Abstract
The oncogenic potential of the HTLV-1 Tax protein involves activation of the NF-κB pathway, which depends on Tax phosphorylation, ubiquitination and sumoylation. We demonstrate that the nuclei of Tax-expressing cells, including HTLV-1 transformed T-lymphocytes, contain a pool of Tax molecules acetylated on lysine residue at amino acid position 346 by the transcriptional coactivator p300. Phosphorylation of Tax on serine residues 300/301 was a prerequisite for Tax localization in the nucleus and correlated with its subsequent acetylation by p300, whereas sumoylation, resulting in the formation of Tax nuclear bodies in which p300 was recruited, favored Tax acetylation. Overexpression of p300 markedly increased Tax acetylation and the ability of a wild type HTLV-1 provirus, –but not of a mutant provirus carrying an acetylation deficient Tax gene–, to activate gene expression from an integrated NF-κB-controlled promoter. Thus, Tax acetylation favors NF-κB activation and might play an important role in HTLV-1-induced cell transformation.
Keywords: HTLV-1, Tax, Acetylation, CBP/p300, NF-κB
Introduction
The human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL) and a virus-associated neurodegenerative disease, HTLV-1-associated myelopathy (HAM)/tropical spastic paraparesis (TSP) (Yoshida et al., 1984; Osame et al., 1986; Poiesz et al., 1980). These diseases have been linked to the expression of the HTLV-1 regulatory protein, Tax (Tanaka et al., 1990; Franchini, 1995; Grassmann et al., 1992; Nerenberg et al., 1987). Tax is a pleiotropic transcription factor, which activates gene expression by assembling complexes including cellular transcription factors and coactivators at the HTLV-1 promoter and at promoters of various cellular genes involved in T-lymphocyte proliferation. Tax interacts with members of the ATF/CREB family of cellular transcription factors and increases their binding affinity to the enhancer sequence made of three 21-bp repeats present in the HTLV-1 5′ LTR. Formation of this complex leads to the recruitment of the transcriptional coactivator cAMP response element-binding protein (CBP), leading to the activation of viral gene expression (Giebler et al., 1997; Harrod et al., 1998; Jiang et al., 1999; Kwok et al., 1996).
Leukemogenesis induced by HTLV-1 has been linked to the ability of Tax to constitutively activate the NF-κB pathway (Huang et al., 2000; Malek et al., 2001; Chu et al., 1999; Harhaj and Sun, 1999; Yamamoto and Gaynor, 2004; Kehn et al., 2004; Ratner et al., 2000), to inhibit DNA repair processes (Jeang et al., 2004; Marriott and Semmes, 2005; Haoudi et al., 2003) and to inactivate the p53 tumor suppressor (Jeong et al., 2005; Ariumi et al., 2000). In addition, Tax affects cell cycle progression by acting at multiple levels including direct interaction with cyclin-dependent kinases and their inhibitors leading to Rb hyperphosphorylation (Neuveut and Jeang, 2000; Kehn et al., 2005; Haller et al., 2002; Suzuki et al., 1996), centrosome amplification and subversion of the mitotic spindle assembly checkpoint (Pumfery et al., 2006; Kasai et al., 2002) and promotion of S-phase entry together with a block in mitosis (Liang et al., 2002; Liu et al., 2005; Kuo and Giam, 2006; Sibon et al., 2006).
Tax undergoes post-translational modifications including phosphorylation, ubiquitination and sumoylation (Peloponese et al., 2004; Lamsoul et al., 2005; Shembade et al., 2007; Bex et al., 1999; Nasr et al., 2006). Tax phosphorylation at serine residues 300 and/or 301 is critical for Tax-mediated activation of gene expression via both the ATF/CREB and NF-κB pathways (Bex et al., 1999; Durkin et al., 2006). Ubiquitination of Tax leads to its cytoplasmic retention and this modification is critical for the activation of the IKK complexes, resulting in RelA translocation to the nucleus (Lamsoul et al., 2005; Nasr et al., 2006; Yu et al., 2008). However, these cytoplasmic events are not sufficient for Tax activation of the NF-κB pathway. In addition, Tax must be sumoylated in the nucleus, which leads to its nuclear retention and the formation of nuclear bodies that include transcription factors such as the NF-κB subunits p50 and RelA, the transcriptional coactivators p300 and CBP as well as components of the transcription and the splicing machineries (Bex et al., 1998). Thus, both ubiquitination and sumoylation act in concert for Tax-mediated activation of gene expression via the NF-κB pathway.
Acetylation is a fourth post-translational modification which modulates transcription factor properties including their DNA binding affinity, their stability and their ability to interact with coactivators and corepressors (Glozak et al., 2005; Calao et al., 2008). This modification may also compete with ubiquitination or sumoylation for overlapping targeted lysine residues. We thus analyzed whether Tax was acetylated. In this work, we demonstrate that the transcriptional coactivator p300 acetylates the lysine residue at amino acid position 346 in the carboxy-terminal domain of Tax and that this modification favors activation of gene expression via the NF-κB pathway, suggesting that the Tax oncogenic potential depends on Tax acetylation.
Results
Tax is acetylated on the lysine residue at amino acid position 346
To test whether Tax was modified by acetylation, 293T cells were transfected with a vector expressing 6His-tagged wild type Tax (Tax-6His) or Tax-6His mutants that had either all (K1–10R) or only some (K9R, K9–10R and K10R) of the ten lysine residues of Tax substituted by arginines. The schematic representation of these mutants is presented in Fig. 1A. Following transfection, cells were lysed in highly denaturing conditions in the presence of deacetylase inhibitors to prevent demodification. The cell extracts were subjected to Ni-NTA pulldown and the purified proteins were analyzed by immunoblotting with a rabbit polyclonal antibody directed against Tax (α-Tax) or a monoclonal antibody directed against acetyllysine (α-Ac) (Fig. 1B).
Fig. 1.
Tax is acetylated on the lysine residue at amino acid position 346. (A) Schematic representation of the Tax protein with the positions of the lysines replaced by arginine residues in the different Tax mutants. (B) 293T cells were transfected with vectors expressing wild type or mutant Tax-6His. The cell extracts were purified by Ni-NTA pulldown followed by Western blotting with the anti-Tax rabbit polyclonal (α-Tax) or the anti-acetyllysine mouse monoclonal (α-Ac) antibodies. (C) 293T cells were transfected with vectors expressing wild type or K10R Tax-6His in the absence or the presence of a vector expressing either HA-SUMO-1 (HA-S1) or HA-Ubiquitin (HA-Ub). The cell extracts were purified by Ni-NTA pulldown followed by Western blotting with anti-Tax, anti-acetyllysine or anti-HA antibodies for the detection of HA-SUMO-1 or HA-Ubiquitin. (D) Cell extracts from CEM T-lymphocytes transfected or not with a vector expressing wild type Tax-6His purified by Ni-NTA pulldown or cell extracts from HTLV-1 infected T-lymphocytes C8166 or HUT102 were immunoblotted with anti-Tax or anti-acetyllysine antibodies. The arrow points to the acetylated form of Tax detected in HTLV-1 transformed T-lymphocytes. (E) 293T cells transfected or not with a vector expressing wild type Tax-6His were fractionated into the cytoplasmic (Cy) and nuclear (Nu) fractions, purified by Ni-NTA pulldown and immunoblotted with anti-Tax or anti-acetyllysine antibodies. The total fractions were also immunoblotted with anti-IKKβ or anti-hnRNP A1 antibodies as controls for the purity of the cytoplasmic and nuclear fractions, respectively.
Expression of Tax caused the appearance of a 40 kDa species, which was recognized by the anti-acetyllysine antibody and comigrated with the 40 kDa Tax species recognized by the anti-Tax antibody. This species was not detected in cells that did not express Tax or in cells expressing mutants that had at least K10 mutated (K1–10R, K10R and K9–10R), whereas mutant K9R was acetylated like wild type Tax. These results indicated that Tax was acetylated and strongly suggested that lysine K10 at amino acid position 346 was the target for this modification. As expected from the mutation of all 10 lysines, no Tax species of molecular mass higher than 40 kDa previously attributed to ubiquitinated and sumoylated Tax molecules (Lamsoul et al., 2005) were detected in cells expressing the K1–10R mutant. By contrast, the ladder of slow migrating forms of Tax was detected in cells expressing the acetylation deficient K9–10R and K10R mutants, suggesting that the defect of Tax acetylation did not alter its ability to be ubiquitinated or sumoylated. This was clearly evidenced by analyzing the sumoylation and ubiquitination status of wild type Tax or the K10R mutant by Ni-NTA pulldown of 293T cells coexpressing either HA-SUMO-1 (HA-S1) or HA-Ub (Fig. 1C), indicating that the acetylation deficient K10R mutant was sumoylated and ubiquitinated. The presence of non-specific bands recognized by the anti-acetyllysine antibody (Fig. 1C, α-Ac) prevented the detection of Tax species of molecular weight higher than 55 kDa that were both acetylated and sumoylated or ubiquitinated. The question whether sumoylated forms of Tax can be acetylated will be discussed further below.
