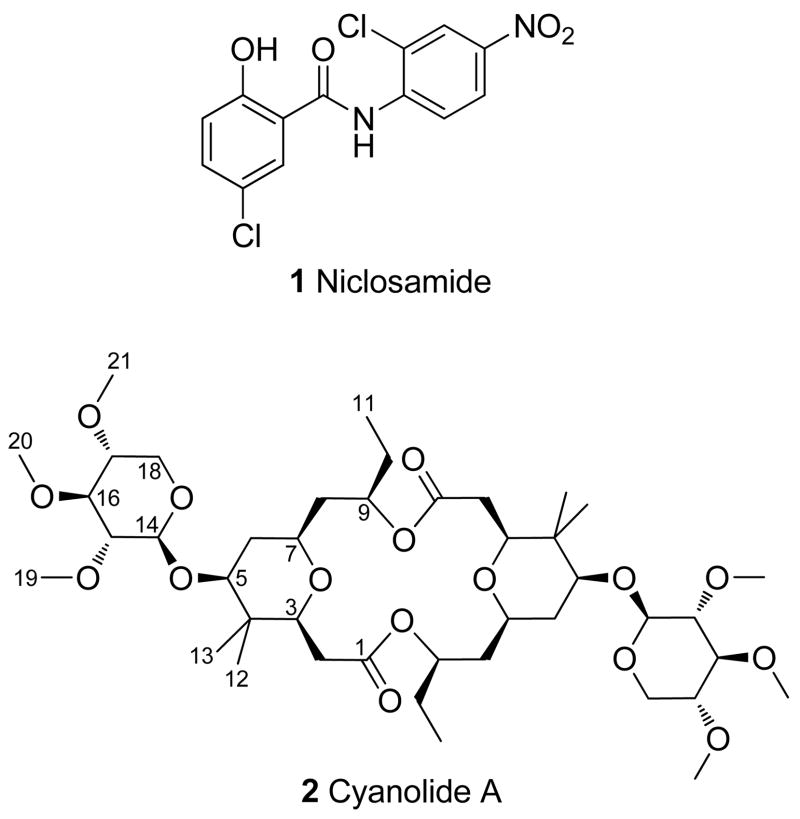
Over the last 50 years, molluscicides have played a critical role in the control of schistosomiasis transmission. Cyanolide A (2), isolated from extracts of a Papua New Guinea collection of Lyngbya bouillonii, is a new and highly potent molluscicidal agent against the snail vector Biomphalaria glabrata (LC50 = 1.2 μM). The structure of cyanolide A (2) was elucidated through extensive NMR spectroscopic analyses, yielding a symmetrical dimer that represents the newest addition to the family of glycosidic macrolides from cyanobacteria.
Schistosomiasis continues to be one of the most prevalent parasitic infections worldwide, with an estimated 207 million people currently infected and 779 million people at risk of infection.1,2 The disease is endemic to many countries with large populations, such as Brazil and China, but by far the majority of infected people (97%) are from the continent of Africa.1 There are five species of Schistosoma known to infect humans, four of which cause the intestinal form of schistosomiasis and one that causes urinary schistosomiasis.3 The pathology of schistosomiasis includes a complex immune response to schistosome eggs that become trapped in tissues.4 This can cause hepatomegaly, splenomegaly, bladder cancer, or kidney malfunction, among other disorders, and can ultimately lead to death.3,4
A major problem in controlling schistosomiasis infection, however, is the complex lifecycle of the worm. The helminths require both an aquatic snail host and a mammalian host to complete their reproductive cycle.3 Thus, eradicating the disease in the mammalian host, with a drug like praziquantel, does not protect against the possibility of re-infection from later exposure to water containing snail vectors (belonging to the genus Biomphalaria, e.g., B. glabrata and B. alexandrina).5,6 Molluscicides have been developed over the last 50 years to kill the intermediary snail host and constitute a crucial method of schistosomiasis control.7 Niclosamide (1) (Bayluscide, LC100 = 4.6 μM) is the most widely used molluscicide available, effectively killing snails at all stages of the lifecycle.3,6,7 However, its high price, poor water solubility, and potential toxicity toward fish are considered major drawbacks.8 Other molluscicides, both synthetic6,7,9 and from plant sources,6,10,11 have been examined, but not one has proved significant enough to replace niclosamide (1).
