Abstract
Diabetic nephropathy (DN) is characterized by a plethora of signaling abnormalities that together ultimately result in the clinical and pathologic hallmarks of DN, namely progressive albuminuria followed by a gradual decline in glomerular filtration rate leading to kidney failure, and accompanied by podocyte loss, progressive glomerular sclerosis and, ultimately, progressive tubulointerstitial fibrosis. Over the past few years, the general understanding of the abnormalities in signaling pathways that lead to DN has expanded considerably. In this review, some of the important pathways that appear to be involved in driving this process are discussed, with special emphasis on newer findings and insights. Newer concepts regarding signaling changes in bradykinin, mTOR, JAK/STAT, MCP-1, VEGF, endothelial nitric oxide synthase, activated protein C and other pathways are discussed.
Keywords: diabetes, glomerulus, matrix proteins, signaling
Diabetic nephropathy (DN) is usually manifested clinically by gradually worsening albuminuria, followed by a decline in glomerular filtration rate, which over years or decades leads to end-stage kidney disease in many patients with Type 1 or Type 2 diabetes. The pathologic correlates of this process are glomerular podocyte damage and loss [1], followed by the gradual, inexorable scarring of the renal glomerulus and then a similar fibrosing process in the tubulointerstitial region. A myriad of abnormalities in signaling pathways have been described that may combine to produce these pathologic correlates of DN. Many studies have attempted to elucidate the molecular signaling mechanisms that lead to DN so that effective therapies and preventative strategies might be developed. Through these efforts the general understanding of the pathogenic signaling factors that lead to progressive DN has expanded considerably over the past decade.
Many cell types in the kidney are involved in progressive DN
Signaling abnormalities that directly enhance mesangial cell matrix protein expression or reduce matrix metalloproteinase expression have been clearly implicated in DN and have been the focus of many studies over the years. More recent reports have indicated that signaling abnormalities may contribute to podocyte damage and loss, which leads directly to glomerulosclerosis [2], and that podocyte loss appears to be a requisite early event in DN [1]. Indeed, podocyte loss in humans with Type 2 diabetes is as good a predictor of progressive nephropathy as is mesangial expansion [3]. Presumably, signals to the mesangium from damaged podocytes, or the hemodynamic factors triggered by podocyte loss, provide stimuli to mesangial cells to react by increases in extracellular matrix (ECM) synthesis or decreases in ECM degradation. Similarly, attention to glomerular endothelial cells in the pathogenesis of glomerulosclerosis has been revived in the last few years. A growing number of models of endothelial dysfunction have resulted in diabetic pathology [4-8], suggesting substantial signaling crosstalk between endothelial and mesangial cells, although there have been few studies documenting relevant signaling abnormalities. Finally, proximal tubule cells, as well as nontubular cells, in the tubulointerstitium develop signaling abnormalities that probably contribute to progressive fibrosis, and although controversy remains, there is evidence that altered proximal tubular cell signaling induces epithelial-to-mesenchymal transition in DN [9].
Since signaling abnormalities in multiple cell types in the kidney appear to contribute to the pathogenesis of DN it is difficult, if not impossible, to establish a hierarchy of involved signaling pathways. Moreover, interactions between the different kidney cell types add further complexity. Finally, changes in both intercellular and intracellular signaling that evolve slowly during the years and decades, over which the disease process unfolds, may be of huge significance and this issue will be critical for understanding how the panoply of signaling abnormalities plays out to induce disease. Despite these complexities the current review will take a more reductionistic and somewhat artificial approach by concentrating on specific signaling abnormalities in individual cell types, with special concentration on signaling abnormalities that have been elucidated during the past several years. In this process we will give short shrift to altered signaling in response to advanced glycation end products (AGEs), reactive oxygen species (ROS), and protein kinase C (PKC) isoforms, as these are all specifically covered by other articles in this issue.
Mesangial cell signaling abnormalities
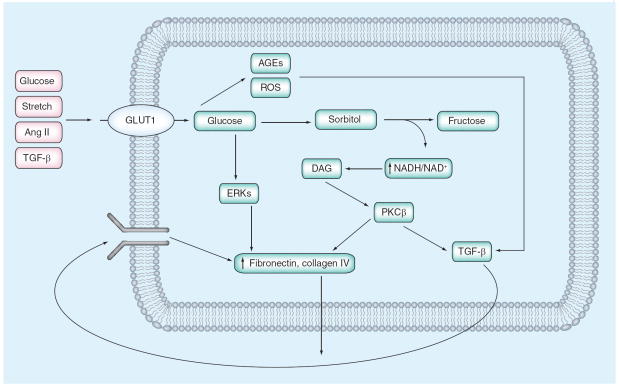
During the 1990s, most studies on signaling abnormalities in DN focused on the role of the mesangial cell in the production of ECM proteins. From these studies a general consensus emerged regarding major signaling mechanisms involved in this process. In this consensus view, high extracellular glucose induces an increase in glucose uptake via the facilitative glucose transporter, GLUT1 [10,11]. The resultant enhancement in glucose metabolic flux leads to activation of a number of metabolic pathways that result in increased AGE and ROS generation [12-14]. In turn, these activate a number of signaling pathways that lead to enhanced ECM production directly via PKC-β stimulation [15,16] of AP-1 transcriptional activation, ERK pathways and, critically, TGF-β1 synthesis [17,18], which in an autocrine and paracrine fashion stimulates its signaling pathways to stimulate ECM protein synthesis (Figure 1). These responses triggered by TGF-β1 appear to be the final common pathway by which mesangial fibrosis occurs. Over the past few years, data from human DN and from animal models of DN have led to an understanding that other, previously unappreciated, signaling pathways may also contribute critically to mesangial cell abnormalities in DN. Some of these more newly appreciated signaling abnormalities are discussed below.