We then checked whether Tax was acetylated in T-lymphocytes, the physiological host of HTLV-1, as well as in HTLV-1 transformed T-lymphocytes. CEM T-lymphocytes transfected or not with a vector expressing Tax-6His or HTLV-1-transformed T-lymphocyte cell lines C8166 or HUT102 were lysed in highly denaturating conditions and the cell extracts were immunoblotted with anti-Tax or anti-acetyllysine antibodies (Fig. 1D). The acetylated form of Tax was detected in CEM cells overexpressing Tax-6His and endogenous Tax expressed in HTLV-1 transformed T-lymphocytes was also acetylated. The lack of a 6His tag on endogenous Tax expressed by the HTLV-1 transformed cell lines prevented its purification by Ni-NTA pulldown, which explains the higher background and the fainter acetylated Tax species on the anti-acetyllysine immunoblot. We were unable to detect the acetylated form of Tax in HTLV-1-transformed T-lymphocyte cell lines following immunoprecipitation. We suspect that acetylation at lysine K346, which is part the Tax immunodominant epitope (K346HFRE-TEV353) (Levin et al., 2002) partly masks this epitope from recognition by anti-Tax antibodies.
Fractionation of Tax-expressing 293T cells was then used to determine the intracellular localization of the acetylated form of Tax. 293T cells were transfected with a vector expressing wild type Tax-6His or with the empty vector as a control. The cell extracts were fractionated into cytoplasmic and nuclear extracts, which were submitted to Ni-NTA pulldown. The purified proteins were analyzed by immunoblotting using anti-Tax or anti-acetyllysine antibodies as well as anti-hnRNP A1 and anti-IKKβ antibodies as controls for the purity of the nuclear and cytoplasmic extracts, respectively (Fig. 1E). Tax was distributed both in the nucleus and in the cytoplasm as described previously (Lamsoul et al., 2005), whereas the acetylated form of Tax was predominantly concentrated in the nucleus.
The transcriptional coactivator p300 is involved in Tax acetylation
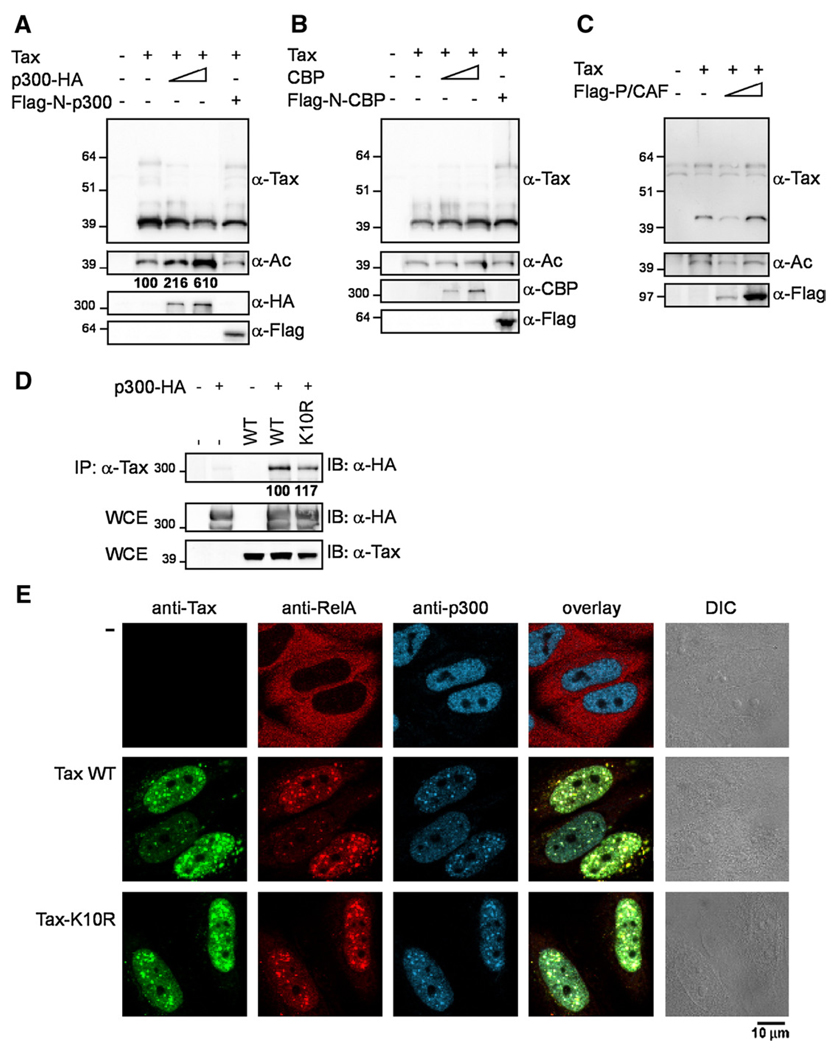
Since Tax interacts with various acetyltransferases including the two related transcriptional coactivators p300 and CBP as well as P/CAF (Harrod et al., 1998; Jiang et al., 1999; Bex et al., 1998; Kwok et al., 1996; Scoggin et al., 2001), we wondered whether one of these enzymes was responsible for Tax acetylation. 293T cells were cotransfected with vectors expressing Tax-6His in combination with increasing amounts of vectors for expression of p300-HA, CBP or Flag-P/CAF. Vectors for expression of the amino-termini of p300 (Flag-N-p300) or CBP (Flag-N-CBP), which interact with Tax but are devoid of acetyltransferase activity, were used as controls. The Tax protein purified by Ni-NTA pulldown as described above was analyzed by immunoblotting for the detection of Tax and acetyllysine (Figs. 2A–C).
Fig. 2.
The transcriptional coactivator p300 is involved in Tax acetylation. 293T cells were cotransfected with a vector expressing Tax-6His (2 µg) in combination with increasing amounts of either (A) a vector expressing p300-HA (0, 0.5 and 1 µg) or Flag-N-p300 (0.5 µg), or (B) a vector expressing CBP (0, 0.5 and 1 µg) or Flag-N-CBP (0.5 µg), or (C) a vector expressing Flag-P/CAF (0, 0.5 and 1 µg). The proteins were purified by Ni-NTA pulldown and immunoblotted with anti-Tax or anti-acetyllysine antibodies. Total extracts were immunoblotted with antibodies directed against HA for the detection of p300-HA, against CBP or against Flag for the detection of Flag-N-p300, Flag-N-CBP or Flag-P/CAF. (D) 293T cells were cotransfected with vectors expressing wild type Tax or the K10R mutant in combination with a vector expressing p300-HA. Extracts were immunoprecipitated (IP) with an anti-Tax monoclonal antibody, and the purified complexes were immunoblotted (IB) with the anti-HA antibody for the detection of p300-HA. The whole cell extracts (WCE: 1/50 of the input) were immunoblotted with antibodies directed against HA or Tax. The numbers under the blot represent the percentages of p300-HA retained in the complexes, calculated by quantitating the p300-HA species present in the immunoprecipitated complexes after subtraction of the background and normalization for equal amount of p300-HA and Tax in the whole cell extracts. (E) HeLa cells transfected with either an empty vector or vectors expressing wild type Tax or the K10R mutant for 30 h were stained by triple immunofluorescence with an anti-Tax monoclonal IgG2a antibody, an anti-RelA rabbit polyclonal serum and an anti-p300 monoclonal IgG1 antibody and analyzed by laser scanning confocal microscopy.
Tax acetylation was stimulated by overexpression of p300-HA in a dose-dependent manner. The anti-Tax immunoblot in Fig. 2A indicated that the increase in the pool of acetylated Tax did not result from an increase in the total Tax pool. By contrast, there was no increase in Tax acetylation when p300 was deleted of its acetyltransferase domain (Flag-N-p300), or when CBP (Fig. 2B) or Flag-P/ CAF (Fig. 2C) were expressed, and the intensity of the acetylated form of Tax was identical in cells expressing full length CBP or just its acetyltransferase-deficient amino-terminus Flag-N-CBP. Total extracts were analyzed by immunoblotting with an anti-HA antibody for the detection of p300-HA, with an anti-CBP or with an anti-Flag antibody for the detection of Flag-P/CAF, Flag-N-p300 or Flag-N-CBP, assessing the expression of these various full length or truncated proteins. The biological activity of the overexpressed CBP and p300 proteins was also assessed by demonstrating that they were both able to induce acetylation of the tumor suppressor p53 (see Supplemental data 1). Thus, the nuclei of Tax-expressing cells, including HTLV-1 transformed T-lymphocytes, contained a form of Tax which was acetylated in vivo by the transcriptional coactivator p300, but not by the other Tax-interacting acetyltransferases CBP or P/CAF.
