Abstract
OBJECTIVE
We have previously demonstrated a widening in the mortality gap between rheumatoid arthritis (RA) subjects and the general population. To further understand this widening in mortality gap, we examined the contribution of rheumatoid factor (RF) positivity on overall mortality trends and cause specific mortality.
METHODS
A population-based RA incidence cohort (1955–1995, and aged ≥18 years) was followed longitudinally until death or 1/1/2006. The underlying cause of death as coded from national mortality statistics and grouped according to ICD-9/10 chapters was used to define cause specific mortality. Expected cause-specific mortality rates were estimated by applying the age-, sex- and calendar year-specific mortality rates from the general population to the RA cohort. Poisson regression was used to model the observed overall and cause specific mortality rates according to RF status, accounting for age, sex, disease duration, and calendar year.
RESULTS
A cohort of 603 subjects (73% female; mean age 58 years) with RA were followed for a mean of 16 years, during which 398 died. Estimated survival at 30 years after RA incidence was 26.0% in RF(+) RA subjects compared to 36.0% expected (p<0.001), while in RF(−) RA subjects, estimated survival was 29.1% compared to 28.3% expected (p=0.9). The difference between the observed and the expected mortality in the RF(+) RA subjects increased over time, resulting in a widening of the mortality gap, while among RF(−) RA subjects, observed mortality was very similar to the expected mortality over the entire time period. Among RF(+) RA subjects, cause specific mortality was higher than expected for cardiovascular (relative risk [RR] 1.50; 95% confidence interval [CI] 1.22, 1.83) and respiratory diseases (RR 3.49; 95% CI 2.51, 4.72). Among RF(−) RA subjects, no significant differences were found between observed and expected cause specific mortality.
CONCLUSION
The widening in the mortality gap between RA subjects and the general population is confined to RF(+) RA subjects and largely driven by cardiovascular and respiratory deaths.
Keywords: Survival, Rheumatoid Factor, Rheumatoid Arthritis, Epidemiology, Trends
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disorder associated with increased mortality. Some studies reported improvements in mortality in RA patients, suggesting that these improvements may, at least in part, be due to increased use of new anti-rheumatic treatment regimens and/or earlier diagnosis of RA in more recent years1–3. Yet, in order to fully understand mortality trends in RA, these must be examined considering the dramatic secular declines in overall mortality in the general population over the past decades4. For example, it may be reasonable to expect that mortality trends in the RA population follow similar patterns to those in the general population. Our recent analysis suggests that this is not the case5. RA subjects did not experience the same improvements in survival as their non-arthritic peers in the general population, resulting in a worsening in the relative mortality in more recent years. Earlier studies suggested that rheumatoid factor (RF) positivity had a significant impact on mortality in RA6–10.
In order to further understand the contributors of this widening of the mortality gap, we examined the impact of RF positivity on overall mortality trends and cause specific mortality.
METHODS
The study was conducted within the population of Rochester, Minnesota (MN). This population is well suited for the investigation of mortality trends because comprehensive medical records are available for all residents seeking medical care from all health care providers for over half a century11, 12. Using the resources of the Rochester Epidemiology Project, virtually all clinically recognized cases of RA and their RF status can be captured along with complete vital status information.
The study population consisted of a previously described inception cohort of all subjects with RA first diagnosed between January 1, 1955 and January 1, 1995 among Rochester, MN residents ≥ 18 years of age13, 14. All subjects fulfilled the 1987 American College of Rheumatology (ACR) criteria for RA15. Incidence date was defined as the first date of fulfillment of (four out of the seven) ACR classification criteria. Data on RF status was collected based on laboratory results available both at baseline and at any time during follow-up. Subject who sero-converted during follow-up were classified as RF(+). All subjects were followed longitudinally through their entire medical records until death or January 1st, 2006 (end of follow-up for the study).