As part of our ongoing NIH-funded International Cooperative Biodiversity Group program in Panama to screen natural product materials for activities to tropical diseases, including malaria, leishmaniasis, Chagas’ disease and schistosomiasis,12 we have used a simple molluscicidal bioassay to screen marine cyanobacterial extracts, and have previously reported the molluscicidal agents barbamide (LC100 = 21.6 μM)13 and tanikolide (LD50 = 31.6 μM).14 Herein, we describe the isolation and structure elucidation of cyanolide A (2), a new molluscicidal agent obtained from a Papua New Guinea collection of Lyngbya bouillonii.
Multiple samples of dark-reddish cyanobacterial mats, tenaciously attached to surrounding corals, were collected by SCUBA from a shallow reef wall outside Pigeon Island, Papua New Guinea. A careful taxonomic characterization using morphological and 16S RNA data allowed us to unequivocally identify this organism as Lyngbya bouillonii (Hoffmann et Demoulin).15 The majority of the biological material was repeatedly extracted with CH2Cl2/MeOH (2:1) to produce 1.22 g of extract, which was then fractionated by silica gel vacuum column chromatography to produce nine fractions (A–I). At this point, the highly bioactive and most spectroscopically intriguing fraction F (74.2 mg) was subjected to a 1H NMR guided fractionation comprised of repetitive silica gel flash column chromatography followed by normal-phased HPLC, to afford 1.2 mg (0.1%) of cyanolide A (2) as the main component. Pure metabolite 2 exhibited significant molluscicidal activity against Biomphalaria glabrata (LC50 = 1.2 μM) as well as modest brine shrimp toxicity (LC50 = 10.8 μM).
HRESIMS of 2 yielded an [M+Na]+ parent ion at m/z 855.4735, consistent with the molecular formula C42H72O16 and 7 degrees of unsaturation. Its IR spectrum indicated a lack of hydroxy groups and the presence of ester and ether linkages (bands at 1740 and 1096 cm−1). The 1H NMR spectrum of 2 in CDCl3 (Table 1, Figure S1 in Supporting Information) displayed a total of eight methines adjacent to oxygen plus one oxygenated methylene exhibiting diastereotopic proton resonances at δa3.97 (dd, J = 5.4, 11.4 Hz) and δb3.09 (H-18b, dd, J = 10.2, 11.4 Hz). Among these, the methine doublet at δ4.24 was quite diagnostic and suggested the presence of a sugar-like moiety comprising a portion of cyanolide A (2). Additional key proton signals included three methoxy singlets at δ3.62, δ3.60 and δ3.46, two shielded methyl singlets at δ0.96 and δ0.88, and a methyl triplet at δ0.85 (J = 7.8 Hz). The readily interpretable 1H NMR spectrum of 2 was completed by four pairs of diastereotopic methylenes in the range δ2.40−1.40, which in combination with all the resonances above, accounted for one half of the 72 protons expected from HRESIMS data. A similar situation was observed in the 13C NMR spectrum of 2 (Table 1, Figure S2), with the appearance of only 21 peaks consistent with one ester carbonyl at δ171.6, one anomeric carbon at δ105.9, 11 oxygenated sp3 resonances between δ85.6 and δ58.8, five methylene peaks ranging δ40.6−28.3, and three methyl carbons at δ22.1, δ13.5 and δ9.4. Thus, we concluded that 2 contained a plane of symmetry and that the NMR data reflected one half of the complete structure. As detailed below, extensive analysis by 2D NMR, including HSQC, HMBC, COSY, and NOESY, revealed the dimeric and symmetric nature of metabolite 2 (Table 1).
Table 1.
NMR Spectrocopic Data (600 MHz, CDCl3) for Cyanolide A (2).
