Abstract
Background:
Nicotine replacement therapies are efficacious for treating nicotine dependence. However, limited data exist on benefits of different NRTs and predictors of treatment outcome. This study compared the effectiveness of transdermal nicotine vs. nicotine lozenge for smoking cessation and identified predictors of treatment response.
Methods:
A randomized, open-label effectiveness trial was conducted at twelve medical sites participating in the National Cancer Institute's Community Clinical Oncology Program. The sample consisted of 642 treatment-seeking smokers randomized to twelve weeks of transdermal nicotine or nicotine lozenge.
Results:
Smoker characteristics were assessed at baseline, and 24-hour point prevalence abstinence confirmed with breath carbon monoxide (CO) was evaluated at end of treatment (EOT) and at a 6-month follow-up. There was a trend for higher quit rates for transdermal nicotine vs. nicotine lozenge at EOT (24.3% vs. 18.7%, p = .10) and 6-months (15.6% vs. 10.9%, p = .10). A logistic regression model of EOT quit rates showed smokers who preferred transdermal nicotine, were not reactive to smoking cues, and did not use nicotine to alleviate distress or stimulate cognitive function had higher quit rates on transdermal nicotine. A logistic regression model of 6-month quit rates showed smokers who preferred transdermal nicotine had higher quit rates on transdermal nicotine, and smokers who used nicotine to alleviate distress or stimulate cognitive processes had lower quit rates on nicotine lozenge.
Conclusions:
Transdermal nicotine may be more effective than nicotine lozenge for smokers who prefer transdermal nicotine and do not smoke to alleviate emotional distress or stimulate cognitive function.
Keywords: smoking cessation, nicotine replacement therapy, nicotine dependence, moderators
1.0 Introduction
Transdermal nicotine, the most widely used tobacco dependence treatment in the United States (Jonk et al., 2005; Pierce and Gilpin, 2002), increases smoking cessation rates vs. placebo (Fiore et al., 2008). Likewise, a relatively new nicotine replacement therapy (NRT), the nicotine lozenge, improves cessation rates vs. placebo (Shiffman et al., 2002a; Stead et al., 2008). However, only 20-25% of smokers who use transdermal nicotine or nicotine lozenges report abstinence 6-months after a quit date (Shiffman et al., 2002a; Stead et al., 2008), and 6-month NRT quit rates are ~10% in the context of over-the-counter use (OTC) (Fagerstrom et al., 1997).
Identifying pre-treatment smoker characteristics related to differential cessation outcomes between these NRTs may help smokers and clinicians select the NRT that provides the best chance for effectiveness. Transdermal nicotine and nicotine lozenges differ in terms of pharmacokinetics and, compared to transdermal nicotine, smokers can titrate dosing with the lozenge. Yet, no clinical trial has directly compared these NRTs or evaluated pre-treatment smoker characteristics as predictors of differential outcomes, which are notable research priorities (Fiore et al., 2008; Stead et al., 2008). Consequently, smokers have little basis on which to select an NRT and physicians have little empirical data on which to base NRT recommendations to their patients who smoke.
Several smoker-related characteristics may be related to differential NRT effectiveness. First, allowing smokers to select their preferred NRT may influence cessation outcome (Leischow et al., 1997); however, a lack of statistical power has prevented examination of interactions between NRT preference and outcome (Shiffman et al., 2003). Reactivity to smoking cues (i.e., degree to which smokers experience urges to smoke when encountering environmental or internal stimuli related to smoking) may also influence NRT effectiveness since acute dosing NRTs (i.e., lozenges) can be dose-titrated during cue exposure unlike transdermal nicotine. In one study, smokers with high cue reactivity showed reduced nicotine craving following cue exposure if they used nicotine gum (an acute dosing NRT like the lozenge) (Shiffman et al., 2003). Lastly, the degree to which smokers use nicotine to alleviate emotional distress or stimulate cognitive function (referred to as self-medicating) may influence NRT responsiveness. Smokers can increase nicotine dose with lozenges during exposure to situations that cause emotional distress or require cognitive enhancement. While self-medicating with nicotine to address cognitive and affective function is cited as important to consider when selecting a treatment for nicotine dependence (Evans and Drobes, 2009; Gehricke et al., 2007), no direct evidence links a smoker's self-medication level with response to NRTs.
This clinical trial compared transdermal nicotine to nicotine lozenge for treating nicotine dependence and evaluated NRT preference, cue reactivity, and self-medication as moderators of NRT effectiveness. This trial was designed as an effectiveness trial to represent “real-world” experience since both NRTs are available OTC.