All subjects (irrespective of residency status) were tracked nationally to ascertain vital status, and death certificates were obtained from the respective states for subjects who were deceased out of Minnesota. The underlying cause of death was coded from national mortality statistics and grouped according to ICD-9 and ICD-10 chapters. The cause-specific death rates from the Minnesota population from 1979 to 2002 were used for comparison.
Statistical Methods
The distribution of survival times following RA incidence date was estimated using the Kaplan-Meier method16. The log-rank test was used to compare survival between RF(−) and RF(+) RA subjects17. Overall and cause specific expected mortality rates were estimated by applying the age-, sex- and calendar year-specific mortality rates from the Minnesota white population (1979–2002) to the RA cohort18. At the time of the analyses, Minnesota life tables were available until the end of 2002. Therefore, we carried forward the 2002 expected mortality rates to 2006. We conducted additional analyses by truncating follow-up in RA cohort at the end of year 2002. This resulted in exclusion of 331 person-years of follow-up and 30 deaths in the RA cohort and had no impact on results reported in this manuscript.
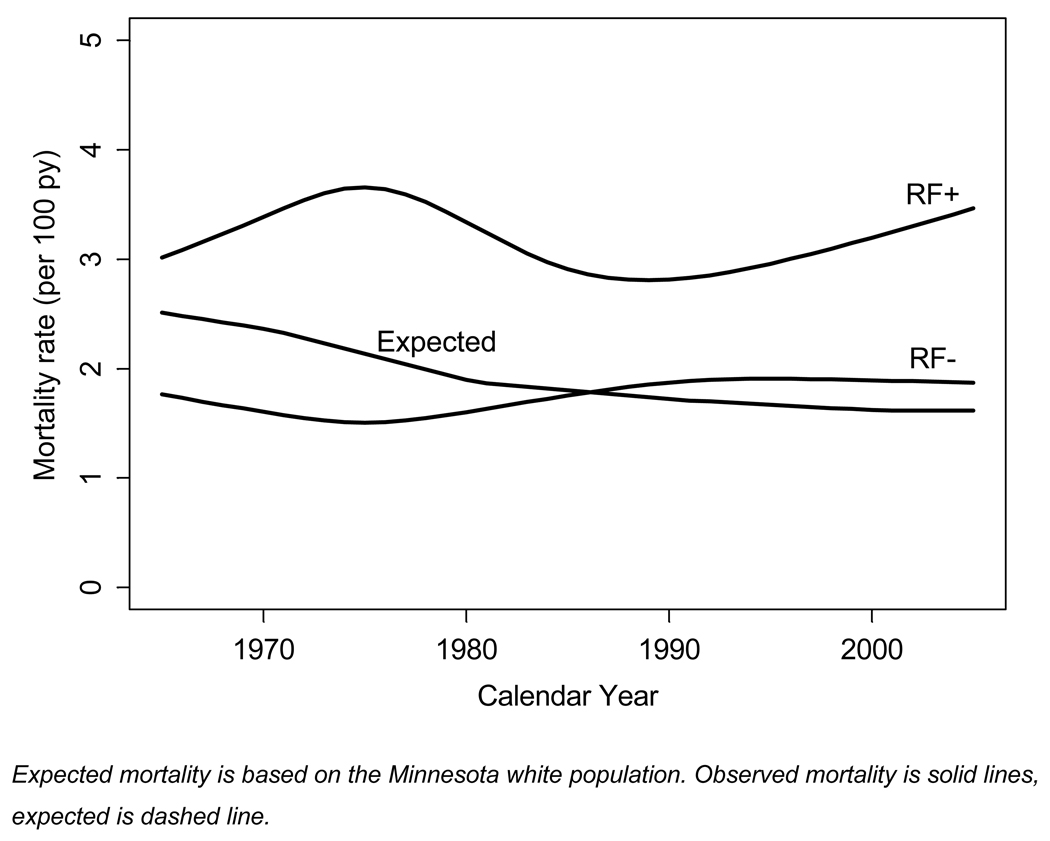
Ninety-five percent confidence intervals for the standardized mortality ratios (SMR) were calculated assuming that the expected rates were fixed and the observed rates followed a Poisson distribution19. Chapters of causes from ICD-9 and ICD-10 were combined for analyses of cause-specific mortality. Poisson regression was used to model the observed mortality rates 20 according to RF status, and accounting for calendar year of follow-up, age, sex and disease duration. In these models, natural splines were used to model the age and calendar year relationships to allow for non-linearity. Predicted mortality rates (shown in Figure 2) were direct standardized to the age and sex distribution of the entire RA cohort.
