Abstract
A series of bivalent hydroxy ether butorphan ligands were prepared and their binding affinities at the opioid receptors determined. Addition of a hydroxy group to a hydrocarbon chain can potentiate binding affinity up to 27 and 86 fold at the mu and kappa opioid receptors, respectively. Two bivalent ligands with sub-nanomolar binding affinity at the mu and kappa opioid receptors were discovered.
The analgesic, euphoric, and addictive properties of analgesic opioids such as morphine are thought to be due primarily to the interaction of the opioid with the mu (μ) opioid receptor.1 It has not proven possible to separate the analgesic and addictive properties of opioid analgesics. Activation though of the kappa (κ) opioid receptor or delta (δ) opioid receptor can modulate the agonist properties of opioid analgesics. Behavioral pharmacological studies in nonhuman primates have shown that dual acting compounds that are agonists or partial agonists at both the μ opioid receptor and κ opioid receptor could be useful as medications in the treatment of drug abuse and as analgesics.2 Opioid receptor dimerization has been suggested to explain the apparent opioid subtype selectivity observed with different pharmacological agents.3, 4 The possibility of the G-protein coupled receptor opioid heterodimers as drug targets has been reviewed.5 Previous reports from our laboratory indicated that the opioid μ partial agonist/κ agonist butorphan (1) (Fig. 1) has a more promising profile of activity than the opioid μ antagonist/κ agonist cyclorphan (2).2, 6
Figure 1.
Structures of Butorphan (1) and Cyclorphan (2)
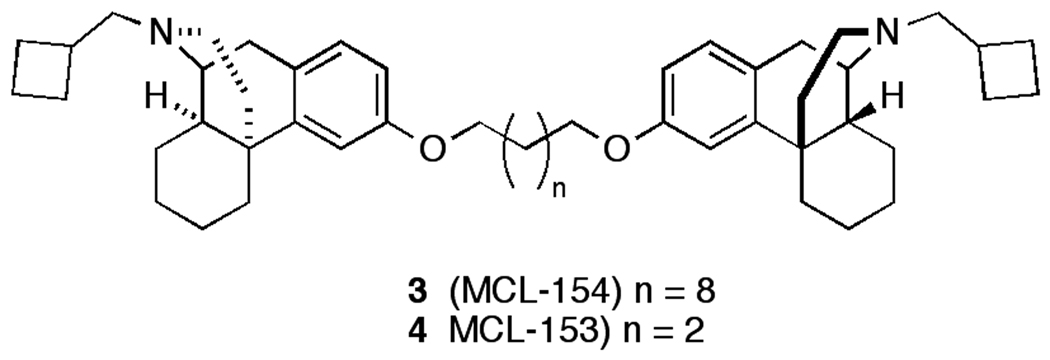
This finding led to the synthesis of a series of homobivalent ligands incorporating butorphan as the pharmacophore connected by linking spacers of various lengths. From these studies it was observed that bivalent ligands connected by an ester linkage retained good binding affinity, selectivity, and potency whereas those connected by an ether linkage (Fig. 2) lost binding affinity.7–9
Figure 2.
Structures of butorphan bivalent ether ligands
We have recently reported where the addition of methyl groups adjacent to the hydrolytically labile ester linkage increased stability while partially affecting binding affinity.10 To further investigate the importance of linker type on binding affinity a series of butorphan bivalent ligands have been prepared where hydroxy ether linkages replaced ether linkages. The incorporation of a hydroxy group was dictated in part to determine if a hydrogen bond donor group (e.g. OH) could help restore the binding affinity that was lost upon conversion of the phenol group to ether.