At this point it was important to demonstrate that the defect of acetylation of the Tax K10R mutant was not simply the result of its inability to interact with p300. To this aim, extracts from 293T cells coexpressing p300-HA and either wild type Tax or the K10R mutant were co-immunoprecipitated with the anti-Tax antibody in conditions that preserved protein interactions and the immunoprecipitated complexes were immunoblotted with the anti-HA antibody to detect the presence of p300-HA in these complexes (Fig. 2D). These results indicated that the K10R mutant interacted with p300 like wild type Tax supporting the idea that lysine K10 was the target for Tax acetylation. We then analyzed the relative intracellular localizations of endogenous p300 and either WT Tax or the K10R mutant. Since the concentration of both p300 and the RelA subunit of NF-κB in Tax nuclear bodies are properties critical for Tax-mediated activation of the NF-κB pathway (Bex et al., 1997, 1998), we simultaneously analyzed the intracellular localization of these proteins by triple immunofluorescence staining with anti-Tax, anti-RelA and anti-p300 antibodies followed by confocal microscopy (Fig. 2E). In HeLa cells that did not express Tax, p300 was predominantly localized in the nucleus, whereas RelA was only detected in the cytoplasm. Expression of Tax led to the formation of nuclear bodies and the recruitment of p300 and RelA in these structures, as previously demonstrated. Mutant K10R had a distribution very similar to that of wild type Tax and this mutant also induced the relocalization of RelA from the cytoplasm to the nucleus and the recruitment of both p300 and RelA in the nuclear bodies. These results indicated that the defect of acetylation did not affect Tax intracellular localization and its capacity to recruit p300 and RelA in the Tax nuclear bodies. They also suggested that cytoplasmic events leading to RelA migration to the nucleus including IKK complex activation and IκBα degradation were not affected by the K10R mutation. Finally, the fact that both p300 and the acetylated form of Tax localized predominantly in the nucleus, strongly suggested that acetylation of Tax by p300 occurred in the nucleus.
Phosphorylation is a prerequisite for Tax acetylation
We then tested whether phosphorylation, ubiquitination or sumoylation affected Tax acetylation. First, we assessed the effect of phosphorylation at serine residues 300/301 on Tax acetylation. For this purpose, we analyzed the acetylation status of two previously described Tax mutants, including the phosphorylation deficient F2 mutant (S300L, S301A), which is unable to activate gene expression via both the ATF/CREB and NF-κB pathways, and the phosphomimetic F9 mutant (S300D, S301D), in which substitution of the serine residues critical for phosphorylation by phosphomimetic aspartic acid residues partly restored transcriptional activities. The schematic representation of these mutants and their transcriptional activities as described in (Bex et al., 1999) are reported in Fig. 3A. 293T cells were transfected with vectors expressing wild type Tax-6His or the F2–6His or F9–6His mutants. The proteins were subjected to Ni-NTA pulldown and the purified proteins were analyzed by immunoblotting with antibodies directed against Tax or acetyllysine (Fig 3B). The phosphorylation deficient F2 mutant was not acetylated, whereas the phosphomimetic mutant F9 was acetylated like wild type Tax. Thus a positive correlation was observed between Tax phosphorylation at serine 300/301 and acetylation at lysine 346.
Fig. 3.
Phosphorylation is a prerequisite for Tax acetylation. (A) Schematic representation of the phosphorylation defective F2 mutant and the phosphomimetic F9 mutant with their ability to activate gene expression via the ATF/CREB and NF-κB pathways as determined in (Bex et al., 1999). (B) 293T cells were transfected with vectors expressing 6His-tagged wild type Tax or mutants F2 or F9 and the proteins were purified by Ni-NTA pulldown and immunoblotted with anti-Tax and anti-acetyllysine antibodies. (C) 293T cells were transfected with vectors expressing the Tax mutants F2 or F9. The cells were stained by dual immunofluorescence with an anti-Tax monoclonal IgG2a antibody and an anti-p300 monoclonal IgG1 antibody and analyzed by confocal microscopy. (D) 293T cells were cotransfected with vectors expressing wild type Tax or the F2 or F9 mutants in combination with a vector expressing p300-HA. Extracts were immunoprecipitated with an anti-Tax monoclonal antibody and the purified complexes were immunoblotted with an anti-HA antibody for the detection of p300-HA. The whole cell extracts WCE (1/50 of the input) were analyzed by immunoblotting with anti-Tax or anti-HA antibodies. Quantitation was performed as described in Fig. 2D. (E) 293T cells were transfected with an empty vector or vectors expressing wild-type Tax or the K10R or F2 mutants and metabolically labelled with either [35S] methionine and [35S] cysteine or [32P] orthophosphate. The cell extracts were immunoprecipitated with a monoclonal antibody directed against Tax and analyzed by electrophoresis on SDS-Page followed by autoradiography.
P300 is localized in the nucleus and acetylation of Tax most likely occurs in the nucleus. It was thus important to test whether mutant F2 was able to translocate to the nucleus. 293T cells transfected with vectors expressing the F2 or F9 mutants were analyzed by dual immunofluorescence staining for the detection of Tax and endogenous p300 (Fig. 3C). Contrary to what was observed for wild type Tax (Fig. 2E), the anti-Tax immunofluorescence staining was only detected in the cytoplasm of the majority of the cells expressing mutant F2, and this mutant did not colocalize with p300, which was only detected in the nuclei, whereas the phosphomimetic F9 mutant was present both in the cytoplasm and in the nucleus, in which it displayed a speckled distribution with p300. We concluded that the inability of the phosphorylation deficient F2 mutant to be acetylated correlated with its inability to migrate to the nucleus and consequently, to meet p300 in the cells. In support to this conclusion, no in vivo complexes between mutant F2 and p300 were detected by in vivo pulldown assays of 293T, coexpressing mutant F2 and p300-HA with the anti-Tax antibody whereas the phosphomimetic F9 mutant formed stable complexes with p300 like wild type Tax (Fig. 3D). Thus, the presence of negative charges provided either by phosphoserine or by aspartic acid residues at position 300 and 301 correlated with the presence of Tax in the nucleus, its colocalization in nuclear bodies with p300, the formation of in vivo complexes with p300 and Tax acetylation. These results suggested that phosphorylation at serine 300 or 301 indirectly controlled Tax acetylation by promoting its presence in the nucleus in which Tax/ p300 complexes proficient for acetylation could be assembled.
Finally, to exclude that the defect of acetylation of the K10R mutant simply resulted from a reduced level of phosphorylation, we analyzed the phosphorylation status of this mutant. 293T cells transfected with vectors expressing wild type Tax or the K10R mutant were cultured in medium containing either [35S] methionine and [35S] cysteine or [32P] orthophosphate. The F2 mutant was included in this study as a negative control for phosphorylation. The Tax proteins were immunoprecipitated with a monoclonal antibody directed against Tax and analyzed by autoradiography (Fig. 3E). Contrary to the F2 mutant and as expected from its ability to migrate to the nucleus (Fig. 2E), mutant K10R displayed 35S and 32P labelled species of intensities similar to wild type Tax. From these results we concluded that acetylation did not control Tax phosphorylation, but that, inversely, phosphorylation was a prerequisite for Tax acetylation.
