Abstract
Recent studies showed that dopamine or D1 receptor-selective agonists increased brain-derived neurotrophic factor (BDNF) mRNA and protein expression in neuronal cultures, and this action was blocked by SCH23390. Moreover, SKF38393 activated Trk receptors and downstream signaling in striatal neurons. This study examined whether dopamine agonists induce the expression of BDNF protein in rat brain tissue. Acute slice preparations were incubated with dopamine agonists in Hibernate A medium and BDNF protein was measured by a sensitive enzyme-linked immunosorbent assay. Results showed that dopamine increased BDNF in tissue slices after 24 h of incubation. Furthermore, SKF38393 produced a significant increase in BDNF protein in striatal and hippocampal tissue slices. These findings suggest that the induction of BDNF expression may constitute a downstream response to D1-like dopamine receptor activation.
Keywords: brain-derived neurotrophic factor, D1-like receptor, dopamine, SKF38393
Introduction
Dopamine is a catecholamine neurotransmitter known to modulate neuronal activity [1–3] as well as key physiological functions related to locomotion, reward, and cognition [4,5]. Dopamine exerts its actions by interacting with five known subtypes of specific membrane receptors belonging to the seven transmembrane domain G protein-coupled family of receptors [6]. Based on their sequence homology and pharmacology, these five subtypes of dopamine receptors are further categorized into two subfamilies of receptors known as D1-like and D2-like receptors [7]. The D1-like subfamily consists of the D1 and D5 receptors, whereas the D2-like subfamily consists of D2, D3, and D4 receptors [7].
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family consisting of nerve growth factor, BDNF, neurotrophin-3 (NT-3), and NT-4/5 [8]. These neurotrophins play important roles in the development, differentiation, maintenance, and survival of distinct and overlapping neuronal populations within the central and peripheral nervous systems [9]. BDNF signaling is initiated by the binding of BDNF to the TrkB receptor. Once activated, the TrkB receptor autophosphorylates specific tyrosine residues within its intracellular domains. The phosphorylated tyrosines serve as protein interaction sites for SHC (SH2-containing adapter protein). Tyrosine phosphorylation of SHC subsequently triggers the phosphorylation of multiple targets that include Raf and MAPK/ERK. Through its high-affinity TrkB receptor, BDNF leads to the activation of three intracellular signal transduction systems, namely, the MAPK signal transduction cascade (MAPK/ERK), PI3K pathway, and phospholipase Cγ [10–12].
An earlier study reported that stimulation with dopamine or a D1 dopamine receptor agonist increased BDNF expression but not TrkB mRNA in neuronal cultures, and this response was attenuated by SCH23390 [6]. Furthermore, it has been reported that striatal neurons incubated with the D1 receptor agonist SKF38393 showed increased sensitivity of TrkB receptors, and this action was accompanied by the phosphorylation of phospholipase Cγ, Akt, and MAPK [13]. In contrast, K-252a, a Trk tyrosine kinase inhibitor, or a dopamine D1 receptor antagonist could block the effects of SKF38393 [13]. These results provide evidence for linking dopamine receptor activation to increased BDNF expression and signaling. However, it remains to be determined whether dopamine or D1 receptor agonists would enhance the expression or action of BDNF protein in native brain tissue. In this study, dopamine and receptor-selective agonists were tested for the effects on BDNF protein expression in slice preparations of rat brain tissue. The results show that dopamine can increase BDNF protein expression in discrete brain tissues, and that this action is mimicked by the D1 agonist SKF38393 but not by the D2 agonist quinpirole.
Materials and methods
Animals
Male Sprague–Dawley rats weighing 175–200 g were obtained from Zivic Laboratories (Pittsburgh, Pennsylvania, USA). The animals were caged in groups of three and housed in climate-controlled facilities with a 12-h light/dark cycle and free access to food and water. Protocols for the care and use of the experimental animals were approved by the Institutional Animal Care and Use Committee and conformed to the NIH Guide for the Care and Use of Laboratory Animals.
Drugs
Dopamine HCl, SKF38393 [(±)-1-Phenyl-2,3,4,5-tetrahydro-( 1H)-3-benzazepine-7,8-diol] and Quinpirole (Sigma Aldrich, St Louis, Missouri, USA) were dissolved and further diluted to use concentrations in sterile Hibernate A medium (Brainbits LLC, Springfield, Illinois, USA).