2.0 Methods
2.1. Participants
Participants enrolled through the National Cancer Institute's Community Clinical Oncology Program (CCOP) coordinated by Fox Chase Cancer Center (FCCC). The CCOP conducts cancer treatment and prevention trials in community settings, thereby bringing potentially advanced interventions to communities that would not typically have access to such treatments. This trial was implemented through: FCCC (Philadelphia, PA), Geisinger Medical Center (Danville, PA), Hematology Oncology Associates of Central New York (Syracuse, NY), Howard University (Washington, DC), Louisiana State University (Shreveport, LA), Main Line Health System (Wynnewood, PA), Medical College of Georgia (Augusta, GA), Meharry Medical College (Nashville, TN), Mount Sinai Medical Center (Miami, FL), North Shore University Hospital (Lake Success, NY), SUNY Downstate Medical Center (Brooklyn, NY), and Virtua Health (Mt. Holly, NJ).
Participants were recruited through media advertisements for free smoking cessation treatment and through physician referrals. To be eligible, participants had to be able to communicate in English, be ≥ 18 years of age, plan to reside in the geographic area for ≥ 6 months, smoke ≥ 10 cigarettes on average/day, and be willing to defer use of other forms of smoking cessation treatments for 6 months. Participants were excluded if they had an allergy to adhesive tape or latex, had been diagnosed with cancer within the previous 5 years, were HIV positive, had taken an antidepressant within the past 6 months, were pregnant, nursing, or planning on becoming pregnant during the course of the study, or were taking bupropion, monoamine oxidase inhibitors, benzodiazepines, or anti-psychotics. Participants were screened for alcohol abuse using the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998), and were excluded if current alcohol dependence/abuse was identified.
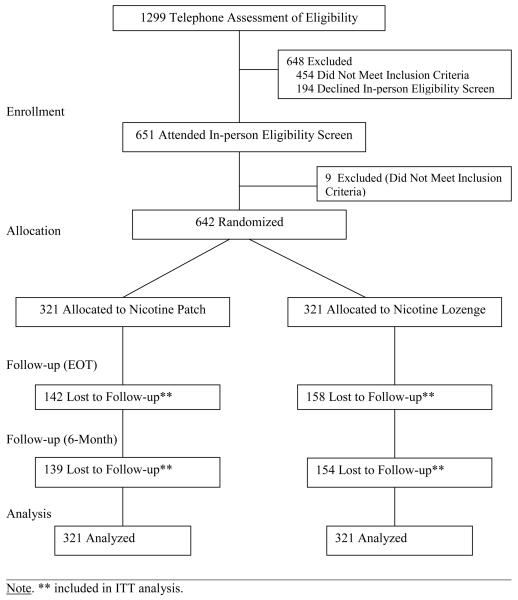
In total, 1299 individuals were screened for this trial over 4 years (Figure 1); 454 individuals were ineligible, 194 refused enrollment, and 651 were randomized. Nine individuals either withdrew from the study prior to treatment or were found to be ineligible after randomization and were removed from the intent-to-treat (ITT) sample. The final ITT sample was 642 (321/arm).
Figure 1.
CONSORT Diagram.
2.2. Study Procedures
This was an open-label, randomized, Phase 4, effectiveness trial of transdermal nicotine (Nicoderm CQ) vs. nicotine lozenge (COMMIT). Randomization was coordinated by FCCC and was stratified at each site. Procedures were standardized through training of site coordinators by FCCC personnel.
Participants contacting sites were screened for eligibility by site coordinators which included a history and physical. Eligible participants completed baseline measures and were randomized to an NRT; all participants received 5 individual smoking cessation counseling sessions. The initial counseling session, conducted in-person by the site coordinator, was about 60 minutes and focused on educating smokers about the benefits of cessation and instructing smokers on techniques for preparing for a designated quit date, which was set for 2 weeks following this session. On the quit date, participants received a second brief counseling session conducted over the phone by trained smoking cessation counselors at FCCC which focused on developing strategies to manage withdrawal symptoms. NRT was initiated at this session. Participants received 3 additional counseling sessions by phone from the FCCC counselor over the subsequent 4 weeks of the trial which focused on relapse prevention.
NRT was provided for 12 weeks. Participants randomized to transdermal nicotine received 12 weeks1 of treatment and the step-down procedure was used; treatment started with the 21mg dose for 6 weeks, followed by 14mg for 2 weeks, and 7mg for 4 weeks. Participants were asked to use 1 patch/day. Participants randomized to nicotine lozenge received 12 weeks of treatment; the 2mg dose was provided to those who said that they do not smoke their first cigarette of the day within 30 minutes of waking and the 4mg dose was provided to those who said that they smoke their first cigarette of the day within 30 minutes of waking. Participants were asked to use about 9 lozenges/day for the first 6 weeks of treatment, about 5 lozenges/day during weeks 7-9 of treatment, and about 3 lozenges/day during weeks 10-12 of treatment.