Figure 1. Simplified model of ‘classic’ mesangial cell signaling abnormalities in early diabetic nephropathy.
High extracellular glucose (and a number of other external signals) leads to increased mesangial cell glucose uptake via enhanced expression of the facilitative glucose transporter, GLUT1, which activates metabolic pathways that result in increased ROS and AGE generation. This in turn activates a number of signaling pathways that augment extracellular matrix production directly via PKC-β stimulation of AP-1 transcriptional activation, ERK pathways and, critically, TGF-β1 synthesis, which stimulates its signaling pathways in an autocrine and paracrine fashion to further enhance extracellular matrix protein synthesis.
AGE: Advanced glycation end product; Ang II: Angiotensin II; DAG: Diacylglycerol; ERK: Extracellular signal-related kinase; PKC: Protein kinase C; ROS: Reactive oxygen species.
Reduced bradykinin signaling
An association between the onset and progression of Type 1 DN in humans and the D allele of the angiotensin converting enzyme (ACE) gene has been reported by several groups [19,20]. In addition, ACE inhibitors and angiotensin receptor blockers are mainstays of renal protection in human DN. However, using knockout and knockin mouse models and computer modeling, Smithies’ group has found that substantial changes in ACE gene dose lead to only modest increases in ACE with minimal effects on blood pressure and angiotensin II levels but, interestingly, led to substantial decreases in bradykinin, suggesting that perhaps bradykinin rather than angiotensin II was more important in renal responses in diabetes [21,22]. Therefore, this group studied the contribution of targeted deletion of the bradykinin 2 receptor on the evolution of DN in Akita mice on a C57BL/6 background [23].
In this model, diabetic homozygous bradykinin 2 receptor knockout mice developed profound mesangial sclerosis that resembled glomerular changes of human diabetic glomerulosclerosis. There were no changes in the glomerular endothelial cells or podocytes. Although the mechanism of this increased glomerulosclerosis phenotype remains unclear, there is normally a high level of bradykinin 2 receptor expression in mesangial cells, and knockout of these receptors was associated with enhanced renal expression of several genes involved in progressive glomerulosclerosis, including TGF-β1, CTGF, a TGF-β effector, and p53 [24]. Although these mice have substantial increases in bradykinin 1 receptors [23], this provided no protection from the effects of bradykinin 2 receptor gene deletion. Similar effects to bradykinin 2 receptor knockout were found in diabetic rats treated with a specific nonpeptidic bradykinin 2 receptor antagonist [25]. This agent reversed almost all the salutary effects of ACE inhibitors on albuminuria, glomerular ERK and TGF-β signaling pathways and glomerular gene-expression changes. It also enhanced oxidative stress in glomeruli from treated diabetic rats. In other studies, bradykinin signaling has been found to inhibit IGF-1 activation of Erk 1 and 2 in cultured mesangial cells [26]. Hence, activation of bradykinin 2 receptors in mesangial cells may dampen activation of ERK- and TGF-β-dependent profibrotic pathways in diabetes and help prevent progression of diabetic glomerulosclerosis.
Reduced nitric oxide signaling
As will be further described under the section on endothelial cell signaling, there appears to be reduced nitric oxide (NO) availability in diabetic glomeruli in humans with DN [27]. Moreover, one of the best experimental animal DN models is the diabetic eNOS-/- mouse [6,7,28-30]. While NO signaling in mesangial cells is complex and incompletely understood, it appears that NO produced in high amounts can stimulate redox signaling and cause downstream damage to cells, but when coupled with soluble guanylate cyclase actually leads to prosurvival and antifibrotic effects in mesangial cells [31]. A very recent report has found that NO suppresses expression of a profibrotic factor known as secreted modular calcium binding protein 1 (SMOC-1) [31]. SMOC-1 was shown to stimulate TGF-β and CTGF expression in mesangial cells. Although these findings need to be confirmed and extended, this work strongly suggests that SMOC-1 is an important mediator of profibrotic factors in the mesangial cell. While SMOC-1 has not been studied in DN, it certainly may play a role in triggering TGF-β-mediated profibrotic responses when either endogenous mesangial cell NO production, largely from inducible NO synthase (iNOS), or exogenous NO delivery, is reduced. Presumably, much of the latter would occur due to decreases in endothelial NOS (eNOS) activity in adjacent endothelial cells.