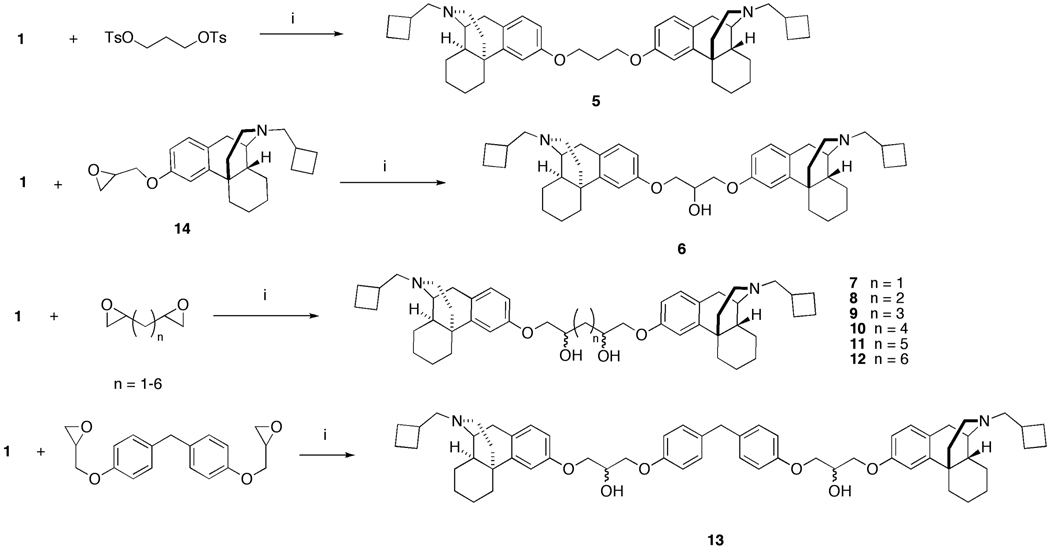
The bivalent ligands were synthesized by coupling butorphan with electrophiles under basic conditions (Scheme 1). Compound 5 was prepared in 66% yield by reacting two equivalents of the sodium salt of butorphan (sodium hydride in dimethylformamide) with the bistosylate of 1,3-propanediol. Its hydroxy derivative, 6, was prepared by reacting butorphan with the glycidyl ether of butorphan (14). The bivalent ligands 7–12 were prepared in variable yields of 15% (10) to 70% (12) by reacting the sodium salt of butorphan with a homologous series of terminal bisepoxides. The difference in yields was due mainly to the need for multiple chromatographic purifications for some compounds. The bisepoxides in turn were prepared by epoxidation of α,ω-diolefins with m-chloroperbenzoic acid under standard conditions in dichloromethane. Bivalent ligand 13 was prepared in 72% yield by reacting two equivalents of butorphan with commercially available bis[4-(glycidyloxy)phenyl]methane. The bivalent ligands were obtained and tested as inseparable diastereomeric mixtures.
Scheme 1.
Synthesis of the bivalent ligands. (i) NaH, DMF, 80 °C, 24–48 hours
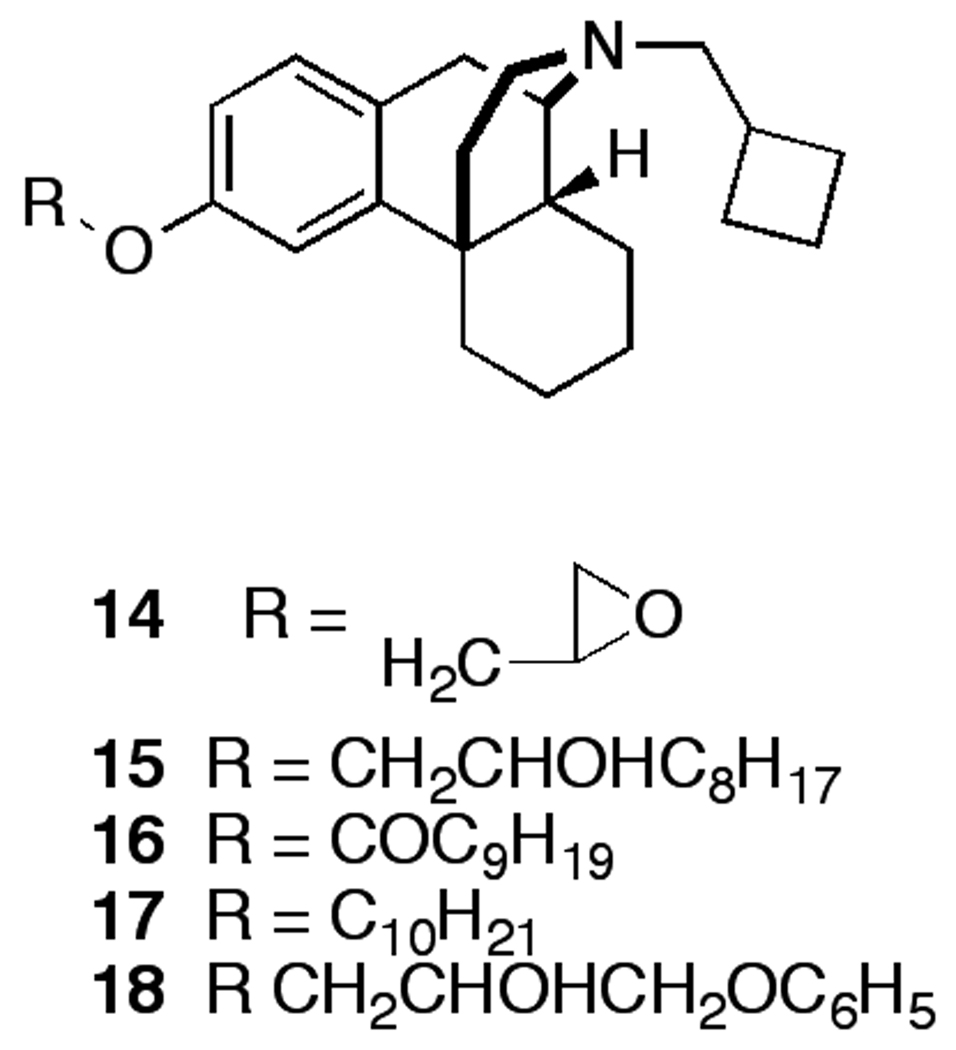
The univalent butorphan compounds (Figure 3) were prepared to investigate the importance of a second butorphan unit on binding affinity to the opioid receptors. Compound 14 is readily prepared in 77% yield by reacting butorphan with epichlorohydrin. Butorphan was reacted with 1,2-epoxydecane, decanoyl chloride, and 1-bromodecane to form 15–17, respectively. Compound 18 was prepared in 50% yield by reacting butorphan with 1,2-epoxy-3-phenoxypropane.
Figure 3.
Structures of univalent butorphan ligands
The affinity and selectivity of the compounds synthesized were evaluated for binding affinities at all three opioid receptor types (μ, δ, and κ) using a previously described procedure (Table 1).9 As shown previously, linking butorphan via an ether hydrocarbon chain greatly reduced the binding affinities relative to butorphan.9 Replacing the ether linkage by an ester linkage restored the binding affinity. The current results show that the binding affinity can be restored upon the introduction of a hydroxy group on the carbon atom beta to the ether linkage. This type of linkage is attractive as it will not be hydrolyzed by esterases thus producing a more chemically and metabolically stable bivalent ligand.
Table 1.