Smoking cessation rate was assessed at the end of treatment (EOT) and 6-months following the target quit-date. Participants were contacted by phone and a standard time-line follow-back measure of smoking was conducted (Brown et al., 1998). Participants who reported no smoking (not even a puff) for the 24 hours prior to the assessment time-point were asked to attend their site to provide a breath sample for verification. Consistent with guidelines (Hughes et al., 2003a), subjects who failed to complete assessments, failed to provide a breath sample, or provided a carbon monoxide (CO) sample ≥10ppm were considered smokers. Subjects who self-reported cessation and had a CO ≤ 10 were considered abstinent (Benowitz et al., 2002). NRT adherence and adverse events were assessed at EOT and 6-months.
2.3. Measures
2.3.1. Demographic Variables
Age, gender, education, race, and ethnicity were recorded for all participants.
2.3.2. Smoking Variables
Smoking history, such as age of initiation, number of years smoked, and nicotine dependence level measured by the 6-item Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) were assessed. Participant preference for the NRT they would receive in this trial (West et al., 2001) and past use of NRTs was measured.
2.3.3. Self-Medication
The 10-item Reasons for Smoking Scale (Horn and Waingrow, 1966) assessed use of tobacco to alleviate negative affect (e.g., when I feel uncomfortable or upset about something I light up a cigarette) and promote cognitive function, as done previously (Lerman et al., 1996). A total score was used by summing the items and a median split created low vs. high self-medication groups. This scale has been related to level of nicotine dependence and smoking behavior and has been shown to be a mediator between depression and smoking (Lerman et al., 1996; Lerman et al., 1998).
2.3.4. Cue Reactivity
The 20-item Temptations scale (Velicer et al., 1990) assessed reactivity to positive affect and social cues (e.g., at a bar or cocktail lounge), negative affect cues (e.g., when things are not going the way I want and I am frustrated), and habit cues (e.g., when I first get up in the morning). A total score was computed by summing items and a median split created low vs. high cue reactivity groups. This scale has been shown to predict relapse to smoking following treatment (Velicer et al., 1990).
2.3.5. Adherence
As done previously (Alterman et al., 1999), adherence to NRT was evaluated using self-report. Participants were asked at each session to report on the number of patches or lozenges used each day. Average number of patches used per week during the first two weeks and average number of lozenges used per day during the first two weeks was calculated as a measure of adherence similar to previous NRT trials (Lerman et al., 2004).
2.3.6. Side Effects
A checklist, similar to that used in previous NRT trials (Lerman et al., 2004), assessed the severity of common side effects related to transdermal nicotine and nicotine lozenges (e.g., skin irritation, mouth soreness). The severity of individual and total side effects reported two weeks following the initiation of NRT were compared across treatment arms.
2.3.7. Abstinence
The primary outcome was CO-confirmed 24 hour point prevalence abstinence at EOT and 6-month assessments (Hughes et al., 2003a). There was no significant difference across treatment arms in the rate of completion of follow-up assessments at EOT (χ2[4] = 3.96, p = .21) or at 6-months (χ2 [4] = 3.68, p = .45).
2.4. Statistical Analysis
Chi-square and ANOVA evaluated the relationship between treatment arm and baseline demographic and smoking variables. Chi-square was used to examine the relationship between treatment arm and 24-hour point prevalence abstinence at EOT and 6-months. Multivariate binary logistic regression models evaluated the effects of treatment arm, pre-treatment smoker characteristics, and interaction terms on EOT and 6-month quit rates. Demographic and smoking-related variables were included in regression models to control for the effects of these variables as covariates. Chi-square tests were used for statistically significant interactions to understand the nature of the interaction effect. Lastly, ANOVA evaluated differences across treatment arms in total and individual side effects.
3.0 Results
3.1. Description of Sample
Participant characteristics are shown in Table 1. Overall, a slightly larger proportion of the sample was female (57%) and over 35% were racial/ethnic minorities, including 12% Hispanic/Latino and 20% African American. The mean age of the sample was 44.7 years (SD = 12.3). Most participants were medium to highly addicted to nicotine (Mean FTND = 5.1, SD = 2.11). The mean number of cigarettes smoked per day was 20.3 (SD = 9.1). The average age that participants began smoking was 16.9 years (SD = 4.9), and the mean number of years smoking since initiation tobacco use was 26.7 (SD = 12.9). Treatment site and variables listed in Table 1 were not related to treatment arm assignment (all p's > .05).
Table 1.