| Carbon | δC, mult.a | δH mult (J in Hz)b | HMBCc | COSY | NOESY |
|---|---|---|---|---|---|
| 1, 1′ | 171.6, qC | ||||
| 2a, 2a′ | 35.0, CH2 | 2.38, dd (1.8, 16.2) | 1, 3, 4 | 2b, 3 | 12, 13 |
| 2b, 2b′ | 2.27, dd (9.0, 16.2) | 1, 3 | 2a, 3 | 12 | |
| 3, 3′ | 80.3, CH | 3.44, dd (1.8, 9.0) | 1, 2, 5, 7, 12 | 2a, 2b | 13 |
| 4, 4′ | 38.6, qC | ||||
| 5, 5′ | 85.4, CH | 3.31, dd (4.8, 11.4) | 4, 12, 13, 14 | 6a, 6b | 13, 14 |
| 6a, 6a′ | 36.9, CH2 | 1.97, ddd (2.0, 4.8, 12.3) | 4, 5, 7 | 5, 6b | |
| 6b, 6b′ | 1.47, ddd (11.8, 11.8, 12.3) | 4, 5, 7, 8 | 5, 6a, 7 | 12 | |
| 7, 7′ | 75.2, CH | 3.47, m | 6b, 8a, 8b | 9 | |
| 8a, 8a′ | 40.6, CH2 | 1.82, dt (9.0, 14.4) | 6, 7, 9, 10 | 7, 8b, 9 | |
| 8b, 8b′ | 1.57, m | 6, 7, 9, 10 | 7, 8a, 9 | ||
| 9, 9′ | 73.8, CH | 4.91, m | 1′, 7, 8, 10, 11 | 8a, 8b | 7, 11 |
| 10a, 10a′ | 28.3, CH2 | 1.59, m | 8, 9, 11 | 11 | |
| 10b, 10b′ | 1.51, m | 8, 9, 11 | 11 | ||
| 11, 11′ | 9.4, CH3 | 0.85, t (7.8) | 9, 10 | 10a, 10b | 9 |
| 12, 12′ | 13.5, CH3 | 0.88, s | 3, 4, 5, 13 | 2a, 2b, 6b, 19 | |
| 13, 13′ | 22.1, CH3 | 0.96, s | 3, 4, 5, 12 | 2a, 3, 5, 14, 19 | |
| 14, 14′ | 105.9, CH | 4.24, d (7.8) | 5, 15, 18 | 15 | 5, 13, 16, 19 |
| 15, 15′ | 84.0, CH | 2.96, dd (7.8, 9.0) | 14, 16, 19 | 14 | 19 |
| 16, 16′ | 85.6, CH | 3.09, dd (9.0, 10.1) | 14, 15, 17, 20 | 14 | |
| 17, 17′ | 79.4, CH | 3.23, ddd (5.4, 10.1, 10.2) | 15, 18, 21 | 18a | |
| 18a, 18a′ | 63.3, CH2 | 3.97, dd (5.4, 11.4) | 14, 16, 17 | 17, 18b | |
| 18b, 18b′ | 3.09, dd (10.2, 11.4) | 14, 16, 17 | 18a | 19 | |
| 19, 19′ | 61.0, CH3 | 3.60, s | 15 | 12, 13, 14, 15, 18b | |
| 20, 20′ | 60.9, CH3 | 3.62, s | 16 | ||
| 21, 21′ | 58.8, CH3 | 3.46, s | 17 |
Recorded at 150 MHz.
Recorded at 600 MHz.
From proton to the indicated carbon.
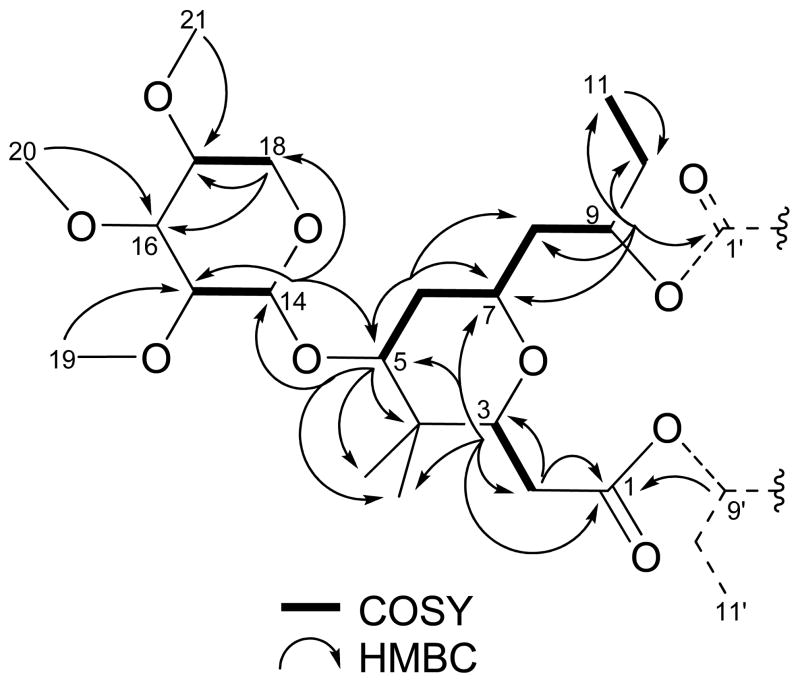
The first substructure comprising 2 was determined to be a 2,3,4-trimethoxy xylose unit on the basis of COSY and HMBC data. Methoxy singlets at δ3.60 (H-19), δ3.62 (H-20) and δ3.46 (H-21) displayed HMBC correlations with methine carbons at δ84.0 (C-15), δ85.6 (C-16) and δ79.4 (C-17), respectively. These were found to be interconnected via reciprocal HMBC correlations (Figure 1), providing evidence for the sequence δ2.96 (H-15), δ3.09 (H-16) and δ3.23 (H-17). This last proton showed a COSY correlation with a resonance at δ3.97 (H-18a), that was part of an oxygenated methylene in which the other proton component resonated at the more shielded shift of δ3.09 (H-18b). Both of these latter resonances, in turn, displayed HMBC correlations with the immediately adjacent oxymethine carbon at δ79.4 (C-17) as well as the oxygenated carbon at δ85.6 (C-16). The H2-18 protons were also correlated by HMBC to an anomeric carbon at δ105.9 (C-14), which must be linked through an oxygen atom to C-18. The xylose backbone was completed by observation of the anomeric doublet proton resonance at δ4.24 (H-14), which displayed COSY correlation with a proton at δ2.96 (H-15), as well as reciprocal HMBC correlations with carbons at δ84.0 (C-15) and δ63.3 (C-18) within the six-membered xylose ring, and a new carbon resonance at δ85.4 (C-5), thus connecting the sugar moiety to the aglycone portion of metabolite 2. All 1H and 13C chemical shifts for this triacetylated xylose moiety were in close agreement with literature values (Tables S1 and S2, Figure S7 in Supporting Information).16