JAK/STAT pathway activation
Many growth factors and agonists, including angiotensin II, act via Janus kinase (JAK)/signal transducers and activation of transcription (STAT) signaling pathways and, therefore, these pathways may be important in the glomerular response to diabetes. Marerro’s group has shown that high glucose levels and diabetes in rodent models leads to tyrosine phosphorylation of JAK2, which, in turn, phosphorylates and activates the transcription factors, STAT1 and STAT3 [32,33]. In cultured mesangial cells, JAK2 activation was shown to mediate collagen IV and fibronectin production, TGF-β activation and cell growth due to angiotensin II administration or exposure to high glucose concentrations [32,34]. Importantly, at least some of the salutary effects of angiotensin receptor blockade and HMG-CoA reductase inhibitor treatment in high glucose conditions and rodent models of DN were mediated by JAK2-inhibition [33,35]. The effects of high glucose and diabetes may be mediated by enhanced production of ROS, as incubation of cultured mesangial cells in high glucose was found to enhance phosphorylation and hence activation of JAK2 and the downstream substrates of JAK2: STAT1, STAT3 and STAT5A/B [33,36]. There has been no report yet on the effects of JAK2 inhibitors on diabetic glomerular fibrosis or tubulointerstitial fibrosis.
We have recently obtained independent confirmation of the potential role of JAK/STAT pathways in human diabetic kidney disease. Using a transcriptomic approach with human cDNA samples derived from glomerular and tubulointerstitial regions from humans with both early and more progressive DN, we found that a host of JAK/STAT genes were expressed at higher levels in both these regions [37]. These results were obtained in screenings designed to identify pathways in which gene expression was altered in humans with DN but not in conventional mouse models of DN, all of which have failed to recapitulate the progressive glomerulosclerosis and tubulointerstitial fibrosis seen in the human disease. JAK1–3 and STAT1 were each expressed at significantly higher levels in glomeruli of patients with DN. Immunohistochemistry showed a strong JAK2 staining in the glomeruli, as well as in proximal tubules, from patients with DN compared with those from healthy controls. By contrast, there was no increase in JAK2 expression in several common mouse models of DN, suggesting one reason for lack of progressive glomerulosclerosis in these models. We also found that increased JAK2 expression in mesangial cells, without direct agonist stimulation, leads to activation of JAK/STAT signaling, as evidenced by enhanced STAT3 phosphorylation, and that JAK2 overexpression alone led to enhanced oxidative stress [37]. Thus, enhanced JAK2 expression and JAK2-mediated signaling in mesangial cells, triggered by high glucose and, possibly, angiotensin II, appears to occur in progressive diabetic glomerulosclerosis in humans and may result in enhanced glomerulosclerosis. JAK2 signaling has been identified in podocytes [38], but nothing is known about the effects of diabetes on JAK/STAT signaling in this cell type.
mTOR activation
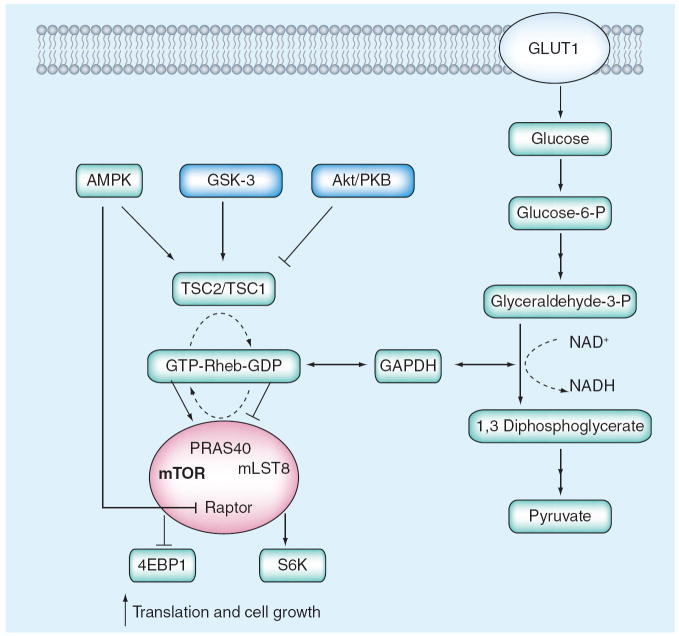
Over the past few years the role of mTOR activation in the pathogenesis of DN has received increased attention. Although enhanced mTOR activity may occur in several cell types in the diabetic kidney, it appears that activation in mesangial cells accounts for at least some of the pathologic changes that result from mTOR activation [39]. Exposure to high glucose and enhanced glycolytic flux induce mTOR activity in several cell types and in the diabetic kidney [40]. This may be due to reduction in AMP-activated protein kinase signaling [41] or due to the decreased interaction of glyceraldehyde 3-phosphate dehydrogenase and Rheb [42], each of which would promote mTOR activation (specifically, the mTORC1 complex) by the small GTPase Rheb (Figure 2). The resultant increase in mTOR activity leads to enhanced cellular metabolism and growth in part via phosphorylation and activation of two major downstream substrates, S6 kinase (S6K)1 and eukaryotic initiation factor 4E binding protein (4EBP)1. mTORC1 phosphorylates S6K1 on threonine 389 thereby activating S6K which in turn stimulates progrowth processes, such as ribosome biogenesis, anti-apoptotic processes and translation of structured 5′ UTR-containing mRNA species [43]. mTORC1 also phosphorylates and causes 4EBP1 to dissociate from eIF4E leading to initiation of cap-dependent translation [43]. In the diabetic glomerulus this enhancement of mRNA translation ultimately contributes to enhanced ECM expansion [40]. In vivo experiments in diabetic animals have found that rapamycin, a specific inhibitor of mTORC1, inhibits glomerular hypertrophy, which occurs quite early in DN, as well as other more specific aspects of DN, such as mesangial expansion [44].