Characteristics of Study Participants (N = 642)
| Variable |
Transdermal Nicotine (N = 321) Mean (SD) or N (%) |
Nicotine Lozenge (N = 321) Mean (SD) or N (%) |
|---|---|---|
| Sex | ||
| Male | 126 (39%) | 151 (47%) |
| Female | 195 (61%) | 170 (53%) |
| Race-Ethnicity | ||
| Black | 64 (20%) | 63 (20%) |
| White | 205 (64%) | 202 (63%) |
| Hispanic | 36 (11%) | 13 (4%) |
| Other* | 16 (5%) | 43 (13%) |
| Marital Status** | ||
| Single | 74 (23%) | 74 (23%) |
| Married | 145 (45%) | 160 (50%) |
| Divorced | 62 (19%) | 65 (20%) |
| Separated | 19 (6%) | 14 (4%) |
| Widowed | 20 (6%) | 8 (3%) |
| Income** | ||
| < $15,000 | 43 (13.5%) | 42 (13%) |
| $15,001-$30,000 | 50 (16%) | 48 (15%) |
| $30,001-$45,000 | 85 (27%) | 61 (19%) |
| $45,001-$60,000 | 43 (13.5%) | 52 (17%) |
| $60,001-$75,000 | 37 (12%) | 33 (11%) |
| > $75,000 | 56 (18%) | 79 (25%) |
| Education** | ||
| < 11 Years | 27 (9%) | 22 (7%) |
| High School Grad | 67 (21%) | 72 (23%) |
| Some College or Voc./Tech. | 150 (47%) | 141 (44%) |
| College Grad | 61 (19%) | 65 (20%) |
| Graduate Degree | 13 (4%) | 20 (6%) |
| Age (Years) | 44.7(12.7) | 44.8 (11.9) |
| Nicotine Dependence (FTND)** | ||
| Very Low (≤ 2) | 41 (13%) | 38 (12%) |
| Low (3-4) | 78 (25%) | 89 (28%) |
| Medium (5) | 58 (18%) | 62 (20%) |
| High (6-7) | 86 (28%) | 86 (28%) |
| Very High (≥ 8) | 49 (16%) | 37 (12%) |
| Number of Cigarettes/Day Past 30 Days** |
20.6 (8.9) | 20.1 (9.3) |
| Age Started Smoking (Years)** | 16.9 (5.1) | 16.9 (4.7) |
| Number of Years Smoked (Years)** |
26.7 (13.2) | 26.8 (12.5) |
| Number of Previous 24-hour Quit Attempts** |
6.1 (12.2) | 4.8 (8.9) |
| Longest Duration of Previous Quit Attempt (Days)** |
333.5 (764.4) | 368.0 (854.9) |
| Previous Use of Nicotine Patch | ||
| Yes | 60 (19%) | 61 (19%) |
| No | 261 (81%) | 260 (81%) |
| Previous Use of Nicotine Lozenge |
||
| Yes | 3 (1%) | 2 (1%) |
| No | 318 (99%) | 319 (99%) |
Note. There were no statistically significant differences across treatment arms (all p's > .05);
Indicates participants who reported Asian American, American Indian or Alaskan Native, Hawaiian or Pacific Islander, More than One Race, or Other;
Indicates missing data (< 18 participants).
3.2. Abstinence
The relationship between treatment arm and EOT abstinence rates approached significance. The quit rate at EOT was 24.3% for transdermal nicotine vs. 18.7% for nicotine lozenge (χ2[4] = 3.00, p = .10). At 6-months, 15.6% of participants on transdermal nicotine were abstinent vs. 10.9% of participants randomized to nicotine lozenge (χ2[4] = 3.01, p = .10).