Assay of brain-derived neurotrophic factor protein levels in tissue slices
Animals were decapitated and frontal cortex, striatum, and hippocampus were dissected out. The brain tissues were cut into 350 × 350 µm slices and washed immediately using Hibernate A medium (Brainbits LLC). Tissue slices were preincubated for 1 h at 37°C and 5% CO2 and washed with Hibernate A medium. A 75-µl aliquot of tissue slices was added to each well of a 24-well plate containing 325 µl Hibernate A. Drugs at indicated concentrations were added in 25-µl volumes to appropriate wells and incubation continued for up to 24 h at 37°C and 5% CO2. Each tissue slice suspension was transferred to a microcentrifuge tube containing ice-cold homogenization buffer [137mM NaCl, 20mM Tris (pH 8.0), 1% Triton X-100, 10% glycerol, 1mM protease inhibitor cocktail (Sigma Aldrich)]. Tubes containing the homogenates were incubated for 20 min at 4°C on a rocking platform and then centrifuged at 17 000g at 4°C for 15 min. The supernatant was transferred to another tube and aliquots of the protein extract were assayed for BDNF protein expression using the Promega BDNF enzyme-linked immunosorbent assay system according to the manufacture protocol (Promega, Madison, Wisconsin, USA). Briefly, 96-well plates were coated with anti-BDNF mAb and nonspecific binding was blocked with Block & Sample buffer (Promega). The immobilized anti-BDNF mAb was incubated with BDNF standard or test samples followed by incubation with anti-human BDNF pAb. After incubation with anti-IgY horseradish peroxidase conjugate, TMB One solution (3,3′,5,5′-tetramethylbenzidine) was added and color detection was achieved at 450 nM using Spectramax Pro plate reader (Molecular Devices, Sunnyvale, California, USA).
Data analysis
Each experiment was performed on multiple occasions so that sample sizes of 6–9 were accumulated. In general, data were analyzed by analysis of variance using GraphPad Prism software (GraphPad Software Inc., San Diego, California, USA), followed post hoc by either the Dunnett’s test to determine which of the tested treatments differed significantly from the respective control group, the Tukey’s test to determine which among the various pairs of treatments or control groups were significantly different, or Bonferonni’s test to compare pairs of control and treatment groups at each dose point. Statistical comparisons were considered significant at a P value of less than 0.05 or better. Subsequently, data were pooled and processed for graphical presentation.
Results
Tissue slices prepared from the rat frontal cortex and incubated with dopamine at various concentrations for 3–24 h showed significant and concentration-dependent increases in BDNF protein expression [analysis of variance F(2,52)=8.89, P<0.01] after 24 h incubation (Fig. 1). Preliminary experiments showed that the slice preparations incubated in Hibernate A medium remain metabolically active after 24 h, as detected by XTT assay (Sigma Aldrich) and by the incorporation of tritiated myo-inositol into inositol phospholipids when the radiolabel was added from the 24th to the 27th hour of incubation (data not shown). Hence, the BDNFobservations are probably physiologic responses to the agonist.
Fig. 1.
Time course for brain-derived neurotrophic factor (BDNF) protein expression in cortical tissue slices. Tissue slices were incubated with dopamine at various concentrations for 3, 12, or 24 h. The data represent the mean±SEM (n =9). Samples collated from three separate experiments and are presented as percentage change in BDNF protein expression compared with control samples. Basal levels of BDNF protein expression varied among the tested brain regions, with the hippocampus having the highest levels of the expressed protein. Slices prepared from the frontal cortex showed a significant increase in BDNF protein expression in response to 300 µM dopamine after 24 h incubation. ***P<0.001 compared with control as determined by one-way analysis of variance followed with Tukey’s post-hoc analysis.