Figure 2.
Observed and expected mortality in rheumatoid factor positive (RF+) and rheumatoid factor negative (RF−) rheumatoid arthritis (RA) subjects
RESULTS
The overall study population comprised a cohort of 603 incident RA subjects (73% female; mean age 58 years). All subjects were followed for a mean of 16 years, during which 398 died. The distribution and characteristics of the study population by RF status are shown in Table 1. For the 393 RF(+) subjects, the mean age of the cohort at RA incidence was 56.8 years and 73.0% were women. Median follow-up for the RF(+) RA cohort was 13.9 years for a total of 6267 person-years. For the 210 RF(−) subjects (Table 1), the mean age at RA incidence was 60.2 years and 73.3% were women. Median follow-up for the cohort was 14.2 years for a total of 3410 person-years. At baseline, RF(+) subjects were younger (p=0.998), more likely to be smokers (0.012) and more likely have nodules (p=0.009). All other baseline characteristics were similar (Table 1). Only 32 (5.3%) RA subjects were lost to follow-up in our study and of these, 18 were RF(+).
Table 1.
Baseline Characteristics of the 603 incident subjects with Rheumatoid Arthritis (Rochester, MN).
| RF(+) RA | RF(−) RA | P value | |
|---|---|---|---|
| Number of Subjects | 393 | 210 | |
| Follow-up, median (years) | 13.9 | 14.2 | |
| Deceased subjects, n (%) | 260 (66.1) | 138 (65.7) | |
| Age at RA incidence, mean (years) | 56.8 | 60.2 | 0.008 |
| Female, n (%) | 287(73%) | 154 (73%) | 0.94 |
| Caucasian, n (%) | 384 (98%) | 207 (99%) | 0.47 |
| Current smoking, No. (%) | 124(32%) | 46 (22%) | 0.012 |
| Disease characteristics | |||
| Nodules, No. (%) | 30(8%) | 5 (2%) | 0.009 |
| Erosions, No. (%) | 25(8%) | 9 (6%) | 0.34 |
| Decalcification, No. (%) | 15(5%) | 9 (6%) | 0.71 |
| Large joint swelling*, No. (%) | 152(42%) | 81 (42%) | 1.00 |
| Hand/wrist swelling, No. (%) | 344(93%) | 185 (93%) | 0.84 |
large joints include elbow, shoulder, hip, and knee joints
During the follow-up period, 260 RF(+) RA subjects died, yielding an age-adjusted overall mortality rate of 4.94 (95% CI 4.32, 5.56) per 100 person-years. Overall mortality for RF(+) RA subjects was significantly higher than the mortality in the general population, with a standardized mortality ratio (SMR) of 1.81 (95% CI 1.60, 2.05). Among the RF(−) RA subjects, 138 subjects died during the follow-up period, yielding an age-adjusted overall mortality rate of 3.18 (95% CI 2.62, 3.74) per 100 person-years. Overall mortality in the RF(−) RA cohort was not different from the general population, with a SMR of 0.99 (95% CI 0.83, 1.17).