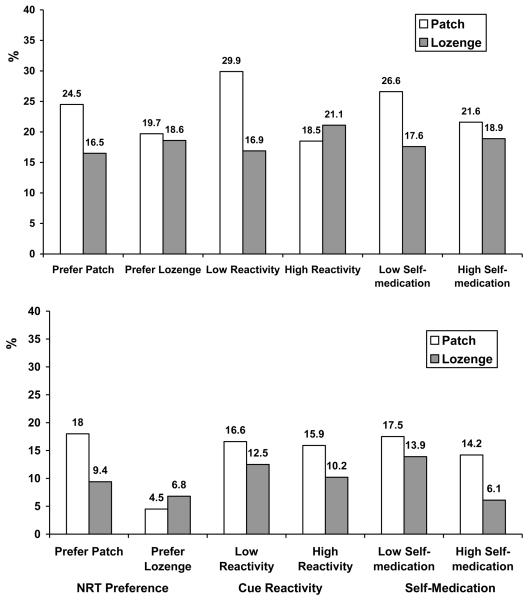
Table 2 and Figure 2 (top) show the results of the multivariate logistic regression for EOT quit rate. Controlling for sex, age, race/ethnicity, education, and level of nicotine dependence, two significant interaction effects were detected and one interaction effect approached statistical significance: treatment arm X NRT preference, treatment arm X cue reactivity, and treatment arm X self-medication. Participants who reported, at baseline, a preference for transdermal nicotine showed significantly higher EOT quit rates if they received the patch (24.5%), versus lozenge (16.5%; χ2 [1] = 4.1, p = .05); participants who reported, at baseline, a preference for nicotine lozenge showed similar EOT quit rates if they received nicotine patch (19.7%), versus nicotine lozenge (18.6%; χ2 [1] = 0.02, p = 1.0). Participants who exhibited, at baseline, low levels of cue reactivity showed significantly higher EOT quit rates if they received the patch (29.9%), versus nicotine lozenge (16.9%; χ2 [1] = 7.6, p = .008); participants who exhibited, at baseline, a high level of cue reactivity showed similar EOT quit rates if they received nicotine patch (18.5%), versus nicotine lozenge (21.1%; χ2 [1] = 0.3, p = .66). Participants who exhibited, at baseline, low levels of self-medication showed a trend for higher EOT quit rates if they received the patch (26.6%), versus nicotine lozenge (17.6%; χ2 [1] = 3.8, p = .06); participants who exhibited, at baseline, a high level of self-medication showed similar EOT quit rates if they received nicotine patch (21.6%), versus nicotine lozenge (18.9%; χ2 [1] = 0.3, p = .58).
Table 2.
Multivariate Logistic Regression Analysis Predicting EOT Smoking Cessation
| Predictor | OR | 95% CI | p |
|---|---|---|---|
| Sex (male = 0; 1 = female) | 0.67 | .38 - 1.01 | .05 |
| Nicotine Dependence | 0.65 | .52 - .81 | .001 |
| Race-Ethnicity (0 = other; 1 = white) | 0.56 | .35 - .92 | .02 |
| Age | 1.03 | 1.01 - 1.05 | .01 |
| Education | 1.16 | .90 - 1.50 | .24 |
| Treatment Arm (0 = patch; 1 = lozenge) | 0.48 | .15 - 1.52 | .21 |
| NRT Preference (0 = patch; 1 = lozenge) | 0.72 | .32 - 1.63 | .43 |
| Cue Reactivity (0 = low; 1 = high) | 0.36 | .15 - .85 | .36 |
| Self-medication (0 = low; 1 = high) | 1.70 | .72 - 4.01 | .23 |
| NRT Preference X Treatment Arm | 3.22 | 1.00 - 10.33 | .05 |
| Cue Reactivity X Treatment Arm | 4.77 | 1.52 - 15.00 | .007 |
| Self-medication X Treatment Arm | 0.38 | .12 - 1.21 | .10 |
Figure 2.
Abstinence Rates at EOT (Top) and 6-Months (Bottom) for Treatment Arm by NRT Preference, Cue Reactivity, and Self-medication.
Note. Sample sizes are as follows. For patch participants: preferred patch = 200; preferred lozenge = 66; low cue reactivity = 157; high cue reactivity = 151; low self-medication = 154; high self-medication = 162. For lozenge participants: preferred patch = 212; preferred lozenge = 59; low cue reactivity = 160; high cue reactivity = 147; low self-medication = 165; high self-medication = 148.
Table 3 and Figure 2 (bottom) show the results of the multivariate logistic regression for 6-month quit rate. Controlling for sex, age, race/ethnicity, education, and level of nicotine dependence, two significant interaction effects were found: treatment arm X NRT preference and treatment arm X self-medication. Participants who reported, at baseline, a preference for transdermal nicotine had significantly higher 6-month quit rates if they received the patch (18%), versus lozenge (9.4%; χ2 [1] = 6.5, p = .01); participants who reported, at baseline, a preference for nicotine lozenge showed similar 6-month quit rates if they received nicotine patch (4.5%), versus nicotine lozenge (6.8%; χ2 [1] = 0.29, p = .71). Participants who exhibited, at baseline, low levels of self-medication showed similar 6-month quit rates if they received the patch (17.5%), versus nicotine lozenge (13.9%; χ2 [1] = 0.78, p = .44); participants who exhibited, at baseline, a high level of self-medication showed significantly lower 6-month quit rates if they received nicotine lozenge (6.1%), versus nicotine patch (14.2%; χ2 [1] = 5.7, p = .02).
Table 3.
