Abstract
Background
The primary objective is to develop an image-guided, non-invasive procedure to create or enlarge an atrial septum defect (ASD) for the treatment of neonates with Hypoplastic Left Heart Syndrome (HLHS) and an intact or restrictive atrial septum. Histotripsy is an innovative ultrasonic technique that produces non-thermal, mechanical tissue fractionation using high intensity ultrasound pulses. This paper reports the pilot in vivo study to create an ASD using extracardiac application of histotripsy in an open chest, canine model.
Methods and Results
In ten canines, the atrial septum was exposed to histotripsy by an ultrasound transducer positioned outside the heart. Ultrasound pulses of 6μs duration at a peak negative pressure of 15 MPa and a pulse repetition frequency of 3.3 kHz were generated by a 1MHz focused transducer. The procedure was guided and monitored by real-time ultrasound imaging. In nine of ten canines, an ASD was produced and shunting across the atrial septum was visualized. Pathology of the hearts showed ASDs with minimal damage to surrounding tissue. No damage was found on the epicardial surface of the heart or other structures.
Conclusions
Under real-time ultrasound guidance, ASDs were successfully created using extracardiac histotripsy in a live canine model. Although further studies in an intact animal model are needed, these results provide promise of histotripsy becoming a valuable clinical tool.
Keywords: Heart defects, congenital, septal defects, surgery, ultrasonics
Hypoplastic Left Heart Syndrome (HLHS) is a rare and complex congenital heart disease 1. If left untreated mortality approaches 95% by the first month of life 2. Surgical palliation of patients with a non-restrictive atrial septum at birth currently increases survival to approximately 80% at hospital discharge after stage I palliation 3-5. However, approximately 6% of HLHS patients have a completely intact atrial septum at birth, and up to 22% have a severely restrictive atrial septum 6, 7. Early survival for this sub-population of patients has been reported to range from 10-48% 7-9. To improve the survival of these neonates, a non-restrictive atrial septum defect (ASD) is required prior to surgical palliation.
Because emergent surgical septectomy usually requires cardiopulmonary bypass and its associated complications, percutaneous catheter approaches have been utilized. Current percutaneous treatment options include variations of catheter-based septostomies, such as Rashkind septostomy, Park blade septostomy, static balloon septoplasty, and atrial septal stent placement 7, 8, 10. These methods have demonstrated effectiveness in ASD creation and/or enlargement and improved survival of patients with HLHS and a restrictive or intact atrial septum 8, 11-13. However, in certain cases these approaches are limited by anatomic challenges and have increased potential for complications 8. Thus, an alternative non-invasive method such as high intensity focused ultrasound (HIFU) for creation of ASDs in this patient population may prove a beneficial innovation.
HIFU can ablate internal organs non-invasively from an extracorporeal position 14. For cardiac applications, researchers have investigated the feasibility of using extracardiac HIFU to ablate cardiac tissue to treat arrhythmias 15-17. Lesions were created inside the beating heart without damaging the intervening heart wall. The main mechanism of these HIFU studies is that continued or long ultrasound pulses can cause heating in the target tissue which results in thermal necrosis. However, ASD creation requires tissue removal that does not occur with HIFU thermal therapy.
Our approach also uses focused ultrasound, but the physical mechanism is different. It depends on precise control of acoustic cavitation to produce non-thermal, mechanical tissue fractionation and removal. This technique has been termed “histotripsy” to reflect the desired end results – soft tissue (“histo”) breakdown (“tripsy”) 7-9. Histotripsy uses very short pulses (≤20μs) at very high pressure to reliably initiate and maintain a cluster of microbubbles (acoustic cavitation) in the target tissue 18. Rapid bubble expansion, contraction and collapse ultimately fractionate tissue to acellular debris 19. Cavitation is a threshold phenomenon, wherein cavitation bubbles are only generated when the ultrasound pressure is above a certain threshold 20. Therefore, tissue damage is limited within the focal zone where the pressure is above the cavitation threshold. Histotripsy suppresses heating by separating short intense pulses with long cool-down time (at least 20 times longer than the pulse duration). The treatment is guided and monitored in real-time by standard ultrasound imaging 21. Previously, histotripsy has been demonstrated to create clearly demarcated perforations in excised porcine atrial tissue 22. This paper reports a pilot study to create an ASD by histotripsy in a live beating heart using an open-chest canine model.
Methods
Canine Surgery
The procedures described here have been reviewed and approved by the University Committee on Use and Care of Animals at the University of Michigan. A total of ten healthy adult 25 – 40 kg mongrel dogs underwent the surgery. The animals were pre-anesthetized with Acepromazine (3mg, IM) and anesthesia was induced with Thiopental (8-12 mg/kg, IV) following IV catheter placement. The dogs were intubated and placed on a surgical table. The animals were maintained on Isoflurane (1-3%) inhalation anesthesia for the duration of the procedure. Each animal was monitored by a pulse-oximeter. A sternotomy incision provided direct access to the heart.
Histotripsy Apparatus
Ultrasound pulses for histotripsy treatment were generated by an external high power ultrasound therapy transducer. The transducer was submerged in a heated, degassed external water bag, which was coupled to the pericardium with a thin plastic membrane and ultrasound coupling gel. To place the ultrasound focus on the atrial septum, the therapy transducer was moved in the water bag to adjust its stand-off distance to the pericardium.