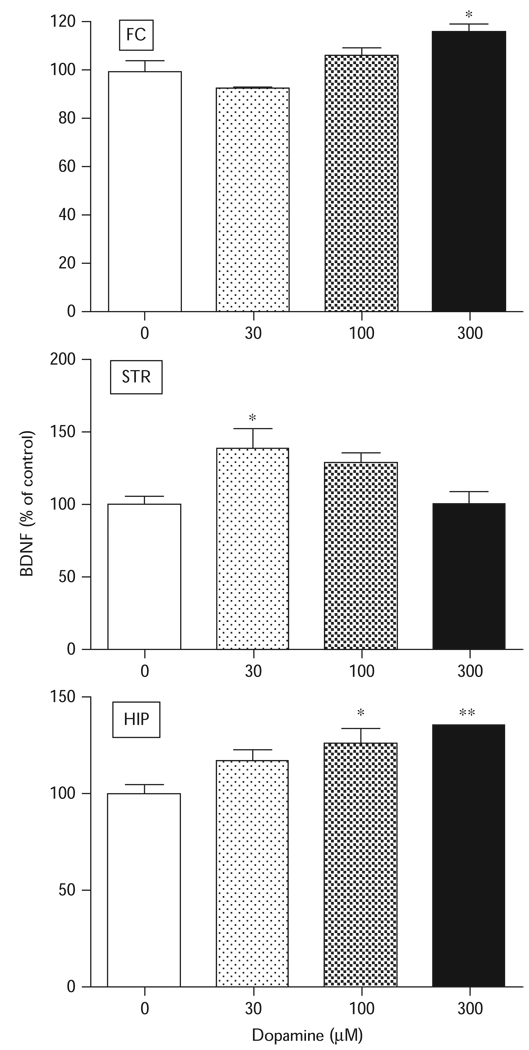
To determine the brain regional dependence of the dopamine response, tissue slices were prepared from the rat frontal cortex, striatum, or hippocampus and tested with various concentrations of dopamine. Dopamine significantly increased BDNF protein expression in all three tissue preparations (Fig. 2). The lowest concentration of dopamine that induced a significant effect in any brain region was 30 µM. Although the effects in the frontal cortex [F(3,11)=10.01, P < 0.01] were significant only at the highest tested concentration of 300 µM, the striatum effects peaked at 30 µM [F(3,11)=4.638, P < 0.05] and declined thereafter. In the hippocampal tissues, dopamine increased BDNF protein expression [F(3,11)=9.901, P<0.01] through to the highest tested concentration of 300 µM (Fig. 2).
Fig. 2.
Brain regional effects of dopamine on brain-derived neurotrophic factor (BDNF) expression in tissue slices. Tissue slices were incubated with dopamine at various concentrations for 24 h and BDNF protein levels were measured by enzyme-linked immunosorbent assay. The data represent the mean±SEM (n=9). Tissue slices prepared from the frontal cortex (FC) showed a significant increase in BDNF protein expression at 300 µM. Treatment of hippocampal (HIP) tissue slices with dopamine produced a significant increase in BDNF protein expression at 100 and 300 µM concentrations. Striatal (STR) tissue slices were more sensitive to dopamine treatment with 30 µM producing a significant increase in BDNF protein expression. *P<0.05; **P<0.01, compared with wells that received no treatment as determined by Tukey’s post-hoc analysis.
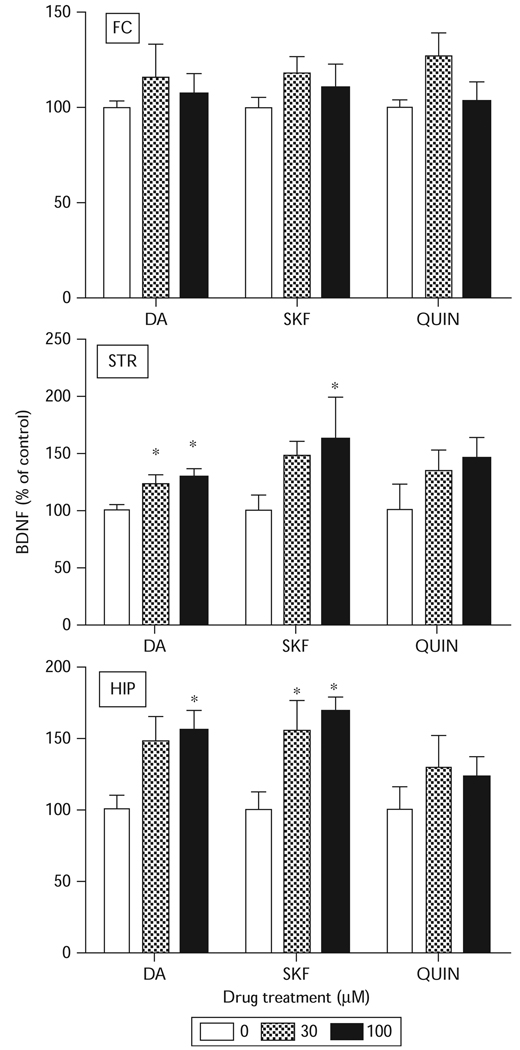
Dopamine receptor subclass-selective agonists also were tested for possible effects on BDNF protein expression. Tissue slices prepared from the frontal cortex showed no change in BDNF protein expression in comparison with control after treatment with SKF38393 or quinpirole (Fig. 3). In hippocampal [F(2,54)=8.74, P<0.05] and striatal tissue slices [F(2,54)=6.65, P<0.05], SKF38393 produced a significant increase in BDNF protein expression, similar to the effects of dopamine. Quinpirole, however, had no effect on BDNF protein expression in any of these brain regions, implying that the response might be mediated through the D1-like subclass of dopamine receptors.