Figure 2. Enhanced mTOR signaling in diabetic glomerulopathy.
Increased glucose uptake into diabetic mesangial cells (and perhaps other renal cells) leads to reduced AMPK activation and reduced association of TSC1 and TSC2, allowing for Rheb activation of mTOR. Increased glycolysis and activation of GAPDH can lead directly to Rheb activation of mTOR by reducing Rheb binding to GAPDH.
AMPK: AMP kinase; S6K: S6 kinase; TSC: Tuberous sclerosis complex.
Plasminogen activator inhibitor-1 activation
Multiple studies have found reductions in plasmin activity, due in part to inhibition of tissue plasminogen activator by enhanced levels of plasminogen activator inhibitor (PAI)-1 in both plasma and kidney of humans with DN and animal models [45,46]. Decreased protease activity of plasmin leads to enhanced accumulation of ECM proteins, such as fibronectin; thus, inhibition of plasminogen activation may contribute to glomerular changes of DN. While circulating levels of PAI-1, plasminogen and other factors are not likely to be affected by signaling abnormalities in kidney cells, local modulation of the system in the glomerulus is almost certainly important for glomerular ECM accumulation. While endothelia and other cells may also participate, most studies have focused on mesangial cells which, when grown in elevated glucose, evidence decreased urokinase plasminogen activator levels and plasmin activity, as well as elevated PAI-1 levels [47,48]. Findings from both cultured mesangial cell studies and glomeruli from diabetic rats suggest that mesangial cell PAI-1 expression is enhanced by ROS induction of TGF-β [49].
miRNA regulation of TGF-β signaling
miRNAs are short noncoding RNAs of 22 nucleotides that have been shown to play important roles in mammalian gene expression. They induce post-transcriptional gene regulation by blocking protein translation (by binding to the 3′UTR of their target genes) or by inducing mRNA degradation and, therefore, have the potential to play central roles in gene regulation in both physiologic and pathophysiologic conditions in a number of disease states [50]. More than 500 human miRNAs have been identified and it is predicted that up to 30% of human protein coding genes may be regulated by miRNAs [50]. Recently, Natarajan’s group has found that expression of miRNA-192 is enhanced in glomeruli from mice with both Type 1 and Type 2 diabetes, as well as by TGF-β treatment of cultured mesangial cells [51]. These investigators found that TGF-β-induced miRNA-192 mediates an increase in collagen 1α2 expression by reducing expression of two E-box repressors of collagen 1α2 gene activation. Since miRNA-192 was increased in tissues from both Type 1 and Type 2 diabetic mice, the authors felt that hyperglycemia may be a common factor in inducing miRNA-192 expression, but the mechanisms of this regulation remain to be elucidated. This appears to be the first demonstration of a functional role for miRNA in diabetic kidney disease. Since miRNA-192 is downstream of TGF-β, its stimulation of ECM synthesis should be a better target for therapy since such interventions could have fewer nonspecific effects than interruption of TGF-β signaling in general. These findings should open up this important regulatory field for further study.
Podocyte signaling abnormalities in DN
Podocyte abnormalities are critical participants in the pathogenesis of DN. Podocytes function in manifold ways to create and preserve the glomerular permselectivity barrier: podocyte interdigitating foot processes are bridged by a sieve-like slit diaphragm; podocytes contribute to the synthesis of the glomerular basement membrane; and podocytes actively crosstalk with glomerular endothelia via VEGF and other paracrine signals. With large plasma proteins continuously traversing the filtration barrier, and the resultant potential to clog this barrier, it is thought that the podocyte maintains the patency of the slit diaphragm and the glomerular basement membrane (GBM) by transcytosis clearance mechanisms [52,53]. Moreover, podocyte contractile properties can modulate the hydraulic pressures sustained by the glomerulus [54]. In diabetes, early podocyte dysfunction is manifested histologycally by broadening of the foot processes and functionally by various alterations of its physiology. The final fate of podocytes in DN is a reduction in total number and density secondary to apoptosis and/or detachment from the GBM. Current evidence indicates that signaling abnormalities in diabetic podocytes mainly revolve around the TGF-β family, Monocyte chemoattractant protein (MCP)-1/cysteine chemokine receptor (CCR)-2 system and VEGF pathways.
Increased TGF-β signaling
Subjected to high glucose and mechanical stretch in diabetes, the glomerular epithelial cell responds by increasing the expression of TGF-β [55], as well as its receptor TGF-βRII [56]. Further activation of TGF-β may occur at the podocyte/GBM interface due to alterations in the integrins that provide adhesion between the two layers. In addition, diabetic podocytes downregulate the more common α3/β1 integrin [57-59] and upregulate the αv/β3 and αv/β5 integrins [60,61]. In particular, the integrin αv subunit is essential for the action of the urokinase receptor, uPAR, in podocytes, the activation of which can lead to effacement of the foot processes and proteinuria [62]. This integrin may also contribute to the bioactivation of TGF-β, evidenced by the ability of αv/β6 integrins to bind and induce traction on the TGF-β large latent complex, thus activating TGF-β via a conformational change [63]. The TGF-β system in turn reduces the binding of podocytes to the GBM by downregulation of the α3β1 integrin [64].