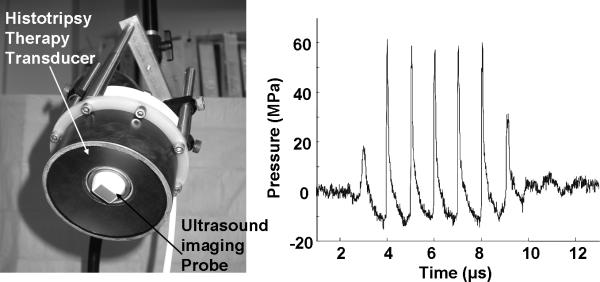
The therapy transducer was designed in our laboratory and fabricated by Imasonic, S.A., Besançon, France. The circular transducer has a center frequency of 1 MHz, a geometric focal length of 90 mm, an outer diameter of 100 mm, and a 40 mm inner hole for housing an ultrasound imaging probe (Fig. 1a). The transducer was driven by a high voltage amplifier built in-house. Ultrasound pressure waveforms of the therapy transducer were measured in degassed water using a fiber optic probe hydrophone 23. The peak negative and positive pressures used in the following experiment were 15 MPa and 61 MPa, respectively (Fig. 1b). Taking into account the attenuation caused by the approximately 1 cm thick atrial wall tissue in the pathway 24, the peak negative pressure reaching the atrial septum was estimated to be 12 MPa, resulting in a mechanical index of 12 25. This compares to the maximum mechanical index of 1.9 used in diagnostic ultrasound 25. The ultrasound pulses were 6 μs in duration (6 cycles at 1MHz) and separated by 300 μs (i.e., pulse repetition frequency = 3.3 kHz). These acoustic parameters have been used successfully to create perforations in the atrial wall tissue in vitro 22.
Fig. 1.
a) The 1 MHz histotripsy therapy transducer with an ultrasound imaging probe inserted in its central hole. b) Ultrasound pressure waveform of the histotripsy pulse at the transducer focus measured by a fiber optic hydrophone.
Histotripsy Treatment
The entire histotripsy procedure was guided by ultrasound imaging. The targeting and monitoring of the procedure was achieved by a 5 MHz phased array ultrasound imaging probe (GE VingMed System FiVe, GE, United Kingdom) inserted into the central hole of the therapy transducer. Another 10 MHz phased array imaging probe was used to achieve higher quality imaging of the atrial septum before and after the procedure by placing it directly on the epicardial surface of the heart.
The first step in the histotripsy procedure was to target the therapy focus to the atrial septum. Prior to the procedure, histotripsy pulses were applied to a water bath, resulting in a bubble cloud and a bright (hyperechoic) zone on a two-dimensional ultrasound image. The front center of the hyperechoic zone was marked as the focal position (Fig. 2). Even though the hyperechoic bubble zone generated in the water could be 7-8 mm wide, the bubble zone created in the blood on the tissue was measured to be 2-4 mm. The therapeutic transducer was then moved by a 3-axis positioning system (Parker Hannifin, Rohnert Park, CA, USA) to align the focus marker on the atrial septum surface in the right atrium. To verify the targeting accuracy, a small number of pulses were applied to produce a hyperechoic zone on the atrial septum.
Fig. 2.
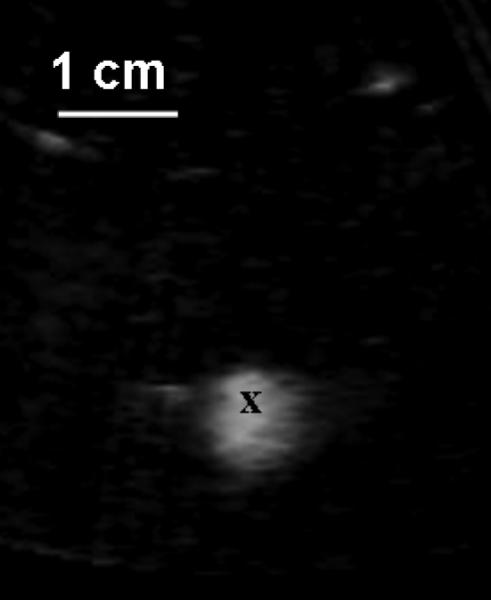
Prior to the treatment, a bubble cloud was generated in a water bath, which was shown as a hyperechoic zone on an ultrasound image and used for target localization. Ultrasound was delivered from the top to the bottom. The front center of the hyperechoic zone was marked as the focal zone (‘x’). The image was collected using a 5 MHz imaging probe inserted in the central hole of the therapeutic transducer.
Once the targeting was accomplished, histotripsy ultrasound exposures of 2 min were applied to the atrial septum at one time. After every 2 min exposure, the atrial septum was scanned by two-dimensional imaging and Doppler color flow mapping to identify whether blood flow was seen across the atrial septum. Repetitive 2 min exposures were delivered to the atrial septum until an ASD was generated.
Post-treatment Process
Within an hour after the ASD creation, animals were euthanized with a lethal dose of Pentobarbitol IV (100 mg/kg). The heart was extracted and fixed in a 10% formalin solution and dissected after a week of fixation. The atrial septum and the rest of the heart were first examined by gross evaluation and then processed with standard hematoxylin and eosin (H&E) staining for pathological evaluation.