Sumoylation improves Tax acetylation
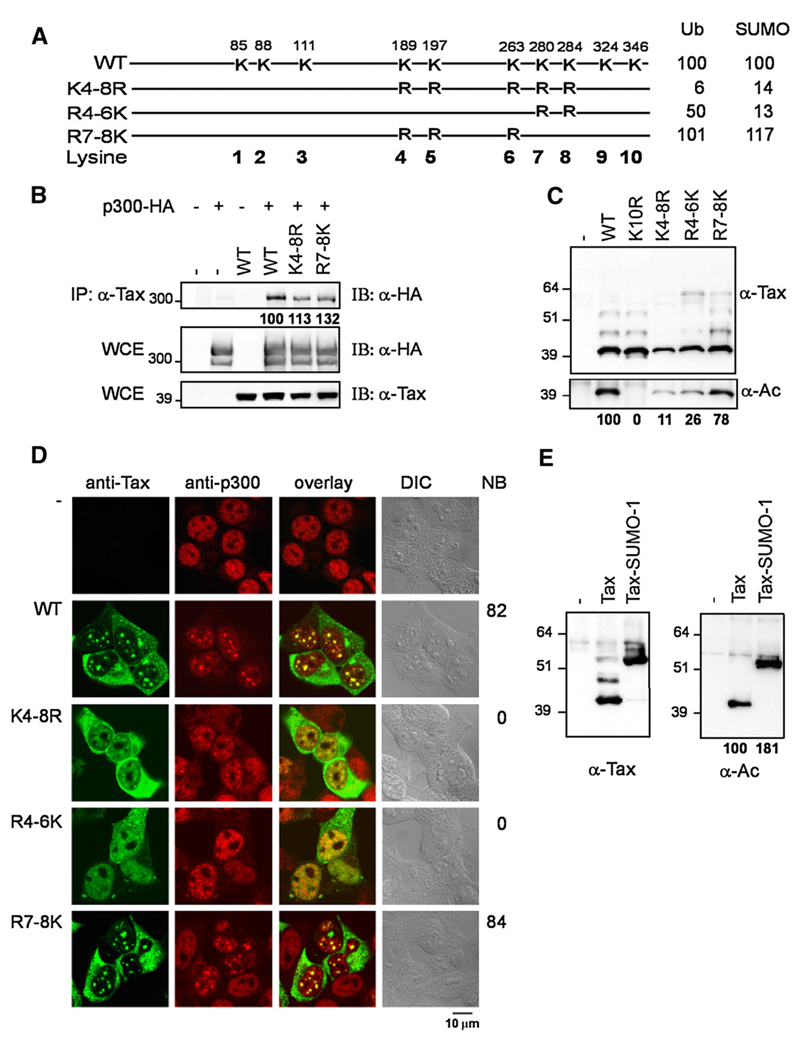
Since formation of the Tax nuclear bodies in which Tax colocalizes with p300 critically depends on Tax sumoylation, we investigated the effect of sumoylation on Tax acetylation. To this end, we analyzed the acetylation status of sumoylation and/or ubiquitination deficient Tax mutants, including mutant K4–8R, which is neither ubiquitinated nor sumoylated as a result of the mutation of the five central lysine residues, mutant R4–6K, in which reintroduction of lysines K4, K5 and K6 in the context of the K4–8R mutant partly restored ubiquitination but not sumoylation and mutant R7–8K, in which reintroduction of lysines K7 and K8 restored both ubiquitination and sumoylation. The schematic representation of these mutants and their ubiquitination and sumoylation status as described in (Lamsoul et al., 2005) are reported in Fig. 4A. First, co-immunoprecipitation studies indicated that these lysine mutants interacted with p300 like wild type Tax, indicating that ubiquitination or sumoylation were not required for Tax interaction with p300 (Fig. 4B). Interestingly, Ni-NTA pulldown assays (Fig. 4C) indicated that the non-ubiquitinated and non-sumoylated mutant K4–8R and the ubiquitinated but non-sumoylated R4–6K mutants were poorly acetylated (11% and 26% relatively to wild type Tax, respectively), whereas the lysine restoration mutant R7–8K, which was fully ubiquitinated and sumoylated, nearly recovered wild type level of acetylation (78% relatively to wild type Tax). We concluded that sumoylation, and to a lesser extent ubiquitination, favored acetylation of Tax without affecting its ability to interact with p300.
Fig. 4.
Sumoylation improves Tax acetylation. (A) Schematic representation of the Tax protein with the positions of the lysine residues replaced by arginines in the different Tax mutants. The ubiquitination and sumoylation status of these mutants as determined in (Lamsoul et al., 2005) are indicated. (B) 293T cells were cotransfected with vectors expressing wild type Tax or the K4–8R or R7–8K mutants in combination with a vector expressing p300-HA. Extracts were immunoprecipitated with an antibody directed against Tax, and the purified complexes were immunoblotted with an anti-HA antibody for the detection of p300-HA. The whole cell extracts WCE (1/50 of the input) were analyzed by immunoblotting with anti-Tax or anti-HA antibodies. Quantitation of immunoprecipitated p300-HA was performed as described in Fig. 2D. (C) 293T cells were transfected with vectors expressing wild type or mutant Tax-6His as indicated. The cell extracts were purified by Ni-NTA pulldown followed by immunoblotting with anti-Tax or anti-acetyllysine antibodies. The numbers under the blot represent the percentages of acetylated forms for each mutant relatively to wild type Tax, calculated after subtraction of the background on the α-Ac immunoblot and normalization to equal amount of the 40 kDa Tax species on the α-Tax immunoblot. (D) 293T cells were transfected with vectors expressing wild type Tax or the Tax mutants as indicated. The cells were stained by dual immunofluorescence with an anti-Tax monoclonal IgG2a antibody and an anti-p300 monoclonal IgG1 antibody. The percentages of cells displaying Tax-containing nuclear bodies (NB) are indicated. (E) 293T cells were transfected with vectors expressing Tax or the Tax–SUMO-1 fusion were submitted to Ni-NTA pulldown followed by immunoblotting with anti-Tax or anti-acetyllysine antibodies.
To better understand the role of sumoylation in Tax acetylation, we asked whether the recruitment of p300 in the nuclear bodies assembled by sumoylated Tax molecules correlated with increased Tax acetylation. We analyzed the relative intracellular localizations of endogenous p300 and wild type Tax or Tax mutants deficient for sumoylation (K4–8R and R4–6K) as well as the sumoylation restoration mutant R7–8K by dual immunofluorescence staining and confocal microscopy, including the fraction of the Tax-expressing cells which displayed Tax nuclear bodies (NB) (Fig. 4D). Wild type Tax was present in discrete nuclear foci in 82% of the Tax-expressing cells and endogenous p300 was concentrated in the Tax nuclear foci as previously demonstrated ((Bex et al., 1998) and see above). Mutant K4–8R, which was neither ubiquitinated nor sumoylated and mutant R4–6K, which was ubiquitinated but not sumoylated, had a diffuse distribution in the cytoplasm and the nucleus without formation of nuclear bodies and p300 had a speckled distribution in the nucleus like observed in cells that did not express Tax. Both of these mutants were poorly acetylated (Fig. 4C). Importantly, restoration of sumoylation by the reintroduction of lysines K7 and K8 (mutant R7–8K) restored the formation of nuclear bodies in which p300 was present in 84% of the Tax-expressing cells and improved the acetylation status to 78%. Thus, a positive correlation was observed between the sumoylation status of Tax mutants, their ability to colocalize with p300 in nuclear bodies and their level of acetylation.
It was thus critical to determine whether sumoylated Tax molecules could be acetylated. To this end, we analyzed the acetylation status of a Tax–SUMO-1 fusion in which SUMO-1 was fused in frame to the carboxy-terminus of Tax. This fusion was previously shown to assemble nuclear bodies in 100% of Tax expressing cells (Lamsoul et al., 2005) and to recruit p300 in these nuclear structures (data not shown). Fig. 4E demonstrates that the constitutively sumoylated Tax–SUMO-1 fusion was acetylated. Furthermore, quantitation of the acetylated forms of Tax and Tax– SUMO-1 on the anti-acetyllysine immunoblot and normalization to equal amounts on the anti-Tax immunoblot indicated that the Tax– SUMO-1 fusion was more acetylated (181%) than non-fused Tax. This observation supports the idea that sumoylation stimulates Tax acetylation.
Acetylation differentially affects Tax-mediated activation of gene expression via ATF/CREB and NF-κB pathways on promoters integrated in the chromatin
We then analyzed the functional consequences of Tax acetylation. To this aim, we introduced the K10R mutation in the background of a cloned HTLV-1 provirus (ACH K10R) and we compared the kinetics of virus production in the culture supernatants of HeLa cells transfected with the cloned ACH WT and ACH K10R proviruses (Fig. 5A). The analysis of the supernatants by an ELISA using a monoclonal antibody directed against the HTLV-1 matrix protein p19 indicated that virus production followed similar kinetics in both cultures, suggesting that the wild type and K10R Tax proteins produced by the two proviruses had comparable abilities to activate transcription from the HTLV-1 promoter.
Fig. 5.
Acetylation differentially affects Tax-mediated activation of gene expression via ATF/CREB and NF-κB pathways on promoters integrated in the chromatin. (A) The supernatants of HeLa 57A cells transfected with the wild type or K10R proviruses were collected at various time points after transfection and analyzed by ELISA with a monoclonal antibody directed against the HTLV-1 matrix protein p19 for the quantitation of virus production. (B) 293T cells were cotransfected for two days with either the HTLV-1 LTR-Luc (ATF/CREB) or the HIV-1 LTR-Luc (NF-κB) reporter plasmids and the vectors carrying either the cloned wild type HTLV-1 provirus (white bars) or the mutated K10R provirus (black bars). ACH WT activated gene expression from the transiently transfected HTLV-1 LTR and HIV-1 LTR 26+/−6 and 12+/−1 fold, respectively. (C) CHOK-1 cells, which contain an HTLV-1 LTR-Luc reporter construct integrated in the chromatin (ATF/CREB) or HeLa 57A cells with an integrated κB-ConA-Luc reporter construct (NF-κB) were transfected for 3 days with the vectors carrying either the cloned wild type HTLV-1 provirus (white bars) or the mutated K10R provirus (black bars). ACH WT activated gene expression from the integrated HTLV-1 LTR and NF-κB-controlled promoters 25+/−3 and 44+/−7 fold, respectively. (D) HeLa 57A cells were cotransfected with the ACH WT or ACH K10R proviruses (100 ng) and increasing amounts of the vector for expression of p300-HA (0, 50, 100, 200, 400 and 800 ng). The values are averages from at least six independent experiments, with the standard deviations indicated.