Figure 1.
Representative COSY and HMBC correlations for the monomer of cyanolide A (2).
One half of the 16-membered central macrolide constituting 2 was assembled via a combination of HMBC and COSY data beginning from the oxymethine at δ3.31 (H-5), directly linked to the xylose residue. This informative proton resonance showed HMBC correlations with a quaternary carbon at δ38.6 (C-4), as well as two methyl resonances attached to this latter carbon at δ13.5 (C-12) and δ22.1 (C-13). The same proton resonance constituted the start of a tightly coupled spin system that included signals for a diastereotopic methylene at δ1.97 (H-6a) and δ1.47 (H-6b), an oxygenated methine at δ3.47 (H-7), a second diastereotopic methylene with protons at δ1.82 (H-8a) and δ1.57 (H-8b), and a final deshielded methine at δ4.91 (H-9). HMBC data among all of these protons confirmed the sequence given by COSY and provided additional connectivity information for methine H-9, which correlated with a carbonyl carbon at δ171.6 (C-1′) and an ethyl branch comprised by a methylene carbon at δ28.3 (C-10, δHa1.59 and δHb1.51) and a methyl at δ9.4 (C-11, δH0.85).
The methyl singlets at δ0.88 (H-12) and δ0.96 (H-13), which formed part of the gem-dimethyl moiety mentioned above, displayed HMBC correlations with a geminal sp3 carbon at δ38.6 (C-4), as well as with vicinal carbon resonances at δ85.4 (C-5) and δ80.3 (C-3). By HMBC from the proton at δ3.44 (H-3), this latter carbon was found to be linked, through an oxygen atom, to the resonance at δ75.2 (C-7). Additional HMBC correlations from H-3 allowed placement of an adjacent methylene carbon at δ35.0 (C-2), which exhibited diastereotopic proton resonances at δ2.38 (H-2a) and δ2.27 (H-2b), followed by a carbonyl signal at δ171.6 (C-1). At this point all of the COSY, HSQC and HMBC NMR data had been fully engaged, but accounted for only one half of the expected atomic composition indicated by HRESIMS measurement. An exhaustive review of the 2D NMR data revealed that the deshielded oxymethine H-9 (δ4.91) was involved in an ester linkage containing also the carbonyl at δ171.6; this linkage would give rise to an 8-membered ring system if only a monomeric chemical entity is considered. However, given that all 1H and 13C NMR resonances were assigned at this point, it was evident that compound 2 was a symmetrical dimer exhibiting a central 16-membered macrocycle fused with two flanking tetrahydropyran rings, each connected to a xylose moiety. Sequential loss of the two sugar units under APCI conditions (fragment ions at m/z 659.0 and m/z 485.0, Figure S7) gave further evidence of the symmetrical dimeric nature of cyanolide (2).