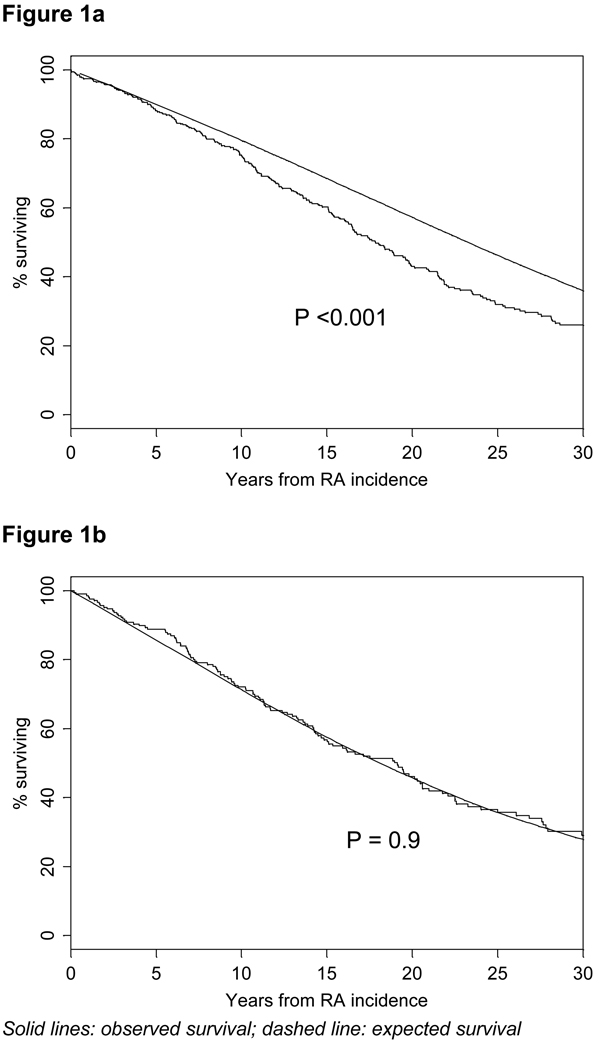
Figures 1a and 1b illustrate observed and expected survival in RF(+) (Figure 1a) and RF(−) RA (Figure 1b) subjects up to 30 years after RA incidence. Estimated survival at 30 years after RA incidence was 26.0% in RF(+) RA subjects compared to 36.0% expected (p<0.001), while in RF(−) RA subjects, estimated survival was 29.1% compared to 28.3% expected (p=0.9) (Figures 1a and 1b).
Figure 1.
Observed survival in rheumatoid factor positive rheumatoid arthritis (RF+ RA) (Figure 1a) and RF− RA (Figure 1b) subjects compared with expected survival based on the Minnesota white population.
Figure 2 illustrates mortality rates in RF(+) RA and RF(−) RA subjects and expected mortality based on the Minnesota white population over calendar years of follow-up. Over the entire time period, the overall mortality rate in RF(+) RA subjects was relatively constant at 3.0–3.2 per 100 person-years. The difference between the observed and the expected mortality rates in the RF(+) RA subjects increased over time, resulting in a widening of the mortality gap. In contrast, among RF(−) RA subjects observed mortality rate was very similar to the expected mortality over the entire time period (Figure 2).
We then examined the cause specific mortality in the two groups in order to examine whether increased mortality was due to specific causes of death. Among RF(+) RA subjects (Table 2), cause specific mortality was higher than expected for circulatory (ICD-9 390-459 and ICD-10 I00-I99) (SMR 1.50; 95% CI 1.22, 1.83) and respiratory diseases (ICD-9 460-519 and ICD-10 J00-J99) (SMR 3.49; 95% CI 2.51, 4.72). Cause specific mortality among RF(+) RA subjects was also higher than expected for diseases of blood and blood forming organs, infectious diseases, genitourinary and gastrointestinal system disorders, but the number of observed and expected events was small. In contrast, no significant differences in cause specific mortality were found for the RF(−) RA subjects when compared to expected cause specific mortality (Table 3). Because the number of observed and expected events was small (≤ 3) and no significant difference was apparent for infectious and parasitic diseases, endocrine disorders, diseases of blood and blood forming organs, diseases of digestive system, and diseases of genitourinary system, data was not included in the table.