Collection of Human HLHS Patient Data
To evaluate the relevance of the canine study to the applicability of histotripsy to human, important geometrical and anatomical data on human HLHS neonatal patients were collected. Available acoustic window position and size, the thickness, size and motion of the atrial septum, the thickness of overlying tissues in the ultrasound beam pathway, and the distance between these tissues were measured by analyzing patient echocardiography data. A total of 47 neonatal HLHS patients with an intact or restrictive (defined by a left atrial to right atrial pressure gradient of > 5mmHg and/or reversal of flow in the pulmonary veins) atrial septum who received ultrasound imaging at Washington University Children's Hospital over the past ten years were included. These neonatal patients weighed 2.5 – 3.9 kg (mean 3.4 kg) and aged 1-3 days. All patients underwent a complete two-dimensional and Doppler study using commercially available Ultrasound Imaging System (Philips Sonos 5500, Siemens Sequoia and GE Vivid 7). The above procedures were approved with full I.R.B approval at Washington University Medical School and the University of Michigan.
Results
In nine of the ten in vivo canine heart experiments, an ASD was successfully generated in 6-16 min of histotripsy treatment (Table 1). The ASD generation was verified using ultrasound imaging, color flow Doppler mapping, gross morphology, and pathological evaluation. All animals survived the immediate procedure and there were no complications such as pericardial effusions or sustained arrhythmias.
Table 1.
Result Summary (all measurements except exposure time are expressed in millimeters)
| Canine Number | Treatment Time | Atrial Septal Axial Motion | ASD |
Hemorrhage |
|||
|---|---|---|---|---|---|---|---|
| Ultrasound (Center)* | Pathology† | Pathology‡ | |||||
| RA side | LA side | RA side | LA side | ||||
| 1 | 6 min | 5.2 | 1.7 | 3.4 | 2.9 | 1.2 | 1.9 |
| 2 | 10 min | 17.1 | No ASD was created | ||||
| 3 | 8 min | 8.4 | 3.3 | 5.1 | 2.2 | 2.3 | 2.1 |
| 4 | 6 min | 10.3 | 5.0 | 5.7 | 2.4 | 4.2 | 2.2 |
| 5 | 10 min | 10.0 | 4.2 | 4.7 | 2.6 | 1.6 | 2.3 |
| 6 | 6 min | 8.7 | 3.0 | 6.6 | 3.0 | 1.6 | 2.0 |
| 7 | 6 min | 9.7 | 3.0 | 4.5 | 2.2 | 2.3 | 1.7 |
| 8 | 6 min | 6.5 | 3.2 | 6.2 | 2.5 | 3.2 | 2.4 |
| 9 | 16 min | 6.1 | 4.0 | No pathology result§ | |||
| 10 | 12 min | 8.1 | 3.6 | 4.1 | 3.3 | 4.0 | 2.6 |
| mean | 8.4 min | 8.1 | 3.4 | 5.0 | 2.6 | 2.6 | 2.2 |
| std | 3.4 min | 3.3 | 0.9 | 1.1 | 0.4 | 1.1 | 0.3 |
”ASD Ultrasound (Center)” column reports the width of the color jet in the center of the ASD measured on Ultrasound Doppler images.
”ASD Pathology RA side” and “LA side” columns show the widths of the acellular zone in the ASD for the right and left atrial surfaces, respectively, measured based on pathology analysis using H&E cross-section slides.
”Hemorrhage Pathology RA side” and “LA side” report the width of the eosinophilic and hemorrhagic rim surrounding the acellular ASD zone for the right and left atrial surfaces, respectively. This width is measured from the outermost edge of the acellular zone to the outermost edge of eosinophilic myocyte and hemorrhage zone on either side of the ASD. For each ASD, this measurement was performed for both sides of the ASD and using multiple slides across the same ASD, and the maximal value of the measurements is reported.
Pathological evaluation on the heart of dog #9 could not be obtained because it was filled with heart worms. However, ultrasound Doppler confirmed the ASD creation.
Ultrasound Imaging
During the procedure, generally a ~2-4 mm wide bubble cloud was created at the focus on the atrial septum. The histotripsy ultrasound pulses went through the right atrium to reach the atrial septum. When the procedure was progressing normally, the bubble cloud appeared as a temporally changing (twinkling) hyperechoic zone on the ultrasound image (Fig. 3a). The ultrasound image was disturbed by a small number of lines due to the interference from the short histotripsy ultrasound pulses, but the image quality was sufficient for real-time monitoring.
Fig. 3.
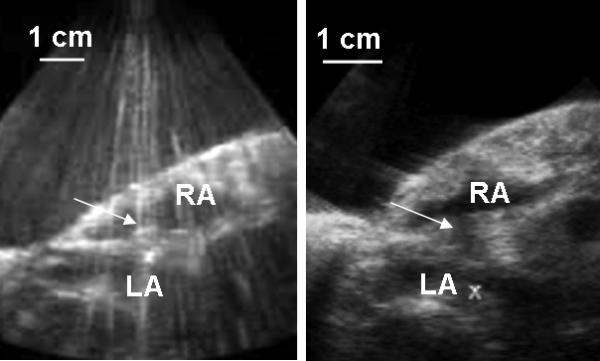
a) During the treatment, a bubble cloud was generated on the atrial septum by histotripsy, which showed as a temporally changing, hyperechoic (bright twinkling) zone on the ultrasound image (marked by the arrow). The ultrasound image has a few disturbed B-scan lines due to the interference from histotripsy pulses. b) Before the ASD creation, erosion of the atrial septum generated by histotripsy appeared as a dark groove in the atrial septum (marked by the arrow). The images were collected using a 5 MHz imaging probe. RA and LA indicate the right atrium and left atrium, respectively.