Multivariate Logistic Regression Analysis Predicting 6-Month Smoking Cessation
| Predictor | OR | 95% CI | p |
|---|---|---|---|
| Sex (male = 0; 1 = female) | 0.61 | .33 - 1.12 | .11 |
| Nicotine Dependence | 0.66 | .50 - .87 | .003 |
| Race-Ethnicity (0 = other; 1 = white) | 0.84 | .51 - 1.74 | .84 |
| Age | 1.04 | 1.01 - 1.06 | .005 |
| Education | 1.34 | .97 - 1.83 | .07 |
| Treatment Arm (0 = patch; 1 = lozenge) | 2.14 | .26 - 17.58 | .48 |
| NRT Preference (0 = patch; 1 = lozenge) | 1.11 | .33 - 3.79 | .87 |
| Cue Reactivity (0 = low; 1 = high) | 0.40 | .13 - 1.24 | .11 |
| Self-medication (0 = low; 1 = high) | 12.01 | 2.36 - 61.06 | .003 |
| NRT Preference X Treatment Arm | 5.75 | 1.00 - 33.09 | .05 |
| Cue Reactivity X Treatment Arm | 1.93 | .47 - 7.85 | .36 |
| Self-medication X Treatment Arm | 0.09 | .01 - .56 | .01 |
3.3 Adherence and Side Effects
During the first 2 weeks of treatment, participants randomized to nicotine lozenges used, on average, 4.8 lozenges/day (SD = 3.3); 27% of the sample randomized to lozenge used 7 or more lozenges per day during this time period. During the same period, participants randomized to transdermal nicotine used, on average, 5.8 patches/week (SD = 2.3); 72% of the participants randomized to transdermal nicotine used 6 or more patches/week for the first 2 treatment weeks.
There was no significant difference in mean total side effects across the treatment arms (F[1, 288] = .284, p = .60). However, significant differences were detected across treatment arms for four individual side effects. Transdermal nicotine participants reported significantly higher mean ratings for sleep problems (M = 1.55, SD = .85) and skin rash (M = 1.32, SD = .65), compared to nicotine lozenge (M = 1.33, SD = .74; M = 1.1, SD = .40; F [1,299] = 5.5, p = .02; F[1,299] = 14.6, p = .001). In contrast, nicotine lozenge participants reported significantly higher mean ratings for excess saliva (M = 1.14, SD = .47) and mouth soreness (M = 1.11, SD = .40), compared to transdermal nicotine (M = 1.05, SD = .27; M = 1.0, SD = .16; F [1,289] = 3.8, p = .05; F[1,299] = 6.7, p = .01). Lastly, there were 11 reported serious adverse events during the trial, but the frequency of events were similar across treatment arms (χ2[1] = .39, p = .73). In the patch arm, two participants reported a stroke, one reported a seizure, and one participant was killed in a car accident. In the lozenge arm, serious adverse events were: heart disease, ulcers, seizure, appendicitis, heart attack, cough, and abscessed tooth. All reports of serious adverse events were considered unrelated to treatment arm allocation given their occurrence either prior to or after treatment except for the report of ulcers (lozenge) and one case of stroke (patch).
4.0 Discussion
There was a non-significant trend for higher cessation rates at EOT and 6-months for transdermal nicotine versus lozenge. At EOT, smokers who expressed a preference for transdermal nicotine or reported low levels of reactivity to smoking cues or smoking to alleviate emotional distress or enhance cognitive function had higher quit rates with transdermal nicotine. At 6-months, smokers who expressed a preference for transdermal nicotine had higher quit rates with transdermal nicotine, whereas participants who exhibited high self-medication showed a significantly lower quit rate on nicotine lozenge. This first direct comparison of nicotine patch to nicotine lozenge suggests that transdermal nicotine yields a modest yet meaningful increase in quit rates in a real-world setting, especially for certain sub-groups of smokers.
A survey of tobacco control scientists indicated that patient preference for treatment modality should be considered in personalizing nicotine dependence treatment (Bader et al., 2009). However, little empirical data exist to support this recommendation. The ability to select a preferred NRT may reduce smoking rates vs. assigning NRT randomly (Leischow et al., 1997). Yet, allocation to one's preferred NRT was not found to influence response to NRT vs. random selection (West et al., 2001). From the present results, NRT preference may influence NRT response in the context of real-world use but only for transdermal nicotine, rather than to NRTs in general. Notably, more than two-thirds of the sample indicated a preference for transdermal nicotine, an imbalance that may have influenced the present outcome. Nevertheless, the relationship between a preference for transdermal nicotine and quit rate on transdermal nicotine was maintained at the 6-month outcome, indicating some sustainability for this relationship.