Ki Values for the inhibition of μ, δ and κ opioid binding to CHO membranes by uni- and bivalent ligands
| Ki (nM) ± SE | Selectivity | |||
|---|---|---|---|---|
| Compound | μ | δ | κ | μ/δ/κ |
| 1a | 0.23 ± 0.01 | 5.9 ± 0.55 |
0.079 ± 0.003 |
3/75/1 |
|
3b (MCL- 154) |
66 ± 4.3 | 2500 ± 91 |
120 ± 8.2 | 1/38/1.8 |
|
4b (MCL- 153) |
29 ± 1.2 | 730 ± 29 | 18 ± 1.3 | 1.6/40/1 |
| 5 | 7.1 ± 0.19 | 180 ± 3.3 | 6.2 ± 0.53 | 1.1/29/1 |
| 6 | 0.95 ± 0.16 | 37 ± 3.3 | 0.99 ± 0.022 | 1/39/1 |
| 7 | 1.2 ± 0.07 | 33 ± 5.0 | 0.17 ± 0.089 | 7/194/1 |
| 8 | 17 ± 1.9 | 420 ± 14 | 12 ± 0.87 | 1.4/35/1 |
| 9 | 12 ± 1.0 | 160 ± 7.5 | 9.8 ± 0.56 | 1.2/16/1 |
| 10 | 3.2 ± 0.41 | 74 ± 1.9 | 3.3 ± 0.23 | 1/23/1 |
| 11 | 4.8 ± 0.16 | 99 ± 5.4 | 3.9 ± 0.12 | 1.2/25/1 |
| 12 | 2.4 ± 0.43 | 27 ± 1.9 | 1.4 ± 0.15 | 1.7/19/1 |
| 13 | 0.24 ± 0.036 |
29 ± 1.4 | 0.34 ± 0.064 | 1/121/1.4 |
| 14 | 7.3 ± 1.0 | 200 ± 21 | 36 ± 0.52 | 1/27/5 |
| 15 | 7.4 ± 0.26 | 52 ± 2.8 | 8.3 ± 0.68 | 1/7/1.1 |
| 16 | 0.21 ± 0.017 |
20 ± 1.2 | 0.2 ± 0.023 | 1/100/1 |
| 17 | 70 ± 7.0 | 920 ± 149 |
52 ± 5.1 | 1.3/180/1 |
| 18 | 6.5 ± 0.28 | 86 ± 11 | 22 ± 1.2 | 1/13/3 |
Figure 4 clearly shows that potent binding affinity can be restored upon introduction of the hydroxy group.
Figure 4.
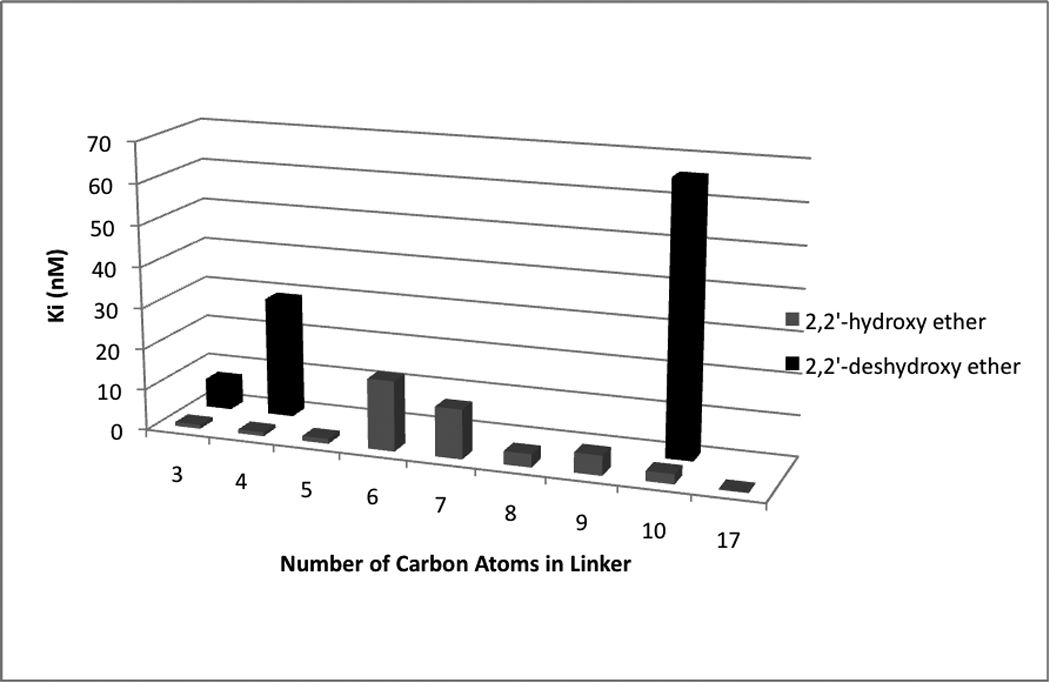
Effect of binding affinity on chain length and presence of a hydroxy group
The most striking results are in the 10-carbon series. The ether based compound 3 had a Ki value of 66 and 120 nM at the μ and κ opioid receptors, respectively; introduction of the beta-hydroxy groups (12) increased binding affinity by 27 and 86 fold at the μ and κ opioid receptors, respectively. Additional evidence of the importance of the hydroxyl group can be seen from the C10 univalent series. Compound 17, the des-hydroxy derivative of 15, has 10 fold weaker binding affinity at the μ opioid receptor. Interestingly, the second butorphan unit in the bivalent ligand does not appear to be necessary for binding affinity. The bivalent monohydroxy ligand 6 has 7.5 fold greater binding affinity at the μ receptor than its des-hydroxy analog 5. However, the univalent hydroxy ether 18 (equal-potent to C3 des-hydroxy 5) has only 7.5 fold weaker binding affinity at the μ opioid receptor than the bivalent hydroxy ether 6. The univalent hydroxy ether analog 15 had approximately 3–6 fold weaker binding affinity at the μ and κ receptors than the bivalent ligand 12. Interestingly, the decyl ester 16 was one of the most potent compounds with sub-nanomolar affinity at the μ and κ opioid receptors suggesting that the presence of a hydrogen-bond donating group is not strictly required for strong binding affinity. The hydrophobicity of substituents attached to the phenolic oxygen has been shown to be important for the binding of morphinans to the opioid receptors.11,12 We have previously shown that strong binding affinity can be obtained with univalent ligands compared to their bivalent analogs.9, 10