The atrial septum was constantly moving during the histotripsy procedure due to the heart contraction and respiration motion. Based on ultrasound image, the atrial septum motion was mostly along the axial direction of the therapy ultrasound beam and perpendicular to the atrial septum, measured to be 8.1 ± 3.3 mm (mean ± standard deviation (std), Table 1). The atrial septum also had motion in and out of the ultrasound image plane in the lateral direction of the ultrasound beam. Unfortunately the extent of lateral motion could not be evaluated precisely using this setup.
As the treatment progressed, the bubble cloud eroded the atrial septum layer-by-layer within the treatment zone. Before the completion of the ASD, the erosion appeared as a dark indentation in the atrial septum on the ultrasound image, with the opening facing the right atrium, which was the ultrasound entrance side (Fig. 3b). When the atrial septum was perforated, the ASD could be seen as a dark channel through the atrial septum by two-dimensional ultrasound imaging (Fig. 4). The ASD creation was further confirmed by color flow Doppler mapping revealing left to right shunting across the atrial septum (Fig. 4). The width of the color jet through the ASD was measured on Doppler images (3.4 ± 0.9 mm) and listed in Table 1
Fig. 4.
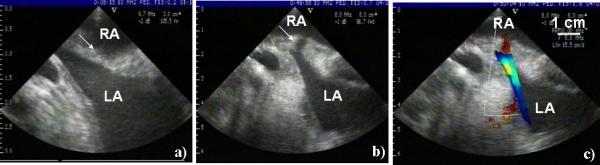
a) Before treatment ultrasound image of the intact atrial septum (marked by arrow). b) Representative ultrasound image of the ASD generated by histotripsy. The ASD appears as a dark channel through the atrial septum (marked by arrow). c) The Doppler color flow mapping showing a blood jet through the ASD. All images were collected using a 10 MHz imaging probe.
Gross Morphology
Hemorrhage indicated by dark colorization was observed on both sides of the treated atrial septae where an ASD was created. The location of the ASD was consistent with that indicated on ultrasound image. The diameter of the dark colorization area (including the ASD) was larger on the right atrial side (~ 4-10 mm in diameter) (Fig. 5a) and smaller on the left atrial side (~2-5 mm in diameter) (Fig. 5b). The cross-section of the atrial septae showed cone-shaped lesions extending all the way through the atrial septum, with a larger opening on the right atrial side compared to the left. These results are consistent with fact that the histotripsy ultrasound pulses reached the atrial septum from the right atrium.
Fig. 5.
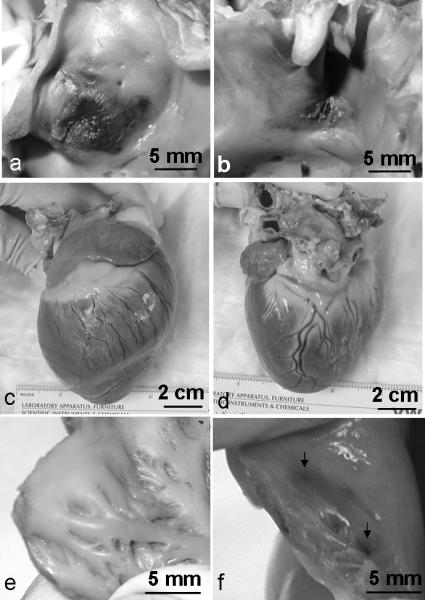
Representative gross morphology of canine ASD and the whole heart treated by histotripsy. a-b): The atrial septum on the RA side (a) shows more hemorrhage than that on the LA side (b). Histotripsy pulses reached the atrial septum from the RA side to the LA side. c-d): Gross morphology of the whole heart at the RV side (c) and the LV side (d). No gross damage to epicardial surface. e-f): Right atrial wall in the ultrasound pathway shows no damage in nine cases (e) and slight hemorrhage (indicated by arrows) in one case (f).
The whole hearts appeared intact from the outside, with no visible damage on the epicardial surfaces (Fig. 5c, d). After dissection, in 9 of 10 cases, no gross damage was observed inside the heart other than in the treatment area in the atrial septum (Fig. 5e). In only one of the nine successful cases, small areas of discoloration were found on the anterior wall of the right atrium (Fig. 5f), which was in the pathway of the histotripsy ultrasound pulses, just opposite the created ASD.
Pathology
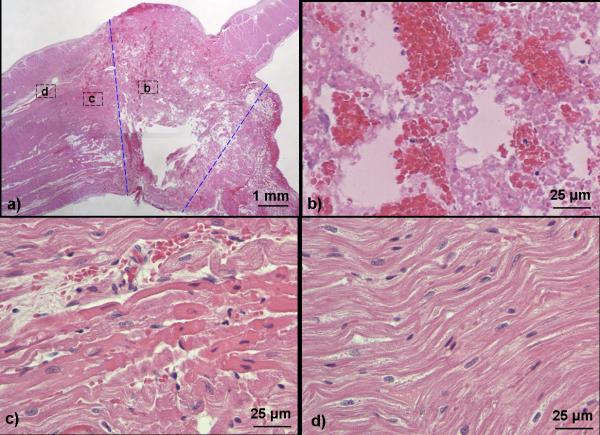
On the H&E stained slides, the central damage zone of the ASD contained acellular tissue debris and red blood cells (Fig. 6a-b). Other than the red blood cells, no discernable intact cellular structures remained. It appeared that variable degrees of thrombus formed within the ASD (Fig. 6b). As all ASDs were patent with blood flow up to the time of euthanasia confirmed by Doppler, the thrombus is likely post-mortem. However, the possibility of pre-mortem thrombus formation partially obstructing the defect cannot be excluded. This necrotic zone extended from the right atrial surface all the way to the left, suggesting a complete penetration through the atrial septum and creation of an ASD. The cross section of the acellular zone was generally cone shaped. The width of the acellular zone measured on H&E cross-section slides were 5.0 ± 1.1 mm on the right atrial surface and 2.6 ± 0.4 mm on the left (Table 1).