Podocyte TGF-β may also participate in GBM thickening. In a high glucose environment podocytes increase their deposition of α1, α3 and α5 chains of collagen IV [56]. Interestingly, TGF-β alone does not replicate the hyperglycemic effect faithfully. It increases α3 collagen IV expression, similar to high glucose, but inhibits the expression of the α1 and α5 chains [56]. Thus, other signals must be necessary to recapitulate the diabetic effects.
Finally, TGF-β has been posited to be the main proapoptotic factor in the diabetic podocyte with the apoptosis cascade initiated once TGF-β concentration exceeds a certain threshold [65]. The podocyte changes linked to TGF-β seem to be mediated largely by Smad-independent pathways. For example, as described below, TGF-β stimulation of the MCP-1/CCR2 loop is mediated by PI3K signaling [66]. A number of downstream mediators have been delineated: the first candidate pathway involves the activation of p38-MAP kinase; this induces Bax protein synthesis and mitochondrial translocation that result in cytochrome C release and caspase activation [67]. p38-MAP kinase activation may be secondary to TGF-β-induced inhibition of PINCH-1–integrin-linked kinase-α-parvin complex (PIP), which could be a podocyte-specific response as TGF-β actually promotes PIP formation in mesangial cells [68]. In addition, Susztak et al. have presented evidence that Notch pathways are significantly upregulated in the diabetic kidney and represent another candidate system for TGF-β-mediated podocyte apoptosis [69]. Inhibition of this signaling with γ-secretase inhibitors may be a potential therapeutic intervention in DN [70]. Alternatively, activation of the inhibitory Smad7 may potentiate podocyte apoptosis through inhibition of the survival factor NF-κB [67]. The activation of the apoptotic machinery by TGF-β may be dependent on the presence of the cyclin-dependent kinase inhibitor p21, since p21-null podocytes are resistant to TGF-β stimuli [71].
In summary, TGF-β is central to the development of podocytopenia in DN via two mechanisms: decreased attachment of the podocyte to the GBM; and activation of apoptosis possibly through multiple redundant signaling pathways. Recent evidence indicates that the TGF-β-mediated decrease in α3/β1 integrin is sufficient to induce podocyte detachment since blocking apoptotic pathways with caspase-3 inhibitors did not affect the integrin downregulation [64]. As detached podocytes may eventually undergo apoptosis, such findings could explain the decreased podocyte density in diabetes, the presence of apoptotic cells in the kidney [69] and the appearance of live podocytes in the urine [8].
Increased MCP-1/CCR2 signaling
Monocyte chemoattractant protein-1 is a cysteine–cysteine ligand chemokine that is gaining interest as a mediator of DN. MCP-1 levels are upregulated in glomeruli and tubules of diabetic rodents [72,73], while its urinary excretion correlates significantly with the degree of proteinuria in human patients [74]. A potent chemoattractant of monocytes and macrophages [73], MCP-1 may play a role in DN that goes beyond inflammation.
Abnormalities in MCP-1 signaling can produce certain features of the podocytopathy seen in diabetes. MCP-1 expression appears to be predominantly localized to the podocytes in diabetic mice [75]. In cell culture studies, podocyte MCP-1 production increases in response to metabolic mediators, such as AGEs and, especially, TGF-β1 [66,76]. We and others have demonstrated that differentiated podocytes maintain the expression of cognate (CCR2, the main receptor of MCP-1 [66,77,78]. Accordingly, podocytes are susceptible to paracrine and autocrine MCP-1/CCR2 signaling [66]. After treatment with exogenous MCP-1, cultured podocytes reorganize their actin cytoskeleton and increase motility [66], effects that are preventable by a neutralizing anti-MCP-1 antibody or a specific inhibitor of CCR2, RS102895. TGF-β also causes similar actin changes and increased cell migration. These TGF-β effects appear to be mediated by the stimulation of an autocrine MCP-1/CCR2 signaling loop within the podocyte and this TGF-β-induced MCP-1/CCR2 signaling relies on PI3K activation. Additional paracrine MCP-1 signaling could arise from other nearby glomerular cells, such as mesangial cells, which also express MCP-1.
In vivo these signaling changes may translate into the effacement of foot processes and adapt the cells to podocytopenia as they move to cover a denuded GBM [66]. In addition, MCP-1/CCR2 may target the slit diaphragm, inducing a rapid downregulation of nephrin through Rho-kinase-dependent mechanisms [78]. The sum of these changes in podocyte function offers a likely explanation for the correlation between MCP-1 and proteinuria in human and animal models of diabetes. Indeed, the genetic knockout of MCP-1 prevents the down-regulation of nephrin and the development of albuminuria in streptozotocin-treated mice [78].