Table 2.
Cause specific mortality among RF(+) RA subjects.
| Cause | No. Observed Events |
Expected No. Events |
SMR (95% Confidence Intervals) |
|---|---|---|---|
| All Causes* | 260 | 143.2 | 1.81 (1.60, 2.05) |
| Circulatory System | 97 | 64.7 | 1.50 (1.22, 1.83) |
| Respiratory System | 42 | 12.0 | 3.49 (2.51, 4.72) |
| Neoplasms | 39 | 35.0 | 1.12 (0.79, 1.52) |
| Musculoskeletal | 18 | 0.8 | 21.4 (12.7, 33.9) |
| Digestive System | 11 | 4.7 | 2.35 (1.17, 4.21) |
| Genitourinary System | 10 | 2.4 | 4.20 (2.01, 7.73) |
| Injury and Poisoning | 7 | 3.6 | 1.94 (0.78, 4.00) |
| Mental | 6 | 3.2 | 1.85 (0.67, 4.02) |
| Endocrine | 5 | 4.4 | 1.13 (0.37, 2.63) |
| Infectious and Parasitic | 5 | 1.2 | 4.10 (1.33, 9.56) |
| Hematological | 4 | 0.55 | 7.27 (1.98, 18.62) |
| Nervous System | 4 | 4.1 | 0.98 (0.27, 2.50) |
Includes 11 deaths of unknown cause and 1 death due to congenital abnormality
Table 3.
Cause specific mortality among RF(−) RA subjects.
| Cause Specific Mortality | No. Observed Events |
Expected No. Events |
SMR (95% Confidence Intervals) |
|---|---|---|---|
| All Causes* | 138 | 139.8 | 0.99 (0.83, 1.17) |
| Circulatory System | 75 | 64.5 | 1.16 (0.91, 1.46) |
| Respiratory System | 10 | 11.0 | 0.91 (0.44, 1.68) |
| Neoplasms | 23 | 24.5 | 0.94 (0.60, 1.41) |
| Injury and Poisoning | 5 | 3.0 | 1.65 (0.53, 3.83) |
| Mental | 5 | 3.0 | 1.67(0.54, 3.90) |
| Endocrine | 4 | 3.5 | 1.16 (0.32, 2.96) |
| Nervous System | 5 | 3.1 | 1.60 (0.52, 3.74) |
Includes 3 deaths of unknown cause, 3 digestive system, 3 genitourinary system, 1 musculoskeletal, and 1 death due to an ill-defined condition
We then examined the specific causes of death for circulatory and respiratory diseases since these causes were the major driver of excess deaths with the highest number of cases. Under circulatory causes, ischemic heart disease (ICD-9 410-414, ICD-10 I20-I25, including myocardial infarction), was the main driver of mortality in both RF(+) and RF(−) RA subjects, with 47 and 32 deaths, respectively. The SMR for ischemic heart disease among RF(+) RA subjects was 1.41; 95% CI 1.04, 1.88 and among RF(−) RA subjects was 1.00; 95% CI 0.68, 1.41. Among respiratory causes, chronic obstructive pulmonary diseases (ICD-9 490-494, 496 and ICD-10 J40-J47) were the most common with 19 and 2 deaths respectively among RF(+) and RF(−) RA subjects. The SMR for chronic obstructive pulmonary diseases among RF(+) RA subjects was 3.36; 95% CI 2.02, 5.24 and among RF(−) RA subjects was 0.49; 95% CI 0.06, 1.77.
DISCUSSION
In this study, we examined the overall and cause specific mortality trends in subjects with RF(+) and RF(−) RA as compared to mortality trends in the population at large. Our results indicate that the widening in the mortality gap between RA subjects and the general population is confined to RF(+) RA subjects only. In contrast, mortality trends for RF(−) RA subjects are essentially identical to the general population. Furthermore, excess mortality in RF(+) RA subjects is due not only to cardiovascular conditions but to various other disorders, including respiratory, hematologic and hematopoietic disorders, infectious diseases, genitourinary and gastrointestinal system disorders. Nevertheless, since cardiovascular and respiratory deaths constitute the majority of deaths (58%) in RA, their impact on excess mortality is greater.