Fig. 6.
Representative H&E stained slides of the canine ASD lesion generated by histotripsy. a): a full view of an ASD lesion. The ASD region containing necrotic acellular debris is approximately outlined by dashed blue lines. The RA is on the top side of the atrial septum, and the LA is at the bottom. b-d): Magnified (90x) views of the atrial septum corresponding to the areas indicated in Fig 6a. b): Center of ASD showing complete fractionation of cellular structures, plus some trapped red blood cells with platelets and fibrin. c): A few hundred microns away from the acellular debris, hemorrhage and structurally intact myocytes with a more eosinophilic hue. d): Completely normal appearing myocytes beyond the eosinophilic myocytes.
Immediately outside the acellular zone was a rim of hemorrhage and structurally intact myocytes with a more eosinophilic hue in comparison to the normal myocytes, suggesting early contraction necrosis (Fig. 6c). These myocytes were likely affected by histotripsy. Beyond this rim of damaged cells were normal appearing myocytes with no further discernable damage (Fig. 6d). The width of the eosinophilic and hemorrhagic rim surrounding the acellular ASD zone was measured from the outermost edge of the acellular zone to the outermost edge of eosinophilic myocyte and hemorrhage zone on either side of the ASD. For each ASD, this measurement was performed for both sides of the ASD for the right and left atrial surfaces and using multiple slides across the same ASD, and the maximal value of the measurements is reported as the hemorrhage width in Table 1.
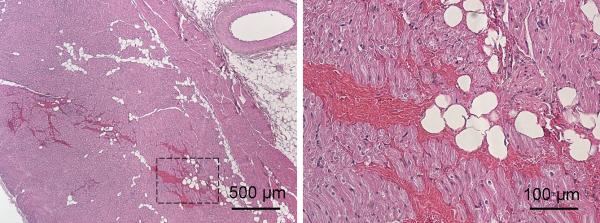
Histology of the atrial wall tissues in the ultrasound pathway appeared normal. In the one case where the anterior wall of the right atrial wall had slight discoloration on morphology, H&E slides showed intact and normal appearing myocytes with slight hemorrhage (Fig. 7).
Fig 7.
H&E stained slides of the right atrial wall that was slightly damaged by the treatment at 4x (a) and 20x (b) magnifications. b) is an expanded view of the area outlined by rectangular lines from a).
The failure case
In the one treatment where an ASD was not generated, ultrasound first went through the right ventricle and then the right atrium to reach the atrial septum. This pathway is different from our other treatments where ultrasound reaches the atrial septum through the right atrium directly. With the pathway used in the failure case, the atrial septum has a significantly larger motion with respect to the ultrasound beam (17.1 mm axial motion). In this case, the atrial septum was eroded, but not perforated. This case was also performed early in the series of experiments, and the treatment procedures were not well developed.
Collection of Human HLHS Patient Data
The measurements based on echocardiograph of 47 human HLHS neonatal patients with an intact or restrictive atrial septum showed a subcostal acoustic window with a length of 5.1 – 6.7 cm. From the subcostal window, ultrasound can access the atrial septum without bone or lung obstruction. To reach the atrial septum, the ultrasound beam would go through the abdominal skin, the right atrial wall, and sometimes a small portion of the liver. The ultrasound beam could be positioned perpendicular to the atrial septum. Measurements on the thickness and size of the atrial septum, the motion of the atrial septum, the thickness of overlying tissues in the ultrasound beam pathway, and the distance between these tissues are reported in Table 2.
Table 2.
Echocardiographic Measurements from 47 human HLHS Neonates with an Intact or Restrictive Atrial Septum (all measurement are obtained from the subcostal window and expressed in millimeters)*
| Measurements | Coronal View | Sagittal View |
|---|---|---|
| Skin to RA wall | 21.5 – 28.0 | 20.7-27.5 |
| RA wall to Atrial Septum | 11.2 – 18.5 | 11.4-17.5 |
| Atrial septum length | 11.2 – 17.4 | 11.5 – 17.2 |
| Atrial Septum thickness | 1.7 – 2.2 | 1.5-2.1 |
| Septum to LA wall | 7.7 – 10.4 | 8.8- 11.4 |
| Septal motion | 1 – 2 | 1-2 |
| RA free wall motion | 1 – 3 | 1-3 |
| LA free wall motion | 0 – 1 | 0-1 |
Measurements were obtained using echocardiography from subcostal coronal and sagittal views and reported in the “Coronal View” and “Sagittal View” columns, respectively. The subcostal window as selected, because a subcostal acoustic window exists in human neonates for ultrasound beam to access the atrail septum without obstructions from lungs or bones.