We then tested the consequences of acetylation on Tax transcriptional activities. 293T cells were transfected for two days with the cloned ACH WT or K10R proviruses, in combination with luciferase reporter constructs under the promoter present in the HTLV-1-LTR (HTLV-LTR-Luc) for the analysis of the ability of Tax to activate gene expression via the ATF/CREB pathway or under the control of the promoter present in the HIV-1-LTR (HIV-LTR-Luc), which contains two NF-κB binding sites, for the analysis of the ability of Tax to activate gene expression via the NF-κB pathway (Fig. 5B). ACH K10R was able to activate gene expression from the transiently transfected ATF/CREB- or NF-κB-controlled promoters at levels comparable (102%+/−13 and 93%+/−4, respectively) to wild type ACH.
Tax activation of gene expression has a more stringent dependency on cofactors on integrated promoters than on transiently transfected promoters (Okada and Jeang, 2002). We thus tested the ability of the cloned wild type and K10R proviruses to activate transcription from integrated ATF/CREB- or NF-κB-controlled promoters. The chromatin of CHOK-1 cells and HeLa 57A cells contains an integrated HTLV-1-LTR-Luc construct or an integrated κB-ConA-Luc construct, respectively. These cells were transfected for three days with the cloned wild type or K10R ACH proviruses (Fig. 5C). The ability of the ACH K10R provirus to activate the integrated NF-κB promoter was reduced by two fold (42%+/−12%) relatively to the wild type ACH, whereas activation of the integrated HTLV-1-Luc reporter gene was not affected (101%+/−14 relatively to wild type ACH). This effect was not the result of a reduced expression of Tax K10R mutant by the K10R ACH provirus since both proviruses produced equal amounts of Tax.
To convincingly demonstrate that the reduced level of activation of the NF-κB pathway by ACH K10R was the results of the defect of Tax acetylation, we tested whether overexpression of p300 could enhance the transcriptional activity of ACH WT,but not the activity of ACH K10R. HeLa 57A cells were cotransfected for three days with either the wild type or the K10R proviruses and increasing amounts of the vector expressing p300-HA (Fig. 5D). Overexpression of p300 increased the ability of ACH WT to activate expression from the integrated NF-κB-controlled promoter in a dose-dependent manner. By contrast, no increase in luciferase activity was observed in HeLa 57A cells coexpressing the K10R provirus and p300. These results indicated that overexpression of p300, resulting in increased acetylation of Tax (Fig. 2A), markedly stimulated activation of gene expression from an integrated NF-κB-controlled promoter and this effect was specific since the defect of acetylation had no consequence on the activation of gene expression via the ATF/CREB pathway. These results also suggest that Tax is poorly acetylated when expressed in HeLa cells unless p300 is overexpressed since expression of wild type Tax only increased by a factor two activation of gene expression from the integrated NF-κB-controlled promoter as compared to the acetylation deficient mutant K10R (Fig. 5C), whereas the effect was much more dramatic (3.5 fold increase) when wild type Tax was coexpressed with p300 (Fig. 5D).
Discussion
In this work, we establish that the Tax oncoprotein of HTLV-1 is acetylated in cells expressing Tax as well as in HTLV-1 transformed T-lymphocytes. This modification targets lysine residue K10 at amino acid position 346 in the carboxy-terminus of Tax, involves the acetyltransferase activity of the transcriptional coactivator p300, and most likely occurs in the nucleus since Tax acetylation depends on its presence in the nucleus and both p300 and the acetylated form of Tax are localized in the nucleus. Our results indicated that Tax acetylation promoted activation of gene expression from a NF-κB-controlled promoter integrated in the chromatin. This was evidenced by the facts that this phenotype was altered in cells transfected with the provirus producing the acetylation deficient K10R mutant Tax and that increased acetylation of Tax following over-expression of p300 promoted the activation of an integrated NF-κB-controlled promoter by a wild type HTLV-1 provirus but not by the K10R mutant provirus.
Acetylation and the subsequent control NF-κB activation is the third phenotype to be attributed to the 13 carboxy-terminal amino-acids of Tax. Besides the lysine K346, this region also includes a motif involved in the interaction of Tax with PDZ-domain proteins (Rousset et al., 1998) and a domain involved in the formation of micronuclei (Semmes et al., 1996). Moreover, these 13 carboxy-terminal amino acids are highly divergent in the less pathogenic but otherwise highly related Tax proteins of the various HTLV-2 isolates (Tax-2), resulting in the inability of Tax-2 to interact with PDZ-domain proteins (Hirata et al., 2004) and to induce the formation of micronuclei (Semmes et al., 1996). Our recent results indicate that the Tax-2 protein of the HTLV-2B isolate is acetylated (Supplemental data 2). It will be critical to determine whether acetylation is involved in the oncogenic potential of Tax.
Our previous studies indicated that Tax-mediated activation of the NF-κB pathway requires both cytoplasmic and nuclear events, including the release of RelA cytoplasmic sequestration by ubiquitinated Tax molecules and the recruitment of RelA in Tax nuclear bodies by sumoylated Tax molecules (Lamsoul et al., 2005). These two steps were carried out by the acetylation deficient mutant K10R, which had wild type abilities to be ubiquitinated and sumoylated, to induce translocation of RelA to the nucleus and to colocalize with RelA and p300 in nuclear bodies. Thus, the transcriptional activity of Tax on an integrated NF-κB-controlled promoter was promoted by Tax acetylation but this step was dispensable for the activation of a transiently transfected promoter. Interestingly, the acetylation deficient K10R Tax mutant stimulated virus production and activated the HTLV-1 promoter as efficiently as wild type Tax.
Tax interacts directly with the two related transcriptional coactivators p300 and CBP and the acetyltransferase activity of these coactivators leading to acetylation of histone tails is critical for activation of the HTLV-1 promoter on chromatin templates (Georges et al., 2002; Lu et al., 2002). Here we show that p300, but not CBP, acetylates Tax in vivo, which specifically promotes activation of gene expression from an integrated NF-κB-controlled promoter. This observation suggests that acetylation of Tax may affect the stability or the chromatin-remodelling activity of transcription complexes on an integrated NF-κB-controlled promoter. It is important to note that the acetyltransferase activities of the highly related CBP and p300 not only depend on phosphorylation events by specific kinases that target non-conserved serine residues of CBP and p300 but that these two transcriptional coactivators display non overlapping activities (Turnell and Mymryk, 2006; Kalkhoven, 2004), which is supported by the fact that p300−/− or CBP−/− knockout mice have different phenotypes (Kung et al., 2000; Yao et al., 1998). Support in favor of the specific role of p300 in Tax acetylation would require knock-down of p300 or CBP.
Both phosphorylation of Tax at serine residues 300/301 and sumoylation at lysine residues 280/284 correlated with increased acetylation of Tax. Contrary to the phosphorylation deficient F2 mutant, the phosphomimetic F9 mutant was found in the nuclei and this mutant was acetylated like wild type Tax, indicating that the translocation of Tax to the nucleus conditioned Tax acetylation. Contrary to the correlation observed between the inability of the phosphorylation deficient F2 mutant to form complexes with p300 in vivo and its lack of acetylation, sumoylation deficient mutants were abletointeract with p300 like wild type Tax, but they were nevertheless poorly acetylated. In addition, restoration of sumoylation in mutant R7–8K, or fusion of SUMO-1 to the carboxy-terminus of Tax, which favored formation of Tax nuclear bodies that included p300, significantly increased Tax acetylation. Because the acetyltransferase activity of p300 is induced by phosphorylation events involving specific kinases such as AKT or HIPK2 (Huang and Chen, 2005; Huang et al., 2007), we hypothesize that nuclear bodies containing Tax and p300 also recruit a kinase involved in the activation of p300 acetyltransferase activity.
We conclude that the ability of Tax to constitutively activate the NF-κB pathway critically depends on a hierarchical sequence of post-translational modifications which differentially controls Tax intracellular localization and its transcriptional activity. Considering the role of acetylated Tax in promoting activation of the NF-κB pathway and the critical role of this pathway in Tax transforming activity, we suggest that Tax acetylation contributes to HTLV-1-induced leukemias/lymphomas.