Fig. 3.
Effects of dopamine receptor agonists on brain-derived neurotrophic factor (BDNF) protein expression in vitro. Tissue slices were incubated with dopamine (DA), SKF383893 (SKF), or quinpirole (QUIN) at indicated concentrations for 24 h and the levels of BDNF protein assayed by enzyme-linked immunosorbent assay. The data represents the mean±SEM (n=9). Treatment of hippocampal (HIP) and striatal (STR) tissue slices with DA and SKF significantly increased BDNF protein expression. *P<0.05 compared with wells that received no treatment as determined by two-way analysis of variance followed by Tukey’s post-hoc analysis. FC, frontal cortex.
In general, basal concentrations of BDNF protein expression were highest in the hippocampus (50 pg/ml), whereas the levels in the striatum (20 pg/ml) and the frontal cortex (25 pg/ml) were similar (Fig. 3). This pattern of basal activity distribution and agonist responsiveness is reminiscent of D1-like receptor induction of phosphoinositide signaling as previously reported for various parameters of this signaling system.
Discussion
A summary of the results shows that dopamine increased BDNF expression in a concentration-dependent manner in cortical, striatal, and hippocampal tissue slices after 24 h of incubation. Furthermore, in hippocampal and striatal tissue slices, SKF38393 produced a significant increase in BDNF protein expression, similar to the effects of dopamine. Quinpirole, however, had no effect on BDNF protein expression in any of these brain regions.
To our knowledge, this is the first study to show the effects of dopamine receptor agonist on BDNF protein expression in brain tissue slices. The advantage of using tissue slices is that it provides a model for the rapid evaluation of cell function at the single cell level, which includes investigation of signaling mechanisms and changes in metabolic processes. What is encouraging is that our results correlate with other studies that have shown that dopamine modulates BDNF protein expression in astrocytes [14] and striatal neurons [6]. Our results also reveal that dopamine increased BDNF protein expression in a time-dependent manner, with 24 h being the interval which produced dose-dependent increases in BDNF protein expression (Fig. 1). Furthermore, experiments conducted to determine the viability of the brain tissue slices after long-term incubation showed that incubation in Hibernate A medium was capable of keeping tissues alive for 24 h or longer (data not shown). Owing to its relatively fast oxidation and enzymatic breakdown, dopamine is not expected to survive in the tissue up to 24 h. Thus, we conclude that this time pattern in protein synthesis reflects the time required for mRNA synthesis and its subsequent translation into BDNF protein. In fact, as others have reported, it takes 3–6 h to obtain drug-induced increases in BDNF mRNA, which must then be followed with protein translation [15].
After determining the dose-related and time-dependent effects of dopamine on modulating BDNF protein expression in cortical tissue slices, we examined and compared dopamine effects on BDNF expression in other brain regions receiving dopaminergic input. We found regional differences in the ability of dopamine to modulate BDNF protein expression (Fig. 2). Dopamine receptors are differentially expressed in various brain regions [16] coupled to intracellular signaling pathways involved in ultimately regulating BDNF protein expression [7,17–19]. Consequently, the regional selectivity of our observations may be because of a combination of overlapping localization of dopamine receptors in the examined brain regions [16], the signaling pathways that they couple to, and the distribution of BDNF expression [20].
