Abstract
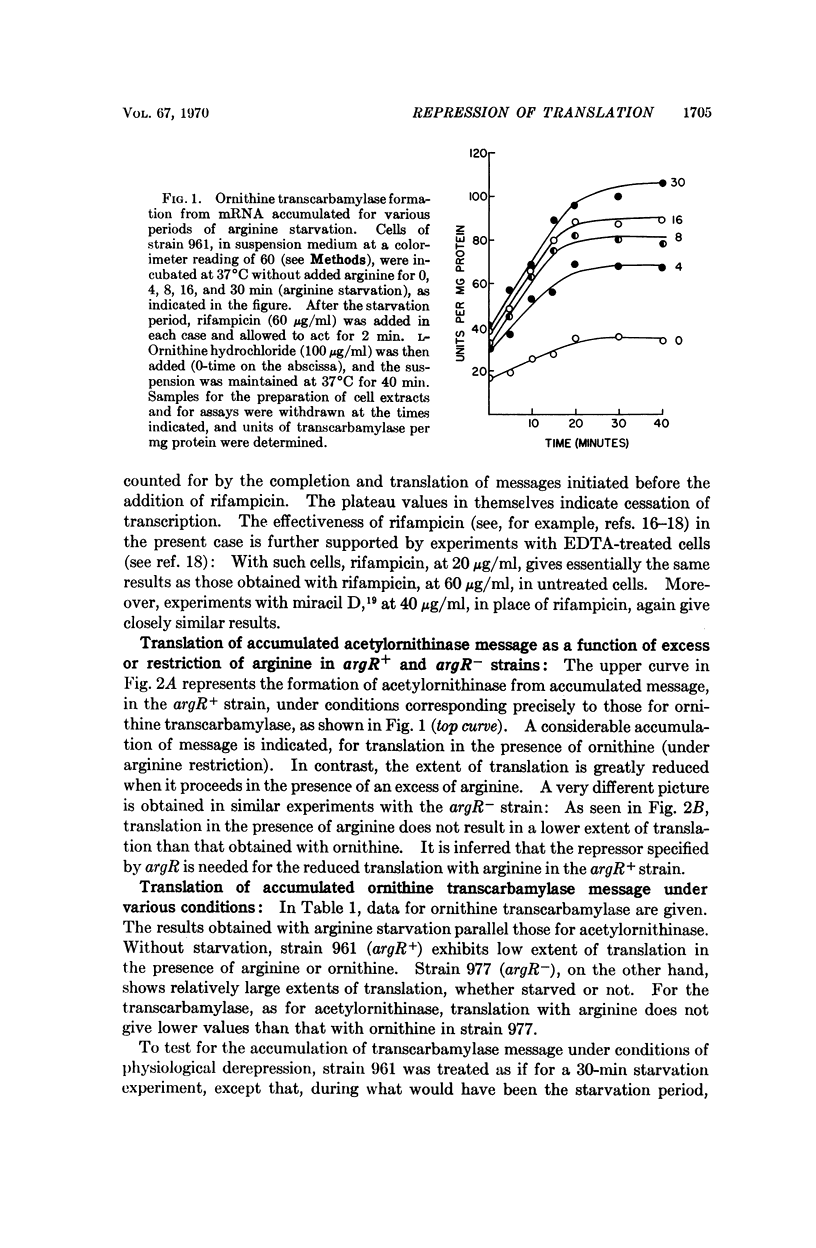
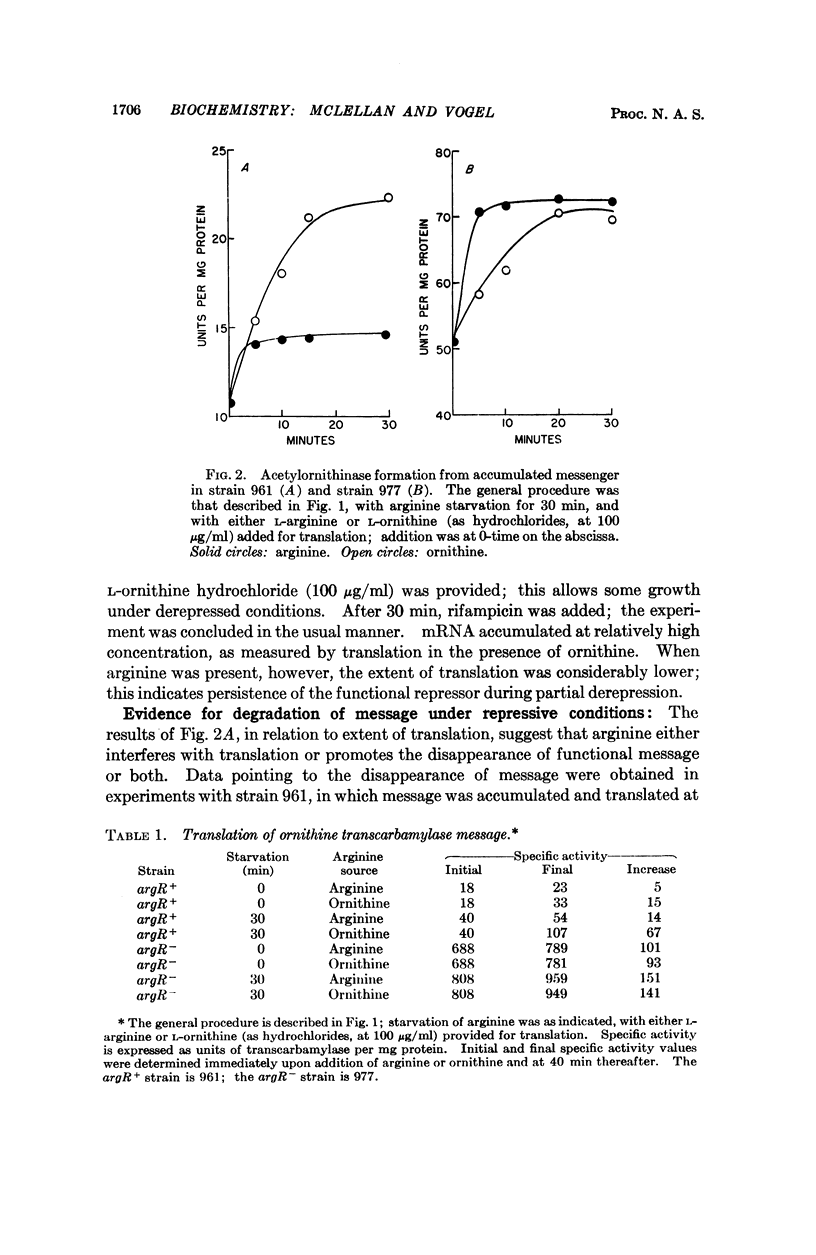
Translation of bacterial mRNA, divorced from transcription, has been obtained for enzymes of arginine synthesis; evidence has been acquired for repression by arginine at the level of translation. mRNAs for acetylornithinase and ornithine transcarbamylase were accumulated by arginine starvation of argR+ and argR- arginine auxotrophs derived from Escherichia coli K12. Further transcription was inhibited with rifampicin or miracil D, and enzyme formation was measured in the presence of either an excess of, or a restricted supply of, arginine. For the argR+ strain 961, little mRNA was found without starvation; for the argR- strain 977, a considerable amount of mRNA was demonstrated even without starvation. There was relatively little translation for the argR+ strain, but not for the argR- strain, in the presence of excess arginine, apparently due to an accelerated degradation of mRNA in the argR+ strain under repressive conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- Baumberg S., Bacon D. F., Vogel H. J. Individually repressible enzymes specified by clustered genes of arginine synthesis. Proc Natl Acad Sci U S A. 1965 May;53(5):1029–1032. doi: 10.1073/pnas.53.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline A. L., Bock R. M. Translational control of gene expression. Cold Spring Harb Symp Quant Biol. 1966;31:321–333. doi: 10.1101/sqb.1966.031.01.042. [DOI] [PubMed] [Google Scholar]
- Edlin G., Stent G. S., Baker R. F., Yanofsky C. Synthesis of a specific messenger RNA during amino acid starvation of Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):257–268. doi: 10.1016/0022-2836(68)90266-0. [DOI] [PubMed] [Google Scholar]