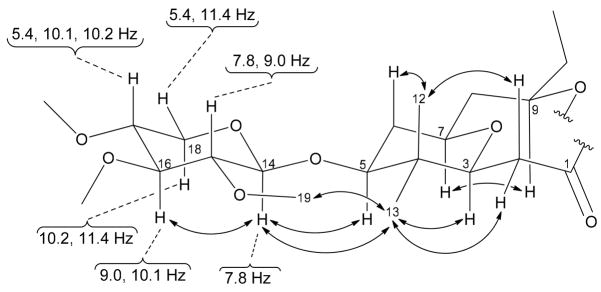
The relative configuration and β-linkage of the two xylose residues was defined by large vicinal coupling constants measured for the axial anomeric proton H-14 (δ4.24, d, J = 7.8 Hz), as well as axial oxymethines H-15 (δ2.96, dd, J = 7.8, 9.0 Hz), H-16 (δ3.09, dd, J = 9.0, 10.1 Hz) and H-17 (δ3.23, ddd, J = 5.4, 10.1, 10.2 Hz) (Figure 2). Thus, all of the methoxy substituents in the sugar were found to be equatorial. NOESY data between H-14 and H-16 provided additional evidence for the sugar configuration defined above. This relative configuration was connected to that of the central macrocycle through two separate points using NOESY. NOE’s were observed between the anomeric proton H-14 (δ4.24) and oxymethine H-5 (δ3.31), as well as between the equatorial methoxy H-19 (δ3.60) and the equatorial methyl H-13 (δ0.96). This latter interaction suggested a locked conformation in 2 wherein the [C-5]-O-[C-14] bridge is prevented from free rotation, most likely due to steric hindrance. Analogous to the xylose residue, a combination of coupling constants and NOESY data led to the assignment of axial positions for methyl H-12 (δ0.88), methylenic protons H-2b (δ2.27) and H-6b (δ1.47), as well as oxymethines H-3 (δ3.44), H-5 (δ3.31), H-7 (δ3.47) and H-9 (δ4.91), all located in the tetrahydropyran ring and the monomeric portion of the central macrocycle comprising cyanolide A. Considering all the data above and assuming a natural D-configuration for the xylose residue, we propose an absolute configuration for cyanolide A (2) as 3S, 3′S, 5S, 5′S, 7R, 7′R, 9R, 9′R, 14S, 14′S, 15R, 15′R, 16S, 16′S, 17R and 17′R. Unfortunately, attempts to experimentally corroborate this proposal were unfeasible due to the limited amount of material available after biological evaluation.
Figure 2.
Coupling constant and NOE data analysis defining the relative configuration of 2.