Materials and methods
Cell culture and transient expression experiments
293T human embryonic kidney and HeLa human cervical epithelial carcinoma cell lines, the non-infected CEM and HTLV-1 transformed C8166 and HUT102 T-lymphocyte cell lines were obtained from the ATCC or ECACC (CRL-11268, CCL-2, CRL-1991, 88051601, TIB-162). The CHOK-1 cells, which contain a HTLV-1-LTR-Luc construct integrated in the chromatin, and HeLa 57A cells, which contain an integrated NF-κB-Luc construct (3Enhancer-κB-ConA-Luc), were kindly provided by J. Nyborg (Lemasson et al., 2006) and R. Hay (Rodriguez et al., 1999), respectively. The 293T, CHOK-1, HeLa 57A and HeLa cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% foetal calf serum, 2 mM l-glutamine, 1% penicillin–streptomycin, and 1 mM sodium pyruvate (Gibco), with the addition of 500 µg of G418 per ml (Calbiochem) for cultures of CHOK-1 and HeLa 57A cells. The T-lymphocyte cell lines CEM, C8166 and HUT102 were maintained in RPMI 1640 medium with Glutamax-I (Gibco) supplemented with 10% foetal calf serum. 293T cells were transfected using FuGENE 6 reagent (Roche) according to the manufacturer's instructions. The CEM T-lymphocyte cell line was transfected by electroporation with a Gene Pulser electroporator (Bio-Rad Laboratories).
Plasmid constructs
The vectors for expression of wild type Tax-6His or lysine to arginine substitution mutants or the Tax–SUMO-1 fusion and the vectors for expression of ubiquitin or SUMO-1 fused at their amino-termini to the influenza virus hemagglutinin epitope (HA-Ub and HA-SUMO-1) were described in (Lamsoul et al., 2005). Three additional mutants, K9R, K10R and K9–10R, were generated by PCR based site-directed mutagenesis (Quick Change kit, Stratagene). The vectors for expression of CBP (Kwok et al., 1994), p300-HA (Hanstein et al., 1996) and Flag-P/CAF (Yang et al., 1996) have been previously described. The vectors for expression of Flag-N-p300 and Flag-N-CBP were constructed by PCR amplification of the sequence coding for the amino-termini of p300 (amino acids 1 to 595) or CBP (amino acids 1 to 616) and cloning of the amplified fragments in vector pJFE14 (Takebe et al., 1988). The cloned HTLV-1 provirus ACH was kindly provided by L. Ratner (Kimata et al., 1994). The ACH K10R mutant provirus was constructed first by replacing the unique SacI–SmaI fragment containing the 3′ end of the tax gene in the wild type ACH provirus deleted of the KpnI fragment, by the PCR amplified SacI–SmaI fragment containing the K10R mutation and then by transferring the unique NsiI–EcoRI fragment containing the mutated K10R tax gene in the full length wild type provirus. Sequencing was used to check for the presence of the K10R mutation and for the correct nucleotide sequence. The K10 codon AAA at nucleotide position 1036 to 1038 in the Tax coding sequence was replaced by the arginine codon CGC. These changes do not affect the coding sequence of other HTLV-1 proteins including Rex or HBZ.
Antibodies
The rabbit polyclonal antibodies directed against HA (Y-11), CBP (A-22) and RelA (C-20), the goat polyclonal antibodies directed against IKKβ (T-20) and hnRNP A1 (Y-15) were purchased from Santa Cruz Biotechnology. The anti-Flag monoclonal IgG1 antibody (M2) was from Sigma, the anti-acetyllysine monoclonal IgG1 antibody was from Cell Signaling Technology and the anti-p300 monoclonal IgG1 antibody (RW-128) was from Upstate. Tax proteins were revealed with the anti-Tax monoclonal antibody from hybridoma 168-A51 (AIDS Research and Reagent Program, National Institutes of Health) or a polyclonal serum obtained by immunizing rabbits with a purified maltose binding protein–Tax fusion produced in bacteria. For quantitation of virus production, cell-free supernatants collected at different time points after transfection were analyzed by Enzyme Linked Immunosorbent Assay (ELISA) using the RetroTek HTLV-I/II p19 Antigen ELISA kit (ZeptoMetrix Corp.) according to the manufacturer's instructions. The concentration of HTLV-1 p19 in specimens was determined by interpolation from a standard curve.
Ni-NTA pulldown assays
293T or CEM T-cells expressing wild type or mutant Tax-6His were lysed 30 h and 48 h after transfection, respectively, and Ni-nitrilotriacetic acid (NTA) pulldown assays were done as described in (Lamsoul et al., 2005). Briefly, cell pellets were lysed under reducing and highly denaturing conditions using buffer A (6 M guanidinium-HCl, 0.1 M Na2HPO4–NaH2PO4, 0.01 M Tris–HCl, pH 8.0, 5 mM imidazole,10 mM β-mercaptoethanol) and incubated with Ni2+-NTA beads for 4 h at 4 °C. The beads were washed with buffer A, B (8M urea, 0.1 M Na2HPO4–NaH2PO4, 0.01 M Tris–HCl, pH 8.0, 10 mM imidazole, 10 mM β-mercaptoethanol), and C (8M urea, 0.1 M Na2HPO4–NaH2PO4, 0.01 M Tris–Cl, pH6.3, 10 mM imidazole, 10 mM β-mercaptoethanol), and the bound proteins were eluted with buffer D (300 mM imidazole, 0.15 M Tris–HCI, pH 6.7, 30% glycerol, 0.72 M β-mercaptoethanol, 5% sodium dodecyl sulfate). All solutions contained a cocktail of inhibitors, including 50 mM NaF, 20 mM β-glycerophosphate, 1 mM orthovanadate, 50 mM N-ethylmaleimide, 0.5 µM trichostatin A (TSA), 5 mM nicotinamide, and the protease inhibitor cocktail (Roche). To detect modified forms of endogenous Tax in HTLV-1 transformed T-lymphocytes cell lines C8166 and HUT102, cells were lysed for 20 min in buffer A. The lysates were precipitated for 10 min in ice in 10% trichloroacetic acid. The purified proteins were submitted to electrophoresis on 4 to 12% bis-Tris NuPAGE gel (Invitrogen), transferred to a Hybond enhanced-chemiluminescence nitrocellulose membrane (Amersham Pharmacia Biotech), and immunoblotted with the primary antibodies and corresponding secondary antibodies conjugated to horseradish peroxidase. The detection was performed using the ECL Advance Western blotting detection kit (Amersham) and quantitation of chemiluminescent signals was performed with the Chemi-Smart 5000 apparatus and Bio-1D software (Vilber Lourmat, France).
Cell fractionation
293T cells expressing Tax-6His were washed in Tp I (20 mM Hepes buffer pH 7.9, 2 mM MgCl2, 1 mM dithiothreitol) containing inhibitors as above and then lysed on ice for 10 min in the same buffer containing 0.2% NP40. The samples were centrifuged to collect the cytoplasmic components. The resulting pellet containing the nuclear fraction was lysed in Tp II (Tp I supplemented with 420 mM KCl, 25% glycerol and 1 mM EDTA) on ice for 20 min and centrifuged for 10 min to collect the nuclear fraction. Finally, each cytoplasmic or nuclear fraction was submitted to Ni-NTA pulldown assays as described above.
Immunocytochemistry and confocal microscopy
Cells cultured on glass coverslips were fixed with Immunohistofix (A phase Inc., Belgium), an aldehyde-free zinc fixative, to stabilize cellular structures for 10 min at room temperature (Pajak et al., 2000) followed by incubation in 100% methanol at −20 °C for 6 min. The cells were washed with PBS (phosphate buffered saline), blocked in PBS containing 0.5% gelatin (Bio-Rad) and 0.25% bovine serum albumin (Gibco), and incubated with the primary antibodies diluted in the blocking solution. The preparations were then washed with PBS containing 0.2% gelatin and incubated with the secondary antibodies, goat anti-rabbit immunoglobulin G (IgG) conjugated to Alexa Fluor 546, goat anti-mouse total IgG or IgG2a conjugated to Alexa Fluor 488 or goat anti-mouse IgG1 coupled to biotin (Southern Biotechnology Associates, Ala.) for dual- or triple- immunofluorescence staining. Samples were washed and, when biotinylated goat anti-mouse IgG1 was used as the secondary antibody, cells were incubated for 20 min with Cy5-conjugated streptavidin (Jackson ImmunoResearch). Controls to test for secondary antibodies cross-reaction demonstrated the desired specificity of the fluorescent reagents. Samples were then mounted in DABCO based medium and analyzed with a laser scanning confocal microscope (LSM510; Zeiss) using a 63× objective and light source wavelengths of 488, 543 and 633 nm.
In vivo incorporation of 32P-orthophosphate or 35S-labeled methionine and cysteine
293T cells were transfected with vectors expressing wild type or mutant Tax for 24 h. Cells were washed twice with PBS and incubated in starvation medium (minimal Eagle medium without methionine and cysteine or without phosphate) for 30 min which was then replaced by the same medium containing either 100 µCi of [35S] methionine and [35S] cysteine (ICN) or 0.5 mCi of [32P] orthophosphate (ICN). The labelling was continued for 1 h at 37 °C. The cells were then washed twice with PBS, and lysed with RIPA buffer (Tris–HCl 50 mM pH 8.0, NaCl 150 mM, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS). The Tax protein was immunoprecipitated with an anti-Tax monoclonal antibody, submitted to electrophoresis on a 10% SDS page gels and analyzed by autoradiography.