Increased Wnt/β-catenin signaling
As a developmental signaling pathway, the Wnt/β-catenin system is essentially silenced in differentiated podocytes. However, its activity is rapidly increased in the context of proteinuric diseases including DN [79]. Using a model of adriamycin-induced nephropathy (AIN), Dai et al. showed that the Wnt/β-catenin pathway is a key element of podocyte dysfunction and albuminuria [79]. Overexpression of Wnt-1 by hydrodynamic-based gene transfer exacerbates podocyte foot processes effacement and albuminuria in AIN. Conversely, inhibition of Wnt signaling improves the podocytopathy. Similarly, activation of β-catenin results in significant albuminuria, whereas podocyte-specific deletion of β-catenin protects mice from AIN. The Wnt/β-catenin pathway appears to contribute to podocyte injury ultimately by downregulating nephrin or redistributing it via a Snail-dependent mechanism [79]. It is important to note that β-catenin also functions as a structural protein by associating with the cadherin complex and densin at the slit diaphragm [80]. In that context, β-catenin nuclear translocation and signaling are actively modulated by integrin-linked kinase (ILK) [81]. With ILK significantly upregulated in the diabetic podocyte [82,83], the ILK/β-catenin pathway may constitute a mechanism by which podocytes sense GBM dysregulation and react by changing their foot process morphology and slit diaphragm composition. Whether this scenario extends to DN remains to be demonstrated.
Increased VEGF signaling
Podocytes are the major source of renal VEGF. In many chronic kidney diseases VEGF levels are low and are associated with impaired angiogenesis with capillary loss. Conversely, in most animal models of DN, VEGF levels are elevated [84]. In humans, VEGF levels and downstream signaling are increased in early stages of DN but then appear to be reduced below normal with progressive DN [85,86]. This early increase in VEGF levels may be due directly to increases in glucose [56,87,88] or to increased signaling via TGF-β [56], angiotensin II [89,90] and/or hypoxia-inducible factor [91]. Although originally viewed as a trophic factor for glomerular endothelial cells (see immediately below, in section on endothelial signaling), VEGF also has critical effects on podocyte physiology and pathophysiology. Secreted VEGF activates podocytes in an autocrine manner, possibly by binding the VEGFR-1 receptor, and induces α3(IV) collagen synthesis via PI3K signaling [92], consistent with the notion that enhanced VEGF levels induce podocyte GBM matrix protein production. Tumstatin, endostatin, and 2-(8-hydroxy-6-methoxy-1-oxo-1H-2-benzopyran-3-yl) propionic acid (NM-3) are anti-angiogenic factors, though not specific inhibitors, that prevent VEGF signaling in the diabetic kidney [93-95]. These agents restore nephrin expression to normal levels and improve albuminuria in diabetes [93-95]. In db/db diabetic mice, inhibition of VEGF signaling by a tyrosine kinase inhibitor, SU5416, significantly ameliorates albuminuria [96]. In parallel, antagonism of VEGF receptors partially restores slit pore density in diabetic mice and prevents nephrin downregulation when assayed by immunofluorescence [96].
While VEGF may play a role in podocyte signaling abnormalities that promote changes of early DN, VEGF also has podocyte protective effects, especially in cell culture [97,98]. Thus, VEGF upregulation in diabetic rodent kidneys can also be seen as a protective mechanism, by promoting cell healing in the face of increased stress in diabetes. Nonetheless, the deleterious effect of VEGF appears to be clear in animal models and may depend on the complexity of the diabetic milieu. Further studies are required to further elucidate the complex role of VEGF signaling in podocytes in DN.
Glomerular endothelial cell signaling abnormalities
As already noted, the role of endothelial cells in the pathogenesis of diabetic nephrosclerosis has recently received increased attention. Endothelial damage is a hallmark of diabetes [99]. In addition, there is evidence that accelerated angiogenesis in the glomerulus may contribute to diabetic glomerular hypertrophy and to ECM expansion [100], just as it does in diabetic retinopathy. Indeed, as noted previously, alterations in levels of angiogenic factors, especially in VEGF, appear to trigger many of the abnormalities in endothelial signaling that occur in DN.
Increased & decreased VEGF signaling & other angiogenic factors
As just discussed, podocytes express and secrete high levels of VEGF. The two VEGF receptors that bind to the predominant isoform of VEGF, VEGF-A, are expressed on the cell surface of glomerular endothelial cells. While VEGF receptors are present on podocytes, as noted earlier, and in mesangial cells [101], it appears that VEGF effects in DN result largely from endothelial signaling since receptor binding in these cells predominates in humans with DN [86]. VEGF has been strongly implicated in endothelial alterations in DN [102].
Since VEGF levels are elevated in animal models of DN (the great preponderance of which are models of relatively early DN), a number of studies have examined the effect of inhibition of VEGF receptor binding or activation [103]. These studies suggest that many aspects of early DN may be forestalled by interrupting VEGF signaling. However, as noted earlier, in humans with progressive DN, the situation is much more complex and it is difficult to predict the direction of VEGF signaling abnormalities [104].