Improvements in life expectancy in the general population during the last few decades have been attributed mainly to reductions in mortality due to cardiovascular diseases and unintentional injury21. Reductions in major cardiovascular risk factors and evidence-based medical therapies for primary and secondary prevention of heart disease were the major contributors of improvements in cardiovascular mortality22. Given these trends in the general population, there are at least two potential explanations for lack of improvements in mortality in RF + RA subjects. First, RA subjects may not have received the same level of primary and secondary prevention interventions as their non-arthritic peers. This is conceivable since under-diagnosis and under-treatment of comorbidities in RA patients has been previously reported, especially in the setting of unrecognized coronary heart disease and heart failure in RA subjects23–25. The second potential explanation is failure of the primary and secondary preventive interventions to provide the same level of beneficial effects in RF(+) RA subjects as in the general population or the RF(−) RA subjects. If true, this would suggest different biological pathways for cardiovascular disease in RF(+) and RF(−) RA subjects which would in turn require different approaches to prevention and treatment. Although earlier studies have reported on seropositivity as a significant predictor of mortality in RA6–10, none had addressed trends over time in comparison to the general population.
Pulmonary involvement is common in RA, including pleural disease, interstitial lung disease, nodular lung disease, bronchiolitis, pulmonary hypertension and small airway diseases26, 27. However, contribution of these conditions to excess mortality in RA is not well defined. In Finland, Sihvonen et al reported an SMR of 2.5 for respiratory diseases but also, acknowledged the difficulties in distinguishing deaths due to respiratory infections (pneumonia, bronchitis) by relying only on the underlying causes of death28. In a more detailed analysis of mortality data from England29, Thomas et al. examined cause-specific mortality in a large cohort of hospitalized subjects with RA which reported SMR for respiratory diseases to be 2.94 for males and 2.37 for females. With respect to individual causes of respiratory deaths, SMR for respiratory infections were 1.9 and 2.4, respectively for males and females. SMR for chronic obstructive pulmonary disease were 1.8 and 2.1 respectively for males and females. Our estimates for RF(+) RA patients are within the same range as these earlier studies. Our results indicate that chronic obstructive pulmonary diseases constitute almost half of all respiratory disorder mortality with a SMR of 3.36; 95% CI 2.02, 5.24 for RF(+) and 0.49; 95% CI 0.06, 1.77 for RF(−) RA subjects. However, changes in the classification of respiratory diseases make it difficult to interpret these findings. Importantly, conversion from ICD-9 to ICD-10 in 1999 affected both the total number of deaths assigned to the respiratory disease chapter (22% decrease) and the numbers assigned to individual causes within this chapter, such as pneumonia, chronic obstructive pulmonary diseases, influenza30. Therefore, these results must be considered only hypothesis generating and more in depth analyses are warranted to fully understand the contribution of specific respiratory conditions to excess mortality in RA.
Cause specific mortality in RF(+) RA subjects was higher than expected for various other diseases that are relatively well known comorbidities in RA, such as infections, gastrointestinal disorders (e.g. ulcers), diseases of the blood and blood forming organs and of the genitourinary system. Although the SMRs are as high as 6-fold for some comorbidities, the relative impact of these conditions on mortality is low due to the limited number of deaths they each contribute.