As shown in Figure 1 there does not appear to be any relationship between linker length and binding affinity. The bivalent ligands that bound strongest to the opioid receptors were at opposite extremes of linker length. Compound 13, with 17 atoms between the phenol oxygens of butorphan, has a Ki value of 0.24 nM at the μ opioid receptor while 6, with only 3 atoms between the phenol oxygens, has a Ki value of 0.95 nM at the μ opioid receptor, a 4.5 fold difference. A similar trend was observed for bivalent ester butorphan ligands.9
In summary, the synthesis and pharmacological evaluation of series of bivalent ligands for the opioid receptors has been reported.13 The introduction of a hydroxy group in long chain univalent and bivalent butorphan ligands can increase binding affinity to the opioid receptors. The results as a whole suggest that univalent butorphan ligands can bind as strongly as bivalent butorphan ligands to the opioid receptors. Their exact mode of binding, whether in the opioid binding pocket or to allosteric sites, is unknown and is under investigation.
ACKNOWLEDGMENT
This work was supported in part by NIH grants R01-DA14251 (J.L.N.), K05-DA 00360 (J.M.B.) and T32 DA007252 (B.S.F.). Levorphanol tartrate was generously donated by Mallinckrodt Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Koob GFLMML. Neurobiology of Addiction. Academic Press; 2006. pp. 121–172. [Google Scholar]
- 2.Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK. Effects of mixed-action kappa/mu opioids on cocaine self-administration and cocaine discrimination by rhesus monkeys. Neuropsychopharmacology. 2003;28:1125–1139. doi: 10.1038/sj.npp.1300105. [DOI] [PubMed] [Google Scholar]
- 3.Portoghese PS, Lunzer MM. Identity of the putative delta1-opioid receptor as a delta-kappa heteromer in the mouse spinal cord. Eur. J. Pharmac. 2003;467:233–234. doi: 10.1016/s0014-2999(03)01599-1. [DOI] [PubMed] [Google Scholar]
- 4.Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E. Portoghese, receptor dimers. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumeyer JL, Bidlack JM, Zong R, Bakthavachalam V, Gao P, Cohen DJ, Negus SS, Mello NK. Synthesis and opioid receptor affinity of morphinan and benzomorphan derivatives: mixed kappa agonists and mu agonists/antagonists as potential pharmacotherapeutics for cocaine dependence. J. Med. Chem. 2000;43:114–122. doi: 10.1021/jm9903343. [DOI] [PubMed] [Google Scholar]