- Faanes R., Rogers P. Roles of arginine and canavanine in the synthesis and repression of ornithine transcarbamylase by Escherichia coli. J Bacteriol. 1968 Aug;96(2):409–420. doi: 10.1128/jb.96.2.409-420.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. Effect of tryptophan starvation on the rate of translation of the tryptophan operon in Escherichia coli. Biochim Biophys Acta. 1970 Apr 15;204(2):624–626. doi: 10.1016/0005-2787(70)90183-8. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Kwan C. N., Apirion D., Schlessinger D. Ribonuclease V of escherichia coli. I. Dependence on ribosomes and translocation. Proc Natl Acad Sci U S A. 1969 Oct;64(2):693–700. doi: 10.1073/pnas.64.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Messenger RNA synthesis during amino acid starvation in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):269–288. doi: 10.1016/0022-2836(68)90267-2. [DOI] [PubMed] [Google Scholar]
- Leisinger T., Vogel H. J. Repression by arginine in Escherichia coli: a comparison of arginyl transfer RNA profiles. Biochim Biophys Acta. 1969 Jun 17;182(2):572–574. doi: 10.1016/0005-2787(69)90212-3. [DOI] [PubMed] [Google Scholar]
- Leisinger T., Vogel R. H., Vogel H. J. Repression-dependent alteration of an arginine enzyme in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Oct;64(2):686–692. doi: 10.1073/pnas.64.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin P., Bauerle R. H. Determinants for regulation and initiation of expression of tryptophan genes. Cold Spring Harb Symp Quant Biol. 1966;31:311–320. doi: 10.1101/sqb.1966.031.01.041. [DOI] [PubMed] [Google Scholar]