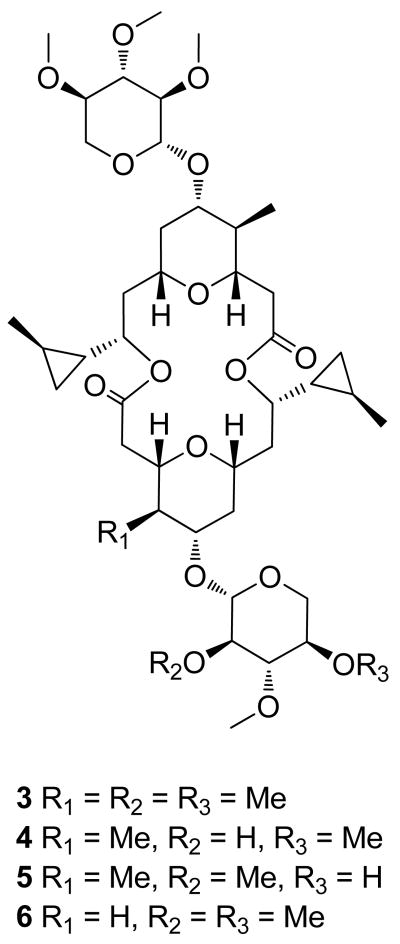
Cyanolide A (2) is structurally most closely related to the marine natural products clavosolides A–D (3–6) isolated from the Philippine sponge, Myriastra clavosa.16 They share a flat dimeric macrocyclic skeleton that is decorated with xylose appendages, the same configuration for their analogous stereocenters throughout the entire molecule, and negative specific rotation values of approximately the same magnitude (Table S3, Supporting Information). The triacetylated xylose residues possess virtually identical 1H and 13C chemical shifts (Tables S1 and S2, Figure S7), which in the case of clavosolides A (3),17 B (4)18 and D (6),19 have been found to have the D-configuration via total synthesis. However, cyanolide A (2) lacks the trans-2-methylcyclopropyl substituents at the C-9/C-9′ position of the clavosolides, instead possessing an ethyl group. Furthermore, rather than having one methyl group at C-4, cyanolide A (2) possesses a dimethyl group at this location. The fact that cyanolide A (2) was isolated from the free living cyanobacteria L. bouillonii suggests that the clavosolides are also cyanobacterial compounds. Indeed, such a possibility was noted by Rao and Faulkner in their initial report on clavosolides A and B.16
Cyanolide A (2) represents the newest addition to the family of glycosidic macrolides from cyanobacteria, a rapidly expanding group of polyketide-derived cyclic compounds exhibiting different ring sizes and complex methylation, glycosidation and unsaturation patterns, even including halogenation.20 We envision that the monomer of 2 originates from a nascent polyketide chain composed of five acetate units which is modified by SAM-derived methylation events giving rise to a gem-dimethyl at C-4 and a methyl extension at C-10. This latter rather unusual methylation is proposed because cyanobacteria do not appear to utilize propionate in the production of their polyketide natural products.21 The high toxicity exhibited by cyanolide A (2) against B. glabrata makes it the most potent molluscicidal agent isolated by our research program so far, and this may relate to its natural function to deter grazing by herbivorous mollusks. Further biological evaluation has revealed that compound 2 is relatively non-cytotoxic when tested to H-460 human lung adenocarcinoma and Neuro-2a mouse neuroblastoma cell lines (up to a maximum test concentration of 35 μM). Thus, cyanolide A represents a promising lead compound for treating waterways infested with schistosomiasis-carrying snails of the genus Biomphalaria.
Experimental Section
General Experimental Procedures
The optical rotation was measured on a JASCO P-2000 polarimeter. UV and IR spectra were recorded on a Beckman DU 800 UV and a Nicolet 100 FT-IR spectrophotometers, respectively. 1H, 13C and 2D NMR spectra were collected at a 1H resonance frequency of either 600 MHz (Bruker Avance III DRX600) or 500 MHz (JEOL ECA500). The Bruker DRX600 was equipped with a 1.7 mm TCI cryoprobe. Chemical shifts were calibrated internally to the residual signal of the solvent in which the sample was dissolved (CDCl3, δH 7.26, δC 77.0). High resolution mass spectra were obtained on a ThermoFinnigan MAT900XL mass spectrometer. LC-MS data was acquired on a ThermoFinnigan Surveyor Plus PDA and pump array, equipped with a LCQ Advantage Max mass detector. APCI MS spectra were recorded on a Finnigan LCQDECA instrument. HPLC was carried out using a dual Waters 515 pump system equipped with a Waters 996 photodiode array detector. Vacuum and flash chromatographic separations were performed using type H (10–40 μ, Aldrich) silica and silica gel 60 (40–63 μ, EMD), respectively. Merck aluminum-supported TLC sheets (silica gel 60 F254) were used for TLC. All solvents were purchased as HPLC grade.
Biological Material
Samples of Lyngbya bouillonii (voucher specimen available from WHG as collection number PNG5-19-05-8) were collected at a depth of 3–10 m from a shallow reef wall outside Pigeon Island, Papua New Guinea (4° 16.063S, 152° 20.266E), in May 2005. Samples were stored in 70% EtOH at −20 °C prior to extraction. Taxonomy was assigned via 16S RNA data (GenBank accession numbers FJ041298 and FJ041299) and by microscopic comparison with the description given by Komárek and Anagnostidis,22 as well as Hoffmann15 (Supporting Information).