Discussion
This study has demonstrated the feasibility of creating an ASD using epicardial application of histotripsy. The treatment targeting and monitoring is guided in real time by ultrasound imaging. The ASD creation is precise and reproducible.
Ultrasound Imaging Guidance
The treatment targeting is guided by a temporally changing, hyperechoic bubble cloud zone on ultrasound image. The location of the bubble cloud indicates the focal position and is used to target the focus to a pre-selected tissue volume such as the atrial septum. The hyperechoic bubble cloud also provides real-time feedback for monitoring the initiation and progress of the entire histotripsy procedure. The location and size of the ASD creation evaluated by ultrasound Doppler matches those found by pathology. This real-time image feedback is unique for histotripsy and an essential feature for this non-invasive therapy technique.
Currently, the imaging probe is housed in the center hole of the therapy transducer which has a 9 cm focal length. Using this setup, the imaging probe needs to be 9 cm away from the target during the treatment. Due to the long stand-off distance, a lower frequency (5MHz) imaging probe was used. As a result, the imaging quality during the treatment is less than desired. To use a higher frequency imaging probe and improve the imaging quality, a therapy transducer with a shorter focal length is needed and currently being developed.
Treatment Accuracy
The ASD generated by histotripsy in this study has a diameter of 2.6 ± 0.4 mm on the LA surface and 5.0 ± 1.1 mm on the RA surface, with a ~1-4 mm rim of injury surrounding the ASD. We did not observe any clear trend on the dependence of the ASD diameter and the width of surrounding injury on the treatment time or motion of the atrial septum. However, only one acoustic parameter set was used here. Our previous in vitro experiments show that acoustic parameters can significantly affect the diameter and boundary condition of ASD 22, 26. For example, shorter pulse duration and lower duty cycle produce smaller ASD and smoother boundary. Further studies are planned to explore the acoustic parameter sets that can minimize the surrounding injury. In this study, the injury to the surrounding tissue may be underestimated, as one hour waiting time between the treatment completion and euthanasia of the animal may not be sufficient for all potential inflammatory effects and myocardial injury to become recognizable by light microscopy. We also plan to perform sub-acute and chronic studies to evaluate whether this injury is reversible.
Even though no clear trend was observed on the effect of septal motion on the surrounding injury, the septal motion probably contributes to generating the collateral damage by exposing surrounding septal tissue to the histotripsy ultrasound beam. In the canine model, the movement of atrial septum along the axial ultrasound beam is 8.1 ± 3.3 mm. There is also movement along the lateral beam estimated up to 4-5 mm. In comparison, the axial and lateral movement of human HLHS patients is measured to be approximately 1-2 mm. With less movement of the atrial septum in human neonates, it is expected that less collateral damage would be produced. At the same time, the critical structures in the neonatal heart are in closer proximity, and higher accuracy will be required.
To reduce the effect of septal motion and further increase the treatment accuracy, there are two possible solutions. First, one could synchronize the histotripsy treatment pulses with the patient's heart beat and respiration, with the pulses being applied only when the atrial septum moves to the same location. This synchronization approach is straightforward to achieve and has been used for HIFU epicardiac ablation 15, 17, but the treatment is prolonged as the time is wasted when the atrial septum moves out of that location. The second approach utilizes motion tracking to maximize the treatment accuracy while minimizing the treatment time. The therapy focal position is steered electronically to track the atrial septum movement in real time 27 and is technically very challenging. We have designed and constructed phased array therapeutic transducers that can electronically steer the transducer focus instantaneously. Other researchers have demonstrated that the movement of the atrial septum can be obtained based on ultrasound imaging 28 We hope to develop and incorporate a motion tracking algorithm in our future ASD applications.
In one case, slight hemorrhage was produced in the right free atrial wall. It occurred likely because some cavitation was induced on the atrial wall when the heart moved significantly. With less motion of the atrial septum in neonatal animals and motion tracking method, we hope to eliminate such events.
Embolization Risk
As histotripsy mechanically erodes the atrial septum, there is a concern that the eroded tissue debris may become hazardous emboli, especially in single ventricle physiology. We measured the size distribution of the tissue debris particles in a separate in vitro study 19. Measurements showed no particles greater than 60 μm in diameter and >80% of the particles were less than 6 μm. Such particles are unlikely to form hazardous emboli, as 100-μm mechanical filters have been successful at preventing embolization in catheter-based thrombolysis procedures 29. However, there may be a high risk for portions of newly developing mural thrombi at the site of tissue injury or necrotic tissue surrounding the ASD to embolize over time. To investigate the risk or embolization during treatment and over time, we plan to use transcranial Doppler during treatments and perform post-procedure diffusion weighted MRI and pathology of the brain, lungs, and kidneys in our future sub-acute and chronic studies.
Technical Challenges
In the canine experiments studied herein, open chest surgery was performed and the transducer was positioned outside the heart. Ultimately to treat neonatal patients non-invasively, the ASD will be created with an extracorporeal application. Since ultrasound does not penetrate dense bone or air found in lungs, an ideal acoustic window to access the atrial septum would have to avoid these structures. Based on echocardiography from neonatal patients, such an acoustic window exists subcostally. The atrial septal length in infants with HLHS ranges from 11.2-17.4 mm (Table 2). This approach, however, requires a smaller therapy transducer with a shorter focal distance than the current transducer. The shorter standoff distance would allow a higher frequency imaging probe and provide better imaging quality for treatment targeting and monitoring. We are in the process of developing a 6 × 4 cm therapy transducer with a 5 cm focal distance. This therapy transducer will house an 8-12 MHz imaging probe and should be suitable for human neonatal use.