Previous research suggests that acute dosing NRTs (e.g., gum, lozenge), which can be dose titrated, may significantly reduce post-cessation craving among smokers who are responsive to smoking cues (Shiffman et al., 2003). Thus, we expected that smokers who were highly reactive to smoking cues would show higher quit rates on nicotine lozenge. We found, however, that smokers who reported low cue reactivity showed significantly higher quit rates on transdermal nicotine; level of cue reactivity did not affect response to nicotine lozenge. The inability for nicotine lozenge to increase quit rates among smokers reactive to cues may have been attributable to low compliance with this NRT; indeed, mean lozenge use in this study resembled the rate of participants considered “low-lozenge users” in a large placebo-controlled trial of lozenges, who showed significantly lower quit rates versus “high-lozenge users” (Shiffman, 2007). The increased effectiveness of nicotine patch among those low in cue reactivity may be explained by the congruence between this sub-group's more consistent smoking habits and the steady dosing of transdermal nicotine. This effect was only evident at EOT, suggesting that the influence of cue reactivity on response to transdermal nicotine may be confined to the treatment phase. Overall, these findings extend the literature on cue reactivity and response to NRT, including previous lab-based studies (e.g., Waters et al., 2004).
Finally, self-medicating with nicotine to alleviate affective distress or stimulate cognitive function is considered central to the effective treatment of nicotine dependence (Evans and Drobes, 2009; Gehricke et al., 2007). Since the dose of nicotine from lozenges can be titrated upwards during acute situations that challenge a smoker's affective state or cognitive abilities, we expected that those who reported high smoking self-medication would show higher quit rates on nicotine lozenge. However, smokers who were low on self-medication showed significantly higher EOT quit rates on transdermal nicotine; there was no significant difference at EOT between high and low self-medication for those treated with nicotine lozenge. The interaction effect for self-medication and treatment arm was significant at 6-months. Quit rate was significantly lower among those high in self-medication treated with lozenge vs. patch. The nicotine lozenge, therefore, may not be effective for smokers who report higher levels of smoking self-medication. Again, the lack of effect for the nicotine lozenge may be attributable to low compliance with this medication, whereas the effect for transdermal nicotine among those low in self-medication may be attributable to congruence between the steady dosing of transdermal nicotine and the consistent self-administration of tobacco among smoker's who do not smoke to enhance cognitive function or alleviate emotional distress.
These results should be viewed in the context of study limitations. First, this was an effectiveness trial, so the study methodology was devised to approximate “real-world” experience. Effectiveness trials emphasize study external validity vs. efficacy trials, which focus on internal validity. The eligibility criteria were minimized, the behavioral counseling was limited, and the treatments were unblinded. The present results should be compared to NRT effectiveness trials and studies of OTC NRT. The quit rates found in this trial converge with OTC NRT trials, which are far lower than efficacy trials (i.e., 1-11%; Hughes et al., 2003b). Second, no placebo was included in this trial, so it is not possible to determine the effectiveness of the NRTs relative to no treatment. The efficacy of these NRTs, relative to placebo, is already established, and this design was selected in accordance with an effectiveness design. Third, the overall reduced effectiveness of the lozenge and the lack of enhanced effectiveness of the lozenge in hypothesized sub-groups may have been due to low compliance among lozenge users. Only about one-quarter of the participants randomized to nicotine lozenge used the approximate recommended dose. We used the original COMMIT lozenge, which has since been enhanced with flavorings to enhance taste and palatability. The present results may be different if the new generation of nicotine lozenges were used. Notably, however, lozenge compliance was similar across participants who preferred the patch vs. the lozenge, across participants low vs. high in cue reactivity, and across participants low vs. high in self-medication (data not shown). Fourth, the nicotine patch and nicotine lozenge have different step-down procedures, which may have also contributed to variation in response to these NRTs. Finally, as is common in effectiveness trials, a substantial proportion of participants were lost to follow-up. However, an ITT approach was used to account for missing outcome data and the rate of missing outcome data compare to recent nicotine dependence clinical trials (Gonzales et al., 2006; Jorenby et al., 2006) and previous effectiveness studies with NRT (Hasford et al., 2003; Shiffman et al., 2002b).
This study provides, for the first time, data on the relative effectiveness in the real-world of nicotine patch and nicotine lozenge for treating nicotine dependence and information that can help smokers and clinicians select an appropriate NRT. Smokers with a preference for transdermal nicotine and smokers who are less reactive to smoking cues or less driven to smoke to augment cognitive function or alleviate distress may be good candidates for transdermal nicotine. Nicotine lozenges, in contrast, were less effective for all smokers and for sub-groups of smokers studied in this trial. Initially, we thought that differences in effectiveness between these NRTs could be attributable to the disparity in the pharmacokinetics between transdermal nicotine and nicotine lozenges, which allows for dose titration with the lozenge. However, expectations of smokers regarding the utility of these NRTs, the situational effectiveness of the lozenge, and compliance barriers with the nicotine lozenge may serve as alternative theoretical explanations for the differences in effectiveness for these NRTs. Additional research is needed to verify these results, including studies that prospectively select treatments based on individual smoker characteristics found in this trial to be related to NRT effectiveness. Such studies may yield methods to personalize treatment selection for nicotine dependence in order to enhance NRT effectiveness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The patch was provided for 12-weeks to equate with manufacturer recommendation of lozenge use.