VEGF signaling in glomerular endothelial cells is initiated by binding of VEGF to both of its receptors, VEGFR1 (or Flt-1) and VEGFR2 (or KDR/Flk-1). VEGFR1 only transduces relatively weak signals for endothelial cell growth and survival that appears to take place via ligand-induced autophosphorylation and coupling to intracellular signal transducers. Autophosphorylation of Y1169 stimulates binding and activation of phospholipase Cg1 and subsequent activation of the MAPK pathway, ultimately resulting in endothelial cell proliferation and permeability. However, this process is muted and other signaling pathways from VEGFR1 have been difficult to unequivocally elucidate from cultured endothelial cell studies [104]. The two major autophosphorylation sites in VEGFR2 result in Src activation and phospholipase Cg activation, respectively. The latter regulates the PKC–Raf–MEK–MAPK cascade and DNA synthesis. VEGFR2 ligand binding also results in activation of PI3K and its downstream effector Akt [104]. It is not known which, if any, of these pathways are involved in the disordered endothelial signaling in DN. Since current evidence suggests that VEGF is elevated in early DN and is reduced in late DN, it is tempting again to speculate that early increases in VEGF signaling in glomerular endothelia lead to increased endothelial growth and possibly proliferation, as well as increased permeability. As VEGF levels fall in later DN, endothelial growth is halted and apoptosis may occur, leading to capillary loss and perhaps contributing to glomerulosclerosis.
Finally, other angiogenic and angiostatic factors may contribute to endothelial alterations in DN. For example, levels of the angiogenic factor, angiopoietin 2, are increased in animal models and humans with early DN [94,105,106], and angiopoietin 2 has been shown to induce albuminuria and endothelial apoptosis as well as to reduce levels of VEGF-A and nephrin in glomeruli when overexpressed specifically in podocytes [107]. How much of the angiopoietin 2 effects are due to specific enhanced signaling from its Tie-2 receptor, which is expressed on glomerular endothelia [108], and how much are due to alterations in nephrin and VEGF-A, remains to be elucidated.
Reduction in eNOS
Endothelial cell-derived vasodilators, such as NO, appear to be important modulators of permeability in the vasculature. As noted above, vascular eNOS activity is altered in diabetes, and functionally significant polymorphisms in the NOS3 gene lead to lower production of NO [109-111] and are associated with the development of advanced nephropathy in patients with Type 1 and Type 2 diabetes [112-114]. Although there is some controversy [115], most reports suggest higher levels of NO production early in diabetes but reduced levels in progressive DN [27], analogous to the pattern with VEGF. Several recent reports that analyzed the effects of diabetes in mice with targeted deletion of the eNOS gene have underlined the critical participation of endothelial responses in diabetic glomerular pathology in animal models [6,7,28,116]. Diabetic eNOS knockout mice developed substantial mesangial expansion and glomerulosclerosis, including nodular sclerosis, as well as other signs of advanced DN, including reduction in GFR. The signaling mechanisms by which reduced NO production results in glomerular pathology are not clear. Resultant hemodynamic effects may play a role, but it seems most likely that paracrine effects between endothelial cells, podocytes, mesangial cells and even resident macrophages account for the bulk of the impressive pathology resulting from altered endothelial NO production. A recent report on the effects of eNOS deletion in db/db C57BLKS mice showed that diabetic eNOS knockout mice developed substantial tubular injury as well as cortical scarring and increased collagen between tubules. These mice also showed increased interstitial macrophages compared with controls [28]. Thus, knockout of eNOS can contribute to both glomerular and tubulointerstitial fibrosis suggesting that disruption of NO signaling in the kidney may be a major factor in the development of DN. Because patients with DN have been found to have high numbers of atubular glomeruli [117] and because eNOS knockout mice also show large numbers of atubular glomeruli [30], it is tempting to speculate that reduction in NO availability to the proximal tubule epithelia is an important determinant of this aspect of DN as well.
Regulation of eNOS in DN may come via VEGF. VEGF stimulates eNOS activity in the glomerulus [102] and may exert some of its protective effects through this mechanism. Nakagawa has suggested an uncoupling of the VEGF–NO axis with high VEGF levels and paradoxically low endothelial NO production [7,102]. However, as was noted above, VEGF receptor binding and signaling appear to parallel that of NO in human DN and, therefore, such uncoupling may not need to be invoked.
The signaling pathways in diabetes that lead to reduced NO production from eNOS are complex, but cultured cell studies by Du et al. showed that hyperglycemia reduces eNOS activity in cultured endothelial cells in part by enhancing mitochondrial production of superoxide [118]. This thereby activates the hexosamine pathway [119], which, in turn, increases eNOS modification by GlcNAc, as well as decreasing phosphorylation of eNOS on Ser1177. Phosphorylation of this site by Akt is required for activation of eNOS [118].