Extraction and Isolation
Approximately 37.9 g (dry wt) of the cyanobacteria were extracted repeatedly with CH2Cl2/MeOH (2:1) to afford 1.22 g of extract. A portion of this material (1.13 g) was fractionated by silica gel vacuum liquid chromatography using a stepwise gradient solvent system of increasing polarity starting from 10% EtOAc in hexanes to 100% MeOH, to produce nine fractions (A–I). The bioactive fraction F (0.0742 g) was subjected to a 1H NMR-guided fractionation using repetitive silica gel column chromatography and isocratic conditions (50 and 60% EtOAc in hexanes, respectively), followed by normal phase HPLC (Phenomenex Luna 10μ Silica 100Å, 250 x 4.60 mm, two columns in series, 40% EtOAc/60% hexanes at 1.5 mL/min, detection at 254 and 280 nm) to yield 1.2 mg of compound 2.
Cyanolide A (2): colorless oil; [α]23D -59 (c 0.6, CHCl3); IR vmax (neat) 2932, 1740, 1463, 1371, 1305, 1255, 1163, 1096 cm−1; 1H and 13C NMR (CDCl3), see Table 1; APCIMS m/z 855.4 [M+Na]+ (9), 833.0 [M+H]+ (49), 659.0 (36), 485.0 (100), 467.1 (11), 242.9 (14), 224.9 (11), 207.0 (7); HRESIMS [M+Na]+ m/z 855.4735 (calcd for C42H72NaO16, 855.4718).
Molluscicidal Assay
The molluscicidal effect of pure cyanolide A (2) was evaluated according to a previously outlined procedure using the test organism Biomphalaria glabrata.23 In all assays performed, a stock solution of 20 mg/mL of pure compound in EtOH was prepared, of which 35 μL was taken and diluted to 7 mL with distilled H2O. The snails were placed in wells of varying concentrations and observed after 24 h. If no heartbeat could be detected upon examination under a dissecting microscope (20X magnification), the snails were assessed as dead.
Supplementary Material
Acknowledgments
We thank R. Grindberg, C. Sorrels and L. Simmons for the collection of L. bouillonii from Papua New Guinea, L. Matainaho for assistance with collection permits in Papua New Guinea, A. Jansma for assistance with the SSPPS Bruker 600 MHz TCI cryoprobe NMR spectrometer, N. Engene for his help with the morphological and taxonomic identification of the source organism, and T. Byrum for running mammalian cell line cytotoxicity measurements. The JEOL NMR spectrometer was supported by the National Science Foundation under CHE-0741968. Support was provided by Fogarty International Center’s International Cooperative Biodiversity Groups program (NIH TW006634) and NIH CA52955.
Footnotes
Supporting Information Available. 1H NMR, 13C NMR, and 2D NMR spectra in CDCl3 for cyanolide A, comparative chemical shift and specific rotation tables for cyanolide A and clavosolides A–D, and morphological characterization and taxonomic identification of PNG5-19-05-8. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Chitsulo L, Engels D, Montresor A, Savioli L. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Report of the Scientific Working Group Meeting on Schistosomiasis; Geneva, Switzerland. November 14–16, 2005. [Google Scholar]
- 4.Gryseels B, Polman K, Clerinx J, Kestens L. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 5.Bergquist R. Acta Trop. 2008;108:65–68. doi: 10.1016/j.actatropica.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro KA, de Carvalho CM, Molina MT, Lima EP, Lopez-Montero E, Reys JR, de Oliveira MB, Pinto AV, Santana AE, Goulart MO. Acta Trop. 2009;111:44–50. doi: 10.1016/j.actatropica.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Perrett S, Whitfield PJ. Parasitol Today. 1996;12:156–159. doi: 10.1016/0169-4758(96)10001-6. [DOI] [PubMed] [Google Scholar]