- Morikawa N., Imamoto F. Degradation of tryptophan messenger. On the degradation of messenger RNA for the tryptophan operon in Escherichia coli. Nature. 1969 Jul 5;223(5201):37–40. doi: 10.1038/223037a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Mosteller R., Baker R. F., Yanofsky C. Direction of in vivo degradation of tryptophan messenger RNA--a correction. Nature. 1969 Jul 5;223(5201):40–43. doi: 10.1038/223040a0. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D., Yanofsky C. Transcription of the tryptophan operon in Escherichia coli: rifampicin as an inhibitor of initiation. J Mol Biol. 1970 Mar;48(3):525–531. doi: 10.1016/0022-2836(70)90064-1. [DOI] [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Stubbs J. D., Hall B. D. Effects of amino acid starvation upon constitutive tryptophan messenger RNA synthesis. J Mol Biol. 1968 Oct 28;37(2):303–312. doi: 10.1016/0022-2836(68)90269-6. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Venetianer P. Level of messenger RNA transcribed from the histidine operon in repressed, derepressed and histidine-starved Salmonella typhimurium. J Mol Biol. 1969 Oct 28;45(2):375–384. doi: 10.1016/0022-2836(69)90112-0. [DOI] [PubMed] [Google Scholar]
- Vogel H. J. REPRESSED AND INDUCED ENZYME FORMATION: A UNIFIED HYPOTHESIS. Proc Natl Acad Sci U S A. 1957 Jun 15;43(6):491–496. doi: 10.1073/pnas.43.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein I. B., Carchman R., Marner E., Hirschberg E. Miracil D: effects on nucleic acid synthesis, protein synthesis, and enzyme induction in Escherichia coli. Biochim Biophys Acta. 1967 Jul 18;142(2):440–449. doi: 10.1016/0005-2787(67)90625-9. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]