In the open-chest model, ultrasound only has to penetrate ~1cm of the right atrial free wall. In the human neonatal patients, using the subcostal window, ultrasound needs to go through ~3-4 cm deep tissue to reach the atrial septum including abdominal skin, liver, and the right atrial free wall (Table 2). Multiple layers of tissue with varying acoustic properties would increase the ultrasound attenuation and aberration and affect the focal volume size and beam profile. Previous studies have shown that histotripsy can produce sufficiently high pressure through 3-4 cm overlying tissue and create accurate lesions in organs such as prostate 30. We have also started experiments in an intact piglet model, and early results show that histotripsy can produce accurate lesions through multiple layers of tissue, including neonatal ribs. We plan to continue our pre-clinical investigations and development of histotripsy in intact animal models.
Clinical Summary.
While previous studies have reported successful cardiac tissue ablation with high intensity focused ultrasound (HIFU) utilizing a mechanism of thermal necrosis, our study is the first to report targeted tissue fractionation and creation of an atrial septal defect (ASD) in a beating heart using extracardiac ultrasound through controlled acoustic cavitation, or histotripsy. In an open-chest canine model, histotripsy generated demarcated tissue destruction through the targeted region of atrial septum with no insult to other cardiac structures and modest damage to adjacent septal tissue. Continued advances in probe design and motion tracking algorithm should minimize this collateral damage. Although further studies are necessary to determine the efficacy and safety of this technology in an intact neonatal animal model, this initial report provides the first evidence that histotripsy may be a useful, minimally invasive clinical tool to create ASDs or other intra-cardiac communications in infants with congenital heart diseases.
Acknowledgements
The authors thank Dr. Kimberly Ives (D.V.M) in her assistance in performing the animal experiments. We thank Dr. Timothy L. Hall in his help with construction of the therapy transducer driving system.
Funding Sources
This research has been funded by grants from the National Institutes of Health R01-HL077629 and The Hartwell Foundation.
Footnotes
Subject Codes: 41) Pediatric and congenital heart disease, including cardiovascular surgery; 130) Animal models of human disease
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zhen Xu, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI
Gabe Owens, Department of Pediatric Cardiology, University of Michigan, Ann Arbor, MI
David Gordon, Department of Pathology, University of Michigan, Ann Arbor, MI
Charles Cain, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI
Achi Ludomirsky, Department of Pediatric Cardiology, New York University, New York, NY
References
- 1.Abu-Harb M, Hey E, Wren C. Death in infancy from unrecognised congenital heart disease. Archives of Disease in Childhood Fetal & Neonatal Edition. 1994;71:3–7. doi: 10.1136/adc.71.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fyler DC. Report of the New England Regional Infant Cardiac Program. Pediatrics. 1980;65:375–461. [PubMed] [Google Scholar]
- 3.Mahle W, Spray T, Wernovsky G, Gaynor J, Cark B. Staged reconstruction for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation. 2000;102:136–141. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 4.Tweddell JS, Hoffman GM, Mussatto KA, Fedderly RT, Berger S, Jaquiss RD, Ghanayem NS, Frisbee SJ, Litwin SB. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106:82–89. [PubMed] [Google Scholar]
- 5.Stasik CN, Gelehrter S, Goldberg CS, Bove EL, Devaney EJ, Ohye RG. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg. 2006;131:412–417. doi: 10.1016/j.jtcvs.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Forbess JM, Cook N, Roth SJ, Serraf A, Mayer JE, Jr, Jonas RA. Ten-Year Institutional Experience With Palliative Surgery for Hypoplastic Left Heart Syndrome : Risk Factors Related to Stage I Mortality. Circulation. 1995;92:262–266. doi: 10.1161/01.cir.92.9.262. [DOI] [PubMed] [Google Scholar]
- 7.Rychik J, Rome JJ, Collins MH, DeCampli WM, Spray TL. The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. Journal of the American College of Cardiology. 1999;34:554–560. doi: 10.1016/s0735-1097(99)00225-9. [DOI] [PubMed] [Google Scholar]
- 8.Gossett JG, Rocchini AP, Lloyd TR, Graziano JN. Catheter-based decompression of the left atrium in patients with hypoplastic left heart syndrome and restrictive atrial septum is safe and effective. Catheter Cardiovasc Interv. 2006;67:619–624. doi: 10.1002/ccd.20630. [DOI] [PubMed] [Google Scholar]