Treatment with dopamine D1-selective agonist SKF38393 increased BDNF protein expression above that observed after dopamine treatment in the striatum and hippocampus, suggesting that the observed increase in BDNF expression may result from dopamine D1 receptor activation (Fig. 3). It seems that this increase in BDNF protein expression, specifically in the striatum, may be responsible for the increase in the level of phosphorylated TrkB in striatal neurons. This is based on studies showing that SKF38393 increased the level of phosphorylated TrkB in striatal neurons, whereas the D2 receptor agonist quinpirole failed to stimulate TrkB phosphorylation [13]. The investigators conclude that acute stimulation of D1 receptors leads to TrkB receptor transactivation. In contrast, TrkB receptor activation could be because of increased activity of other neurotrophins, such as NT-4 [21].
Conclusion
The dopaminergic system serves as the reward system of the brain and therefore plays a central role in the addictive processes [22]. BDNF, also, has been implicated in the actions of various addictive drugs, including cocaine [23,24]. Overall, the results of this study suggest that dopamine D1 receptor signaling can contribute to increase BDNF protein synthesis, which could potentially initiate BDNF/TrkB activity. This finding could have implications for the selective role of D1-like receptor signaling and of BDNF in regulating dopamine-mediated behaviors such as reward, cognition, and motor activity. Further clarification of the mechanisms underlying dopamine–BDNF signaling could lead to the uncovering of biomarkers and targets for the development of novel pharmacotherapeutics for drug addiction or other dopamine-associated brain disorders.
Acknowledgement
This research was supported by grant #DA017614 from the US National Institutes of Health.
References
- 1.Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- 2.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 3.Bustos G, Abarca J, Campusano J, Bustos V, Noriega V, Aliaga E. Functional interactions between somatodendritic dopamine release, glutamate receptors and brain-derived neurotrophic factor expression in mesencephalic structures of the brain. Brain Res Brain Res Rev. 2004;47:126–144. doi: 10.1016/j.brainresrev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, et al. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 5.Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, et al. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci. 2003;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuppers E, Beyer C. Dopamine regulates brain-derived neurotrophic factor (BDNF) expression in cultured embryonic mouse striatal cells. Neuroreport. 2001;12:1175–1179. doi: 10.1097/00001756-200105080-00025. [DOI] [PubMed] [Google Scholar]
- 7.Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay RM, Altar CA, Cedarbaum JM, Hyman C, Wiegand SJ. The therapeutic potential of neurotrophic factors in the treatment of Parkinson’s disease. Exp Neurol. 1993;124:103–118. doi: 10.1006/exnr.1993.1181. [DOI] [PubMed] [Google Scholar]
- 9.Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology. 2005;20:70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- 10.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 12.Heerssen HM, Segal RA. Location, location, location: a spatial view of neurotrophin signal transduction. Trends Neurosci. 2002;25:160–165. doi: 10.1016/s0166-2236(02)02144-6. [DOI] [PubMed] [Google Scholar]
- 13.Iwakura Y, Nawa H, Sora I, Chao MV. Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons. J Biol Chem. 2008;283:15799–15806. doi: 10.1074/jbc.M801553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juric DM, Miklic S, Carman-Krzan M. Monoaminergic neuronal activity up-regulates BDNF synthesis in cultured neonatal rat astrocytes. Brain Res. 2006;1108:54–62. doi: 10.1016/j.brainres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- 16.Jackson DM, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol Ther. 1994;64:291–370. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 17.Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- 18.Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci. 2002;22:8911–8921. doi: 10.1523/JNEUROSCI.22-20-08911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhen X, Uryu K, Wang HY, Friedman E. D1 dopamine receptor agonists mediate activation of p38 mitogen-activated protein kinase and c-Jun aminoterminal kinase by a protein kinase A-dependent mechanism in SK-N-MC human neuroblastoma cells. Mol Pharmacol. 1998;54:453–458. doi: 10.1124/mol.54.3.453. [DOI] [PubMed] [Google Scholar]
- 20.Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 21.Freeman AY, Pierce RC. Neutralization of neutrophin-3 in the ventral tegmental area or nucleus accumbens differentially modulates cocaine-induced behavioral plasticity in rats. Synapse. 2002;46:57–65. doi: 10.1002/syn.10123. [DOI] [PubMed] [Google Scholar]
- 22.Nestler EJ, Berhow MT, Brodkin ES. Molecular mechanisms of drug addiction: adaptations in signal transduction pathways. Mol Psychiatry. 1996;1:190–199. [PubMed] [Google Scholar]
- 23.Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall FS, Drgonova J, Goeb M, Uhl GR. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28:1485–1490. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]