- 8.Dai JR, Wang W, Liang YS, Li HJ, Guan XH, Zhu YC. Parasitol Res. 2008;103:405–412. doi: 10.1007/s00436-008-0988-2. [DOI] [PubMed] [Google Scholar]
- 9.Gawish FA, El-Sherbini SA, Aly HF. J Appl Sci Res. 2009;5:46–56. [Google Scholar]
- 10.Socolsky C, Borkosky SA, Asakawa Y, Bardon A. J Nat Prod. 2009;72:787–790. doi: 10.1021/np800724h. [DOI] [PubMed] [Google Scholar]
- 11.Rawi SM, El-Gindy H, Abd-El-Kader A. Ecotoxicol Environ Saf. 1996;35:261–267. doi: 10.1006/eesa.1996.0109. [DOI] [PubMed] [Google Scholar]
- 12.Kursar TA, Caballero-George CC, Capson TL, Cubilla-Rios L, Gerwick WH, Gupta MP, Ibañez A, Linington RG, McPhail KL, Ortega-Barría E, Romero LI, Solis PN, Coley PD. BioScience. 2006;56:1005–1012. [Google Scholar]
- 13.Orjala J, Gerwick WH. J Nat Prod. 1996;59:427–430. doi: 10.1021/np960085a. [DOI] [PubMed] [Google Scholar]
- 14.Singh IP, Milligan KE, Gerwick WH. J Nat Prod. 1999;62:1333–1335. doi: 10.1021/np990162c. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann L, Demoulin V. Belg J Bot. 1991;124:82–88. [Google Scholar]
- 16.(a) Rao MR, Faulkner DJ. J Nat Prod. 2002;65:386–388. doi: 10.1021/np010495l. [DOI] [PubMed] [Google Scholar]; (b) Erickson KL, Gustafson KR, Pannell LK, Beutler JA, Boyd MR. J Nat Prod. 2002;65:1303–1306. doi: 10.1021/np020193z. [DOI] [PubMed] [Google Scholar]
- 17.(a) Barry CS, Elsworth JD, Seden PT, Bushby N, Harding JR, Alder RW, Willis CL. Org Lett. 2006;8:3319–3322. doi: 10.1021/ol0611705. [DOI] [PubMed] [Google Scholar]; (b) Smith AB, 3rd, Simov V. Org Lett. 2006;8:3315–3318. doi: 10.1021/ol0611752. [DOI] [PubMed] [Google Scholar]; (c) Son JB, Kim SN, Kim NY, Lee DH. Org Lett. 2006;8:661–664. doi: 10.1021/ol052851n. [DOI] [PubMed] [Google Scholar]; (d) Chakraborty TK, Reddy VR, Gajula PK. Tetrahedron. 2008;64:5162–5167. [Google Scholar]; (e) Carrick JD, Jennings MP. Org Lett. 2009;11:769–772. doi: 10.1021/ol8028302. [DOI] [PubMed] [Google Scholar]
- 18.Son JB, Hwang MH, Lee W, Lee DH. Org Lett. 2007;9:3897–3900. doi: 10.1021/ol7015115. [DOI] [PubMed] [Google Scholar]
- 19.Seden PT, Charmant JP, Willis CL. Org Lett. 2008;10:1637–1640. doi: 10.1021/ol800386d. [DOI] [PubMed] [Google Scholar]
- 20.(a) Klein D, Braekman JC, Daloze D, Hoffmann L, Demoulin V. J Nat Prod. 1997;60:1057–1059. doi: 10.1021/np9900324. [DOI] [PubMed] [Google Scholar]; (b) Luesch H, Yoshida WY, Harrigan GG, Doom JP, Moore RE, Paul VJ. J Nat Prod. 2002;65:1945–1948. doi: 10.1021/np0202879. [DOI] [PubMed] [Google Scholar]; (c) Tan LT, Marquez BL, Gerwick WH. J Nat Prod. 2002;65:925–928. doi: 10.1021/np010526c. [DOI] [PubMed] [Google Scholar]; (d) Teruya T, Sasaki H, Kitamura K, Nakayama T, Suenaga K. Org Lett. 2009;11:2421–2424. doi: 10.1021/ol900579k. [DOI] [PubMed] [Google Scholar]
- 21.Tidgewell K, Clark BR, Gerwick WH. Comprehensive Natural Products Chemistry. Vol. 8. Pergamon Press; 2009. (in press) [Google Scholar]
- 22.Komárek J, Anagnostidis K. In: Susswasserflora von mitteleuropa. 2. Budel B, Krienitz L, Gartner G, Schagerl M, editors. Vol. 19. Elsevier GmbH; Munchen: 2005. p. 627. [Google Scholar]
- 23.Hostettmann K, Kizu H, Tomimori T. Planta Med. 1982;44:34–35. doi: 10.1055/s-2007-971396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.