In vivo coimmunoprecipitation
293T cells were cotransfected with vectors for expression of wild type or mutant Tax and a plasmid expressing p300-HA. 24 h after transfection, cells were washed with 1 ml of ice cold PBS containing 1 mM PMSF, 50 mM NaF, 20 mM β-glycerophosphate and lysed for 20 min on ice in lysis buffer (25 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1 mM PMSF, 50 mM NaF, 20 mM β-glycerophosphate, 0.5 µM TSA and 5 mM nicotinamide). Cell extracts were then immunoprecipitated with an anti-Tax monoclonal antibody using gamma-bind plus sepharose (Amersham Biosciences). The beads were washed with lysis buffer four times and eluted in SDS-PAGE loading buffer. The immunoprecipitated proteins were analyzed by immunoblotting and quantitation of the chemiluminescent signals was performed as described above.
Luciferase assays
Tax-mediated transactivation of the transiently transfected HTLV-1 and HIV-1 promoters via the activating transcription factor (ATF)/ CREB and NF-κB pathways, respectively, was assayed by dual-luciferase assays. 293T (1.25×105 cells) were transfected into 12-well plates with 100 ng of phRG-TK (Promega), which was used for monitoring transfection efficiency, 500 ng of HTLV-1-LTR- or HIV-1-LTR-luciferase reporter plasmids, and 500 ng of the vector carrying the cloned wild type or K10R ACH proviruses. For luciferase assays using integrated promoters, CHOK-1 or HeLa 57A cells were transfected with 100 ng of phRG-TK and 500 ng of the wild type or K10R mutant proviruses alone or in combination with increasing amounts of the vector coding for p300-HA (50, 100, 200, 400 and 800 ng). Total amounts of DNA were equalized by adding the empty vector into the transfection mixture. Cells were lysed and subjected to luciferase assay with a TD-20/20 luminometer (Turner Designs) using the dual-luciferase reporter assay system (Promega). The results represent the averages and the standard deviations of at least six independent experiments.
Acknowledgments
We greatly appreciate the gift of CHOK-1 cells from J. Nyborg (Colorado State University, Fort Collins, USA), HeLa 57A cells from R. Hay (University of St. Andrews, United Kingdom) and the cloned HTLV-1 provirus ACH from L. Ratner (Washington University School of Medicine, St Louis, USA). This work was supported by grants from the Belgian National Fund for Scientific Research and FNRS-Télévie and from the Internationale Brachet Stiftung.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.virol.2008.12.043.
References
- Ariumi Y, Kaida A, Lin JY, Hirota M, Masui O, Yamaoka S, Taya Y, Shimotohno K. HTLV-1 tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene. 2000;19(12):1491–1499. doi: 10.1038/sj.onc.1203450. [DOI] [PubMed] [Google Scholar]
- Bex F, McDowall A, Burny A, Gaynor R. The human T-cell leukemia virus type 1 transactivator protein Tax colocalizes in unique nuclear structures with NF-kappaB proteins. J. Virol. 1997;71(5):3484–3497. doi: 10.1128/jvi.71.5.3484-3497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bex F, Yin MJ, Burny A, Gaynor RB. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol. Cell. Biol. 1998;18(4):2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bex F, Murphy K, Wattiez R, Burny A, Gaynor RB. Phosphorylation of the human T-cell leukemia virus type 1 transactivator tax on adjacent serine residues is critical for tax activation. J. Virol. 1999;73(1):738–745. doi: 10.1128/jvi.73.1.738-745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calao M, Burny A, Quivy V, Dekoninck A, Van Lint C. A pervasive role of histone acetyltransferases and deacetylases in an NF-kappaB-signaling code. Trends Biochem. Sci. 2008;33(7):339–349. doi: 10.1016/j.tibs.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Shin YA, Yang JM, DiDonato JA, Ballard DW. IKKgamma mediates the interaction of cellular IkappaB kinases with the tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem. 1999;274(22):15297–15300. doi: 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- Durkin SS, Ward MD, Fryrear KA, Semmes OJ. Site-specific phosphorylation differentiates active from inactive forms of the human T-cell leukemia virus type 1 Tax oncoprotein. J. Biol. Chem. 2006;281(42):31705–31712. doi: 10.1074/jbc.M607011200. [DOI] [PubMed] [Google Scholar]
- Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86(10):3619–3639. [PubMed] [Google Scholar]
- Georges SA, Kraus WL, Luger K, Nyborg JK, Laybourn PJ. p300-mediated tax transactivation from recombinant chromatin: histone tail deletion mimics coactivator function. Mol. Cell. Biol. 2002;22(1):127–137. doi: 10.1128/MCB.22.1.127-137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebler HA, Loring JE, van Orden K, Colgin MA, Garrus JE, Escudero KW, Brauweiler A, Nyborg JK. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactiva-tion. Mol. Cell. Biol. 1997;17(9):5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski JG, Haseltine WA, Ramstedt U. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 1992;66(7):4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller K, Wu Y, Derow E, Schmitt I, Jeang KT, Grassmann R. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 2002;22(10):3327–3338. doi: 10.1128/MCB.22.10.3327-3338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstein B, Eckner R, DiRenzo J, Halachmi S, Liu H, Searcy B, Kurokawa R, Brown M. p300 is a component of an estrogen receptor coactivator complex. Proc. Natl. Acad. Sci. U. S. A. 1996;93(21):11540–11545. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haoudi A, Daniels RC, Wong E, Kupfer G, Semmes OJ. Human T-cell leukemia virus-I tax oncoprotein functionally targets a subnuclear complex involved in cellular DNA damage-response. J. Biol. Chem. 2003;278(39):37736–37744. doi: 10.1074/jbc.M301649200. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Sun SC. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 1999;274(33):22911–22914. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- Harrod R, Tang Y, Nicot C, Lu HS, Vassilev A, Nakatani Y, Giam CZ. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol. Cell. Biol. 1998;18(9):5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Higuchi M, Niinuma A, Ohashi M, Fukushi M, Oie M, Akiyama T, Tanaka Y, Gejyo F, Fujii M. PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein augments the transforming activity in a rat fibroblast cell line. Virology. 2004;318(1):327–336. doi: 10.1016/j.virol.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Huang TT, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc. Natl. Acad. Sci. U. S. A. 2000;97(3):1014–1019. doi: 10.1073/pnas.97.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Chen CC. Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol. Cell. Biol. 2005;25(15):6592–6602. doi: 10.1128/MCB.25.15.6592-6602.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Ju TK, Hung MC, Chen CC. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol. Cell. 2007;26(1):75–87. doi: 10.1016/j.molcel.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang KT, Giam CZ, Majone F, Aboud M. Life, death, and tax: role of HTLV-I oncoprotein in genetic instability and cellular transformation. J. Biol. Chem. 2004;279(31):31991–31994. doi: 10.1074/jbc.R400009200. [DOI] [PubMed] [Google Scholar]
- Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. A novel NF-kappaB pathway involving IKKbeta and p65/RelA Ser-536 phosphorylation results in p53 Inhibition in the absence of NF-kappaB transcriptional activity. J. Biol. Chem. 2005;280(11):10326–10332. doi: 10.1074/jbc.M412643200. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lu H, Schiltz RL, Pise-Masison CA, Ogryzko VV, Nakatani Y, Brady JN. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 1999;19(12):8136–8145. doi: 10.1128/mcb.19.12.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoven E. CBP and p300: HATs for different occasions. Biochem. Pharmacol. 2004;68(6):1145–1155. doi: 10.1016/j.bcp.2004.03.045. [DOI] [PubMed] [Google Scholar]
- Kasai T, Iwanaga Y, Iha H, Jeang KT. Prevalent loss of mitotic spindle checkpoint in adult T-cell leukemia confers resistance to microtubule inhibitors. J. Biol. Chem. 2002;277(7):5187–5193. doi: 10.1074/jbc.M110295200. [DOI] [PubMed] [Google Scholar]
- Kehn K, Berro R, de La FC, Strouss K, Ghedin E, Dadgar S, Bottazzi ME, Pumfery A, Kashanchi F. Mechanisms of HTLV-1 transformation. Front. Biosci. 2004;9:2347–2372. doi: 10.2741/1401. [DOI] [PubMed] [Google Scholar]