References
- Alterman AI, Gariti P, Cook TG, Cnaan A. Nicodermal patch adherence and its correlates. Drug Alcohol. Depend. 1999;53:159–165. doi: 10.1016/s0376-8716(98)00124-0. [DOI] [PubMed] [Google Scholar]
- Bader P, McDonald P, Selby P. An algorithm for tailoring pharmacotherapy for smoking cessation: results from a Delphi panel of international experts. Tob. Control. 2009;18:34–42. doi: 10.1136/tc.2008.025635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol. Addict. Behav. 1998;12:101–112. [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict. Biol. 2009;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Tejding R, Westin A, Lunell E. Aiding reduction of smoking with nicotine replacement medications: hope for the recalcitrant smoker? Tob. Control. 1997;6:311–316. doi: 10.1136/tc.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Dolan Mullen P, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Clinical Practice Guideline. U.S. Department of Health and Human Services, Public Health Service; Rockville, MD: 2008. Treating tobacco use and dependence: 2008 Update. [Google Scholar]
- Gehricke JG, Loughlin SE, Whalen CK, Potkin SG, Fallon JH, Jamner LD, Belluzzi JD, Leslie FM. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine Tob. Res. 2007;9:S523–S536. doi: 10.1080/14622200701685039. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hasford J, Fagerstrom KO, Haustein KO. A naturalistic cohort study on effectiveness, safety and usage pattern of an over-the-counter nicotine patch. Cohort study on smoking cessation. Eur. J. Clin. Pharmacol. 2003;59:443–447. doi: 10.1007/s00228-003-0629-8. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Horn D, Waingrow S. Some dimensions of a model for smoking behavior change. Am. J. Public Health. 1966;56:21–56. doi: 10.2105/ajph.56.12_suppl.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob. Res. 2003a;5:13–25. [PubMed] [Google Scholar]
- Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tob. Control. 2003b;12:12–27. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonk YC, Sherman SE, Fu SS, Hamlett-Berry KW, Geraci MC, Joseph AM. National trends in the provision of smoking cessation aids within the Veterans Health Administration. Am. J. Manag. Care. 2005;11:77–85. [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Rigotti NA, Azoulay S, Watsky EJ. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Leischow SJ, Valente SN, Hill AL, Otte PS, Aickin M, Holden T, Kligman E, Cook G. Effects of nicotine dose and administration method on withdrawal symptoms and side effects during short-term smoking abstinence. Exp. Clin. Psychopharmacol. 1997;5:54–64. doi: 10.1037//1064-1297.5.1.54. [DOI] [PubMed] [Google Scholar]
- Lerman C, Audrain J, Orleans CT, Boyd R, Gold K, Main D, Caporaso N. Investigation of mechanisms linking depressed mood to nicotine dependence. Addict. Behav. 1996;21:9–19. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Lerman C, Caporaso N, Main D, Audrain J, Boyd NR, Bowman ED, Shields PG. Depression and self-medication with nicotine: The modifying influence of the dopamine D4 receptor gene. Health Psychol. 1998;17:56–62. doi: 10.1037//0278-6133.17.1.56. [DOI] [PubMed] [Google Scholar]
- Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain-McGovern J. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Ann. Intern. Med. 2004;140:426–433. doi: 10.7326/0003-4819-140-6-200403160-00009. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Gilpin EA. Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA. 2002;288:1260–1264. doi: 10.1001/jama.288.10.1260. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weille E, Herqueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction. 2007;102:809–814. doi: 10.1111/j.1360-0443.2007.01791.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Arch. Intern. Med. 2002a;162:1267–1276. doi: 10.1001/archinte.162.11.1267. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Rolf CN, Hellebusch SJ, Gorsline J, Gorodetzky CW, Chiang YK, Schleusener DS, Di Marino ME. Real-world efficacy of prescription and over-the-counter nicotine replacement therapy. Addiction. 2002b;97:505–516. doi: 10.1046/j.1360-0443.2002.00141.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shadel WG, Niaura R, Khayrallah MA, Jorenby DE, Ryan CF, Ferguson CL. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacol. (Berl) 2003;166:343–350. doi: 10.1007/s00213-002-1338-1. [DOI] [PubMed] [Google Scholar]
- Stead L, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2008;21:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict. Behav. 1990;15:271–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J. Consult. Clin. Psychol. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Nilsson F, Foulds J, May S, Meadows A. Individual differences in preferences for and responses to four nicotine replacement products. Psychopharmacol. (Berl) 2001;153:225–230. doi: 10.1007/s002130000577. [DOI] [PubMed] [Google Scholar]