- 7.Mathews JL, Fulton BS, Negus SS, Neumeyer JL, Bidlack JM. In vivo characterization of (−)(−)MCL-144 and (+)(−)MCL-193: isomeric, bivalent ligands with mu/kappa agonist properties. Neurochemical Res. 2008;33:2142–2150. doi: 10.1007/s11064-008-9752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathews JL, Peng X, Xiong W, Zhang A, Negus SS, Neumeyer JL, Bidlack JM. Characterization of a novel bivalent morphinan possessing kappa agonist and micro agonist/antagonist properties. J. Pharmacol. Exp. Ther. 2005;315:821–827. doi: 10.1124/jpet.105.084343. [DOI] [PubMed] [Google Scholar]
- 9.Neumeyer JL, Zhang A, Xiong W, Gu XH, Hilbert JE, Knapp BI, Negus SS, Mello NK, Bidlack JM. Design and synthesis of novel dimeric morphinan ligands for kappa and mu opioid receptors. J. Med. Chem. 2003;46:5162–5170. doi: 10.1021/jm030139v. [DOI] [PubMed] [Google Scholar]
- 10.Decker M, Fulton BS, Zhang B, Knapp BI, Bidlack JM, Neumeyer JL. Univalent and Bivalent Ligands of Butorphan: Characteristics of the Linking Chain Determine the Affinity and Potency of Such Opioid Ligands. J. Med. Chem. 2009;52:7389–7396. doi: 10.1021/jm900379p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulton BS, Knapp BI, Bidlack JM, Neumeyer JL. Synthesis and pharmacological evaluation of hydrophobic esters and ethers of butorphanol at opioid receptors. Bioorg. Med. Chem. Lett. 2008;18:4474–4476. doi: 10.1016/j.bmcl.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wentland MP, VanAlstine M, Kucejko R, Lou R, Cohen DJ, Parkhill AL, Bidlack JM. Redefining the structure-activity relationships of 2,6-methano-3-benzazocines. 4. Opioid receptor binding properties of 8-[N-(4'-phenyl)-phenethyl)carboxamido] analogues of cyclazocine and ethylketocycalzocine. J. Med. Chem. 2006;49(5):635–639. doi: 10.1021/jm060278n. [DOI] [PubMed] [Google Scholar]
- 13.Bis-((−)-N-cyclobutylmethylmorphinan-3-oxy) decane-2,9-diol (12) To butorphan (83 mg, 0.27 mmol) in DMF (3 mL) was added hexane washed sodium hydride (10 mg, 0.42 mmol) and the slurry was stirred at room temperature for thirty minutes. To the resultant solution was added 1,9-diepoxydecane (23 mg, 0.13 mmol) in DMF (0.5 mL) and the solution was heated at 80 °C for 48 hours. The reaction was cooled to room temperature, quenched with water, and extracted twice with ethyl acetate. The organic layers were combined and washed three times with brine, dried over sodium sulfate, filtered, and concentrated in vacuo to give 98 mg of a light yellow foam. The compound was purified by flash chromatography (EtOAc/Et3N = 100/1) to give 73 mg (71%) as an oil. 1H-NMR (CDCl3, 300 MHz): δ 7.02 (d, J = 9 Hz, 2H), 6.82 (s, 2H), 6.73 (d, J = 8.4 Hz, 2H), 4.1 - 3.9 (m, 4H), 3.8 - 3.7 (m, 2H), 2.92 (d, J = 18 Hz, 2H), 2.77 (bd s, 2H), 2.56 - 2.06 (m, 14H), 2.05 - 1.0 (m, 46H) ppm. 13C-NMR (CDCl3, 75 MHz) δ 157.1, 142.3, 130.8, 128.7, 111.9, 111.5, 72.5, 70.4, 61.8, 56.1, 46.1, 45.3, 42.2, 38.0, 36.9, 35.2, 33.4, 29.8, 28.1, 27.1, 26.8, 25.7, 24.3, 22.5, 19.1 ppm. Anal. Calcd for C52H76N2O4 HCl: C, 75.28; H, 9.35; N, 3.38. Found: C, 75.29; H, 9.28; N, 3.48.