- Kehn K, Fuente CL, Strouss K, Berro R, Jiang H, Brady J, Mahieux R, Pumfery A, Bottazzi ME, Kashanchi F. The HTLV-I Tax oncoprotein targets the retinoblastoma protein for proteasomal degradation. Oncogene. 2005;24(4):525–540. doi: 10.1038/sj.onc.1208105. [DOI] [PubMed] [Google Scholar]
- Kimata JT, Wong FH, Wang JJ, Ratner L. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology. 1994;204(2):656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- Kung AL, Rebel VI, Bronson RT, Ch’ng LE, Sieff CA, Livingston DM, Yao TP. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 2000;14(3):272–277. [PMC free article] [PubMed] [Google Scholar]
- Kuo YL, Giam CZ. Activation of the anaphase promoting complex by HTLV-1 tax leads to senescence. EMBO J. 2006;25(8):1741–1752. doi: 10.1038/sj.emboj.7601054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok RP, Lundblad JR, Chrivia JC, Richards JP, Bachinger HP, Brennan RG, Roberts SG, Green MR, Goodman RH. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370(6486):223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- Kwok RP, Laurance ME, Lundblad JR, Goldman PS, Shih H, Connor LM, Marriott SJ, Goodman RH. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380(6575):642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- Lamsoul I, Lodewick J, Lebrun S, Brasseur R, Burny A, Gaynor RB, Bex F. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein. Mol. Cell. Biol. 2005;25(23):10391–10406. doi: 10.1128/MCB.25.23.10391-10406.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasson I, Polakowski NJ, Laybourn PJ, Nyborg JK. Tax-dependent displacement of nucleosomes during transcriptional activation of human T-cell leukemia virus type 1. J. Biol. Chem. 2006;281(19):13075–13082. doi: 10.1074/jbc.M512193200. [DOI] [PubMed] [Google Scholar]
- Levin MC, Lee SM, Morcos Y, Brady J, Stuart J. Cross-reactivity between immunodominant human T lymphotropic virus type I tax and neurons: implications for molecular mimicry. J. Infect. Dis. 2002;186(10):1514–1517. doi: 10.1086/344734. [DOI] [PubMed] [Google Scholar]
- Liang MH, Geisbert T, Yao Y, Hinrichs SH, Giam CZ. Human T-lymphotropic virus type 1 oncoprotein tax promotes S-phase entry but blocks mitosis. J. Virol. 2002;76(8):4022–4033. doi: 10.1128/JVI.76.8.4022-4033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hong S, Tang Z, Yu H, Giam CZ. HTLV-I Tax directly binds the Cdc20-associated anaphase-promoting complex and activates it ahead of schedule. Proc. Natl. Acad. Sci. U. S. A. 2005;102(1):63–68. doi: 10.1073/pnas.0406424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Pise-Masison CA, Fletcher TM, Schiltz RL, Nagaich AK, Radonovich M, Hager G, Cole PA, Brady JN. Acetylation of nucleosomal histones by p300 facilitates transcription from tax-responsive human T-cell leukemia virus type 1 chromatin template. Mol. Cell. Biol. 2002;22(13):4450–4462. doi: 10.1128/MCB.22.13.4450-4462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek S, Chen Y, Huxford T, Ghosh G. IkappaBbeta, but not IkappaBalpha, functions as a classical cytoplasmic inhibitor of NF-kappaB dimers by masking both NF-kappaB nuclear localization sequences in resting cells. J. Biol. Chem. 2001;276(48):45225–45235. doi: 10.1074/jbc.M105865200. [DOI] [PubMed] [Google Scholar]
- Marriott SJ, Semmes OJ. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. OncoGene. 2005;24(39):5986–5995. doi: 10.1038/sj.onc.1208976. [DOI] [PubMed] [Google Scholar]
- Nasr R, Chiari E, El Sabban M, Mahieux R, Kfoury Y, Abdulhay M, Yazbeck V, Hermine O, de The H, Pique C, Bazarbachi A. Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-kappa B activation. Blood. 2006;107(10):4021–4029. doi: 10.1182/blood-2005-09-3572. [DOI] [PubMed] [Google Scholar]
- Nerenberg M, Hinrichs SH, Reynolds RK, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237(4820):1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- Neuveut C, Jeang KT. HTLV-I Tax and cell cycle progression. Prog. Cell Cycle Res. 2000;4:157–162. doi: 10.1007/978-1-4615-4253-7_14. [DOI] [PubMed] [Google Scholar]
- Okada M, Jeang KT. Differential requirements for activation of integrated and transiently transfected human T-cell leukemia virus type 1 long terminal repeat. J. Virol. 2002;76(24):12564–12573. doi: 10.1128/JVI.76.24.12564-12573.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Pajak B, De Smedt T, Moulin V, De Trez C, Maldonado-Lopez R, Vansanten G, Briend E, Urbain J, Leo O, Moser M. Immunohistowax processing, a new fixation and embedding method for light microscopy, which preserves antigen immunoreactivity and morphological structures: visualisation of dendritic cells in peripheral organs. J. Clin. Pathol. 2000;53(7):518–524. doi: 10.1136/jcp.53.7.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloponese JM, Jr, Iha H, Yedavalli VR, Miyazato A, Li Y, Haller K, Benkirane M, Jeang KT. Ubiquitination of human T-cell leukemia virus type 1 tax modulates its activity. J. Virol. 2004;78(21):11686–11695. doi: 10.1128/JVI.78.21.11686-11695.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 1980;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumfery A, de La FC, Kashanchi F. HTLV-1 Tax: centrosome amplification and cancer. RetroVirology. 2006;3:50. doi: 10.1186/1742-4690-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L, Portis T, Robek M, Harding J, Grossman W. Studies of the immortalizing activity of HTLV type 1 Tax, using an infectious molecular clone and transgenic mice. AIDS Res. Hum. Retrovir. 2000;16(16):1647–1651. doi: 10.1089/08892220050193092. [DOI] [PubMed] [Google Scholar]
- Rodriguez MS, Thompson J, Hay RT, Dargemont C. Nuclear retention of IkappaBalpha protects it from signal-induced degradation and inhibits nuclear factor kappaB transcriptional activation. J. Biol. Chem. 1999;274(13):9108–9115. doi: 10.1074/jbc.274.13.9108. [DOI] [PubMed] [Google Scholar]
- Rousset R, Fabre S, Desbois C, Bantignies F, Jalinot P. The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. OncoGene. 1998;16(5):643–654. doi: 10.1038/sj.onc.1201567. [DOI] [PubMed] [Google Scholar]
- Scoggin KE, Ulloa A, Nyborg JK. The oncoprotein Tax binds the SRC-1-interacting domain of CBP/p300 to mediate transcriptional activation. Mol. Cell. Biol. 2001;21(16):5520–5530. doi: 10.1128/MCB.21.16.5520-5530.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmes OJ, Majone F, Cantemir C, Turchetto L, Hjelle B, Jeang KT. HTLV-I and HTLV-II Tax: differences in induction of micronuclei in cells and transcriptional activation of viral LTRs. Virology. 1996;217(1):373–379. doi: 10.1006/viro.1996.0126. [DOI] [PubMed] [Google Scholar]
- Shembade N, Harhaj NS, Yamamoto M, Akira S, Harhaj EW. The human T-cell leukemia virus type 1 Tax oncoprotein requires the ubiquitin-conjugating enzyme Ubc13 for NF-kappaB activation. J. Virol. 2007;81(24):13735–13742. doi: 10.1128/JVI.01790-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibon D, Gabet AS, Zandecki M, Pinatel C, Thete J, Delfau-Larue MH, Rabaaoui S, Gessain A, Gout O, Jacobson S, Mortreux F, Wattel E. HTLV-1 propels untransformed CD4 lymphocytes into the cell cycle while protecting CD8 cells from death. J. Clin. Invest. 2006;116(4):974–983. doi: 10.1172/JCI27198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15(7):1607–1614. [PMC free article] [PubMed] [Google Scholar]
- Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol. Cell. Biol. 1988;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. U. S. A. 1990;87(3):1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnell AS, Mymryk JS. Roles for the coactivators CBP and p300 and the APC/C E3 ubiquitin ligase in E1A–dependent cell transformation. Br. J. Cancer. 2006;95(5):555–560. doi: 10.1038/sj.bjc.6603304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 2004;29(2):72–79. doi: 10.1016/j.tibs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382(6589):319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- Yao TP, Oh SP, Fuchs M, Zhou ND, Ch’ng LE, Newsome D, Bronson RT, Li E, Livingston DM, Eckner R. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93(3):361–372. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc. Natl. Acad. Sci. U. S. A. 1984;81(8):2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Minoda Y, Yoshida R, Yoshida H, Iha H, Kobayashi T, Yoshimura A, Takaesu G. HTLV-1 Tax-mediated TAK1 activation involves TAB2 adapter protein. Biochem. Biophys. Res. Commun. 2008;365(1):189–194. doi: 10.1016/j.bbrc.2007.10.172. [DOI] [PubMed] [Google Scholar]