The observed differences in mortality trends between RF(+) and RF(−) RA subjects could possibly be explained by differences in smoking rates over time in the two groups. Poorer survival among the RF(+) RA subjects, relative to the general population could potentially be due to an increased prevalence of smoking and smoking-related diseases among RA subjects only if (a) the prevalence of smoking in RA patients remained stable, despite a significant decline in the general population, or (b) improvements in survival in the general population are largely due to decline in prevalence of smoking. We examined the prevalence of smoking over time in our RA cohort and compared it to population-based matched controls. Among RF(+) RA subjects, the prevalence of smoking declined from 35.2% between 1955–1984 to 22.0% after 1985. Among matched non-RA controls, the prevalence of smoking declined similarly, ie.: from 28.6% between 1955–1984 to 17.3% after 1985. Therefore, although the prevalence of smoking in RF(+) RA subjects is higher than non-RA control subjects, there was a proportionate decline in prevalence of current smokers in both RA and non-RA subjects. Furthermore, only 12% of the decline in overall mortality in the general population is attributed to decline in smoking prevalence22. Therefore, smoking is unlikely to be a major determinant of the observed trends.
Several potential limitations should be considered when interpreting our results. Misclassification of causes of death is a potential limitation when relying on only the underlying cause of death. A detailed analysis of individual causes of death, rather than ICD chapters, would provide more accurate information on contributors of excess mortality in RF(+) RA subjects. Yet, this study spans over a 50 years, a period that includes major switches in coding systems for mortality data. During this 50 year period, comparable cause specific mortality rates for the general population are available only for the underlying causes of death. Our findings may not be generalizable to non-white individuals because the Rochester population during the calendar years under investigation was predominantly white. The local population is socio-economically similar to American whites11 and the incidence of RA and cause specific mortality rates in local residents resemble that for other white populations31. Nevertheless, the generalizability of the study findings to populations with more diverse sociodemographic populations is unknown. Although follow-up for RA subjects extended to 2005, the incident RA subjects are limited to those diagnosed prior to 1995. Therefore, we cannot extrapolate our findings to subjects diagnosed after 1995 who may have been treated earlier, more aggressively and with newer medications. Although we report on RF positivity as the major determinant of mortality, RF status may simply be an indicator of pathological processes causally associated with mortality in RA subjects. Our population-based design, standardized approach for case ascertainment, long and complete follow-up of all subjects and availability of general population cause specific mortality rates throughout the entire study period are major strengths of this study.
In conclusion, the widening in the mortality gap between RA subjects and the general population is confined to RF(+) RA subjects and is driven largely by cardiovascular and respiratory disorders. The reason for the marked difference between mortality of RF(+) and RF(−) RA subjects should be addressed in future studies in order to understand the clinical and biological implications of RF status and to identify the therapeutic strategies that have the potential to reduce excess mortality in RA.
Acknowledgments
Funding Source: This work was supported in part by a grant from the National Institutes of Health, NIAMS (R01 AR46849) and the National Institutes of Health (AR-30582) US Public Health Service.
Footnotes
Financial Disclosures: None.
REFERENCES
- 1.Lindqvist E, Eberhardt K. Mortality in rheumatoid arthritis patients with disease onset in the 1980s. Ann Rheum Dis. 1999;58(1):11–14. doi: 10.1136/ard.58.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroot EJ, van Leeuwen MA, van Rijswijk MH, et al. No increased mortality in patients with rheumatoid arthritis: up to 10 years of follow up from disease onset. Annals of the Rheumatic Diseases. 2000;59(12):954–958. doi: 10.1136/ard.59.12.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornadal L, Baecklund E, Yin L, Granath F, Klareskog L, Ekbom A. Decreasing mortality in patients with rheumatoid arthritis: results from a large population based cohort in Sweden 1964–95. Journal of Rheumatology. 2002;29(5):906–912. [PubMed] [Google Scholar]
