warden PJ, Williams RO, Maini RN, Feldmann M. Suppression of collagen-induced arthritis by continuous administration of IL-4. J Immunol. 1997;159:5687–5696. [PubMed] [Google Scholar]
- Miossec P, Chomarat P, Dechanet J, Moreau JF, Roux JP, Delmas P, Banchereau J. Interleukin-4 inhibits bone resorption through an effect on osteoclasts and proinflammatory cytokines in an ex vivo model of bone resorption in rheumatoid arthritis. Arthritis Rheum. 1994;37:1715–1722. doi: 10.1002/art.1780371202. [DOI] [PubMed] [Google Scholar]
- Miossec P, Briolay J, Dechanet J, Wijdenes J, Martinez-Valdez H, Banchereau J. Inhibition of the production of proinflammatory cytokines and immunoglobulins by interleukin-4 in an ex vivo model of rheumatoid synovitis. Arthritis Rheum. 1992;35:874–883. doi: 10.1002/art.1780350805. [DOI] [PubMed] [Google Scholar]
- Hidaka T, Kitani A, Hara M, Harigai M, Suzuki K, Kawaguchi Y, Ishizuka T, Kawagoe M, Nakamura H. IL-4 down-regulates the surface expression of CD5 on B cells and inhibits spontaneous immunoglobulin and IgM-rheumatoid factor production in patients with rheumatoid arthritis. Clin Exp Immunol. 1992;89:223–229. doi: 10.1111/j.1365-2249.1992.tb06936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapenko A, Wendler J, Lipsky PE, Kalden JR, Schulze-Koops H. Altered memory T cell differentiation in patients with early rheumatoid arthritis. J Immunol. 1999;163:491–499. [PubMed] [Google Scholar]
- Schulze-Koops H, Lipsky PE. Anti-CD4 monoclonal antibody therapy in human autoimmune diseases. Curr Dir Autoimmun. 2000;2:24–49. doi: 10.1159/000060506. [DOI] [PubMed] [Google Scholar]
- Schulze-Koops H, Kalden JR. In: Biological Therapy in Rheumatology. Smolen JS, Lipsky PE, editor. London: Martin Dunitz Publishers; 2003. Targeting T cells in rheumatic diseases; pp. 3–24. [Google Scholar]
- Panayi GS. Targeting of cells involved in the pathogenesis of rheumatoid arthritis. Rheumatology. 1999;38(Suppl 2):8–10. [PubMed] [Google Scholar]
- Yocum DE. T cells: pathogenic cells and therapeutic targets in rheumatoid arthritis. Semin Arthritis Rheum. 1999;29:27–35. doi: 10.1016/s0049-0172(99)80035-3. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Lubberts E, Helsen MM, Saxne T, Coenende Roo CJ, Heinegard D, van den Berg WB. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res. 1999;1:81–91. doi: 10.1186/ar14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis N, Boissier MC, Ferrara P, Blankenstein T, Fradelizi D, Fournier C. Attenuation of collagen-induced arthritis in mice by treatment with vector cells engineered to secrete inter-leukin-13. Eur J Immunol. 1996;26:2399–2403. doi: 10.1002/eji.1830261020. [DOI] [PubMed] [Google Scholar]
- Bruneau JM, Yea CM, Spinella-Jaegle S, Fudali C, Woodward K, Robson PA, Sautes C, Westwood R, Kuo EA, Williamson RA, Ruuth E. Purification of human dihydro-orotate dehydrogenase and its inhibition by A77 the active metabolite of leflunomide. Biochem J. 1726;336:299–303. doi: 10.1042/bj3360299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova P, Skapenko A, Herrmann ML, Schleyerbach R, Kalden JR, Schulze-Koops H. Restriction of de novo pyrimidine biosynthesis inhibits Th1 cell activation and promotes Th2 cell differentiation. J Immunol. 2002;169:3392–3399. doi: 10.4049/jimmunol.169.6.3392. [DOI] [PubMed] [Google Scholar]
- Kang BY, Chung SW, Im SY, Choe YK, Kim TS. Sulfasalazine prevents T-helper 1 immune response by suppressing interleukin-12 production in macrophages. Immunology. 1999;98:98–103. doi: 10.1046/j.1365-2567.1999.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin A, Loubet-Lescoulie P, Lambert N, Yassine-Diab B, Abbal M, Mazieres B, de Preval C, Cantagrel A. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: evidence of increased inter-leukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse transcriptase-polymerase chain reaction. Arthritis Rheum. 1998;41:48–57. doi: 10.1002/1529-0131(199801)41:1<48::AID-ART7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- de Groot K, Gross WL. Wegener's granulomatosis: disease course, assessment of activity and extent and treatment. Lupus. 1998;7:285–291. doi: 10.1191/096120398678920118. [DOI] [PubMed] [Google Scholar]
- Morinobu A, Wang Z, Kumagai S. Bucillamine suppresses human Th1 cell development by a hydrogen peroxide-independent mechanism. J Rheumatol. 2000;27:851–858. [PubMed] [Google Scholar]
- Almawi WY, Melemedjian OK, Rieder MJ. An alternate mechanism of glucocorticoid anti-proliferative effect: promotion of a Th2 cytokine-secreting profile. Clin Transplant. 1999;13:365–374. doi: 10.1034/j.1399-0012.1999.130501.x. [DOI] [PubMed] [Google Scholar]
- Ranges GE, Sriram S, Cooper SM. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985;162:1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor MK, Sriram S, Hardy R, Herzenberg LA, Lanier L, Lim M, Steinman L. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985;227:415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- Wofsy D, Seaman WE. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J Immunol. 1987;138:3247–3253. [PubMed] [Google Scholar]
- Schulze-Koops H, Davis LS, Haverty TP, Wacholtz MC, Lipsky PE. Reduction of Th1 cell activity in the peripheral circulation of patients with rheumatoid arthritis after treatment with a non-depleting humanized monoclonal antibody to CD4. J Rheumatol. 1998;25:2065–2076. [PubMed] [Google Scholar]
- Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–255. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland LW, Alten R, Van Den Bosch F, Appelboom T, Leon M, Emery P, Cohen S, Luggen M, Shergy W, Nuamah I. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum. 2002;46:1470–1479. doi: 10.1002/art.10294. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- Kavanaugh AF, Lightfoot E, Lipsky PE, Oppenheimer-Marks N. The role of CD11/CD18 in adhesion and transendothelial migration of T cells: analysis utilizing CD18 deficient T cell clones. J Immunol. 1991;146:4149–4156. [PubMed] [Google Scholar]
- Kavanaugh AF, Davis LS, Nichols LA, Norris SH, Rothlein R, Scharschmidt LA, Lipsky PE. Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule 1. Arthritis Rheum. 1994;37:992–999. doi: 10.1002/art.1780370703. [DOI] [PubMed] [Google Scholar]