- 9.Glatz JA, Tabbutt S, Gaynor JW, Rome JJ, Montenegro L, Spray TL, Rychik J. Hypoplastic left heart syndrome with atrial level restriction in the era of prenatal diagnosis. Ann Thorac Surg. 2007;84:1633–1638. doi: 10.1016/j.athoracsur.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 10.Atz AM, Feinstein JA, Jonas RA, Perry SB, Wessel DL. Preoperative management of pulmonary venous hypertension in hypoplastic left heart syndrome with restrictive atrial septal defect. American Journal of Cardiology. 1999;83:1224–1228. doi: 10.1016/s0002-9149(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 11.Cheatham JP. Intervention in the critically ill neonate and infant with hypoplastic left heart syndrome and intact atrial septum. Journal of Interventional Cardiology. 2001;14:357–366. doi: 10.1111/j.1540-8183.2001.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 12.Vlahos AP, Lock JE, McElhinney DB, van der Velde ME. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: outcome after neonatal transcatheter atrial septostomy. Circulation. 2004;109:2326–2330. doi: 10.1161/01.CIR.0000128690.35860.C5. [DOI] [PubMed] [Google Scholar]
- 13.Pedra CA, Neves JR, Pedra SR, Ferreiro CR, Jatene I, Cortez TM, Jatene M, Souza LC, Assad R, Fontes VF. New transcatheter techniques for creation or enlargement of atrial septal defects. Catheter Cardiovasc Interv. 2007;70:731–739. doi: 10.1002/ccd.21260. [DOI] [PubMed] [Google Scholar]
- 14.Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans. Med. Electron. 1960;7:166–181. doi: 10.1109/iret-me.1960.5008041. [DOI] [PubMed] [Google Scholar]
- 15.Strickberger SA, Tokano T, Kluiwstra JU, Morady F, Cain CA. Extracardiac ablation of the canine atrioventricular junction using high intensity focused ultrasound. Circulation. 1999;100:203–208. doi: 10.1161/01.cir.100.2.203. [DOI] [PubMed] [Google Scholar]
- 16.Ninet J, Roques X, Seitelberger R, Deville C, Pomar JL, Robin J, Jegaden O, Wellens F, Wolner E, Vedrinne C, Gottardi R, Orrit J, Billes MA, Hoffmann DA, Cox JL, Champsaur GL. Surgical ablation of atrial fibrillation with off-pump, epicardial, high-intensity focused ultrasound: results of a multicenter trial. J Thorac Cardiovasc Surg. 2005;130:803–809. doi: 10.1016/j.jtcvs.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Otsuka R, Fujikura K, Abe Y, Okajima K, Pulerwitz T, Engel DJ, Muratore R, Ketterling JA, Kalisz A, Sciacca R, Marboe C, Yi G, Wang J, Homma S. Extracardiac ablation of the left ventricular septum in beating canine hearts using high-intensity focused ultrasound. J Am Soc Echocardiogr. 2007;20:1400–1406. doi: 10.1016/j.echo.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z, Raghavan M, Hall TL, Chang C-W, Mycek M-A, Fowlkes JB, Cain CA. High Speed Imaging of Bubble Clouds Generated in Pulsed Ultrasound Cavitational Therapy - Histotripsy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2007;54:2091–2101. doi: 10.1109/TUFFC.2007.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z, Fan Z, Hall TL, Winterroth F, Fowlkes JB, Cain CA. Size Measurement of Tissue Debris Particles Generated from Mechanical Tissue Fractionation by Pulsed Cavitational Ultrasound Therapy - Histotripsy. Ultrasound Med. Biol. 2009;35:245–255. doi: 10.1016/j.ultrasmedbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowlkes JB, Crum LA. Cavitation threshold measurements for micro-second length pulses of ultrasound. J. Acoust. Soc. Am. 1988;83:2190–2201. doi: 10.1121/1.396347. [DOI] [PubMed] [Google Scholar]
- 21.Roberts WW, Hall TJ, Ives K, Wolf JJS, Fowlkes JB, Cain CA. Pulsed cavitational ultrasound : a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. Journal of Urology. 2006;175:734–738. doi: 10.1016/S0022-5347(05)00141-2. [DOI] [PubMed] [Google Scholar]
- 22.Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, Cain CA. Controlled ultrasound tissue erosion. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2004;51:726–736. doi: 10.1109/tuffc.2004.1308731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons JE, Cain CA, Fowlkes JB. Cost-effective assembly of a basic fiber-optic hydrophone for measurement of high-amplitude therapeutic ultrasound fields. J. Acoust. Soc. Am. 2006;119:1432–1440. doi: 10.1121/1.2166708. [DOI] [PubMed] [Google Scholar]
- 24.Duck FA. Physical Properties of Tissue. Academic Press Inc.; 1990. [Google Scholar]
- 25.AIUM . Acoustic Output Labeling Standard for Diagnostic Ultrasound Equipment: A Standard for How Manufacturers Should Specify Acoustic Output Data, Revision 1. American Institute of Ultrasound in Medicine; Laurel, MD: 2008. [Google Scholar]
- 26.Xu Z, Fowlkes JB, Ludomirsky A, Cain CA. Investigation of intensity threshold for ultrasound tissue erosion. Ultrasound Med. Biol. 2005;31:1673–1682. doi: 10.1016/j.ultrasmedbio.2005.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pernot M, Tanter M, Fink M. 3-D real-time motion correction in high-intensity focused ultrasound therapy. Ultrasound Med. Biol. 2004;30:1239–1249. doi: 10.1016/j.ultrasmedbio.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Linguraru MG, Kabla A, Marx GR, del Nido PJ, Howe RD. Real-time tracking and shape analysis of atrial septal defects in 3D echocardiography. Acad Radiol. 2007;14:1298–1309. doi: 10.1016/j.acra.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eskandari M. Cerebral embolic protection. Semin Vasc Surg. 2005;18:95–100. doi: 10.1053/j.semvascsurg.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Lake AM, Hall TL, Kieran K, Fowlkes JB, Cain CA, Roberts WW. Histotripsy: minimally invasive technology for prostatic tissue ablation in an in vivo canine model. Urology. 2008;72:682–686. doi: 10.1016/j.urology.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]