- 4.Jemal AWE, Hao Y, Thun M. Trends in the Leading Causes of Death in the United States, 1970–2002. JAMA. 2005 September 14;294(10):1255–1259. doi: 10.1001/jama.294.10.1255. 2005. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez A, Maradit Kremers H, Crowson CS, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007 Oct 29;56(11):3583–3587. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis & Rheumatism. 1994;37(4):481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 7.Turesson C, Jacobsson L, Bergstrom U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatology. 1999;38(7):668–674. doi: 10.1093/rheumatology/38.7.668. [DOI] [PubMed] [Google Scholar]
- 8.Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DPM. Mortality in early inflammatory polyarthritis: Cardiovascular mortality is increased in seropositive patients. Arthritis & Rheumatism. 2002;46(8):2010–2019. doi: 10.1002/art.10419. [DOI] [PubMed] [Google Scholar]
- 9.Heliovaara M, Aho K, Knekt P, Aromaa A, Maatela J, Reunanen A. Rheumatoid factor, chronic arthritis and mortality. Annals of the Rheumatic Diseases. 1995;54(10):811–814. doi: 10.1136/ard.54.10.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. Journal of Rheumatology. 1997;24:445–451. [PubMed] [Google Scholar]
- 11.Melton L. History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 12.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;Vol 30:819–834. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Gabriel SE, Crowson CS, Maradit Kremers H, et al. Survival in Rheumatoid Arthritis: A Population-Based Analysis of Trends over 40 Years. Arthritis & Rheumatism. 2003;48(1):54–58. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- 14.Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis & Rheumatism. 2002;46(3):625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 15.Arnett FCES, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 17.Peto R, Peto J. Asymptotically efficient rank invariant procedures (with discussion) Journal of the Royal Statistical Society, Series A. 1972;135:185–207. [Google Scholar]
- 18.Therneau TM. Rochester, MN: Mayo Clinic Department of Health Sciences Research; Technical Report Series No. 63. Expected Survival Based on Hazard Rates (Updated) 1999
- 19.Cox DR. Some simple approximate tests for Poisson variates. Biometrika. 1953;40:354–360. [Google Scholar]
- 20.McCullagh P, Nelder JA. Generalized linear models. Vol 1. New York: Chapman and Hall; 1983. [Google Scholar]
- 21.Health, Nutrition & Population statistics (NPStats) World Bank. Available at: http://devdata.worldbank.org/hnpstats/query/default.html.
- 22.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007 Jun 7;356(23):2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 23.Maradit Kremers HM, Bidaut-Russell M, Scott CG, Reinalda MS, Zinsmeister AR, Gabriel SE. Preventive medical services among patients with rheumatoid arthritis. J Rheumatol. 2003 Sep;30(9):1940–1947. [PubMed] [Google Scholar]
- 24.Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005 Feb 3;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 25.Davis J, Roger VL, Crowson CS, Maradit Kremers H, Therneau TM, Gabriel SE. Management and Outcomes of Heart Failure in Rheumatoid Arthritis. under review. 2007 [Google Scholar]
- 26.Matteson EL. Extra-articular features of rheumatoid arthritis and systemic involvement. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 3rd ed. Vol 1. New York: Mosby; 2003. pp. 781–792. [Google Scholar]
- 27.Young A, Koduri G, Batley M, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology. 2007 February 1;46(2):350–357. doi: 10.1093/rheumatology/kel253. 2007. [DOI] [PubMed] [Google Scholar]
- 28.Sihvonen S, Korpela M, Laippala P, Mustonen J, Pasternack A. Death rates and causes of death in patients with rheumatoid arthritis: a population-based study. Scand J Rheumatol. 2004;33(4):221–227. doi: 10.1080/03009740410005845. [DOI] [PubMed] [Google Scholar]
- 29.Thomas E, Symmons DP, Brewster DH, Black RJ, Macfarlane GJ. National study of cause-specific mortality in rheumatoid arthritis, juvenile chronic arthritis, and other rheumatic conditions: a 20 year followup study. J Rheumatol. 2003 May;30(5):958–965. [PubMed] [Google Scholar]
- 30.Brock A, Griffiths C, Rooney C. The impact of introducing ICD-10 on analysis of respiratory mortality trends in England and Wales. Health Stat Q. 2006 Spring;(29):9–17. [PubMed] [Google Scholar]
- 31.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheumatic Diseases Clinics of North America. 2001;27(2):269–281. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]