Reduced levels of thrombomodulin & activated protein C
Another piece of evidence underlining the importance of altered endothelial cell signaling responses in the evolution of DN, a recent study elegantly elucidated the effects of the thrombo-modulin/activated protein C pathway on glomerular endothelial cells in diabetes [5]. Protein C is activated by binding of thrombin to its receptor, thrombomodulin. Activated protein C has been shown to have anti-inflammatory, anti-apoptotic, anti-thrombotic and fibrinolytic effects [120]. In diabetic patients, function of the endothelial thrombomodulin–protein C system is impaired [121], and in diabetic mice, glomerular endothelial thrombomodulin A expression and, therefore, protein C activation are substantially reduced [5]. The signaling mechanisms for these events are as yet unclear. The role of activated protein C in glomerular injury in diabetes was explored using two mouse models, one with impaired protein C activation and one expressing a hyperactivatable protein C mutation. Mesangial ECM expansion was enhanced in the diabetic mice with impaired protein C activation and was completely prevented in those with the hyperactivatable protein C mutation. Moreover, mice with impaired protein C activation demonstrated enhanced endothelial and podocyte apoptosis whereas the hyperactivatable protein C mice had fewer apoptotic cells. In an interesting experiment, the investigators showed that they could inhibit glomerular mesangial expansion by a generalized apoptosis inhibitor, minocycline. All of these effects were shown to be independent of any changes in coagulation due to altered protein C activation. In addition, activated protein C was shown to prevent high glucose-induced apoptosis of glomerular endothelial cells and podocytes, but not mesangial cells, in vitro. In summary, a single comprehensive study has established that a reduction in protein C activation promotes glomerular capillary dysfunction and apoptosis of endothelial cells and podocytes in experimental diabetes. Moreover, activated protein C may be a mediator of crosstalk from endothelial cells to podocytes, and possibly also to mesangial cells, to modify nephropathy. Obviously, these findings need to be corroborated in other models and in humans with both early and progressive DN, but when such confirmatory studies are reported, new modalities of treatment that induce protein C activation could be tested.
Tubulointerstitial cell signaling abnormalities
As noted previously, while it is probable that pathogenic changes of early DN are concentrated in the glomerulus, it is clear that progressive DN and renal failure does not take place without tubulointerstitial fibrosis [122-124]. Thus, signaling abnormalities in tubulointerstitial cells are likely to be critical for progressive DN. However, given the absence of good animal models of progressive DN and tubulointerstitial fibrosis, progress in this area has been delayed. A number of studies have suggested that albuminuria, due to altered glomerular permeability, can trigger a profibrotic response in proximal tubule cells and the surrounding tubulointerstitial compartment. Data derived largely from cultured cell experiments indicate that albumin can bind to receptors on proximal tubular cells and be endocytosed. Both albumin binding and endocytosis appear to trigger proinflammatory and profibrotic responses in proximal tubular cells that can lead to enhanced tubulointerstitial fibrosis via a variety of mechanisms [46,125-128], most of which ultimately enhance TGF-β signaling. Although research in this area is now moving rapidly, there appears to be no current consensus regarding the major signaling abnormalities in proximal tubule or other tubulointerstitial cells.
Enhanced inflammation & NF-κB activation
One area for which there is growing consensus is that inflammatory mechanisms play a critical role in progressive tubulointerstitial injury in DN [72,75,129,130]. Macrophage accumulation in the tubulointerstitium of animals with diabetes leads to tubular damage and increased numbers of myofibroblasts, ultimately resulting in enhanced fibrosis [72,75,131]. Activation of NF-κB, a key inflammatory signaling molecule, appears to be an important part of this process. For example, activated NF-κB has been detected in proximal tubular cells in the urinary sediment of many patients with Type 2 diabetes and in diabetic rat kidneys and human diabetic kidney [132]. Another study used a sophisticated modular systems biology approach to demonstrate a prominent inflammatory signature in the tubulointerstitial tissue of patients with progressive DN. Using computational promoter analysis the investigators were able to identify a specific set of genes, especially chemokine genes, containing a specific NF-κB promoter module (NFKB_IRFF_01), that were activated in progressive DN. The NFKB_IRFF_01 module has a NF-κB binding site on the plus DNA strand and interferon regulatory factor binding site on the minus DNA strand, separated by 14–24 bp. Thus, by integrating gene-expression profiling with promoter modeling they were able to define a central role for inflammation in human DN in proximal tubules. In addition, this work underlined the central role of the NF-κB/IRF module as a potential ‘master switch’ activating this response [129].
The importance of inflammatory mechanisms in the fibrotic responses in DN is also underscored by the evidence that many therapeutic agents that prevent or retard progression of human DN are potent anti-inflammatory agents [133-135]. In addition, AGEs and oxidant stress, which are critical factors in the progression of DN, augment and are augmented by inflammatory mechanisms of injury in the kidney [136,137]. Finally, it should be stressed that both glomerular and tubulointerstitial cells produce a multitude of inflammatory mediators in the diabetic milieu, especially as injury proceeds, which can augment inflammatory damage to the tubulointerstitium.
Expert commentary
A simple model of the pathogenesis of DN (Figure 1) has been confounded by a host of recent reports, each of which underscores the complexity and inter-relatedness of the mechanisms of these critical aspects of DN. Undoubtedly, other pathways not yet elucidated or described in this review will be found to contribute importantly to this complication. Conversely, many of the pathways described in this review interact in manifold ways and in multiple cell types, as noted above in the inter-relationships between eNOS, VEGF, JAK/STAT signaling and inflammation. Finally, some of the mechanisms that induce DN may be the same and some may differ from those involved in other chronic renal diseases. Thus, it is unlikely that modulation of a single pathway will lead to prevention or cure of this complex chronic complication in the majority of sufferers.
The focus on candidate pathways, while informative, has clearly limited the ability to expand our understanding of the disease processes. A more unbiased approach to identification of pathways and networks of abnormalities in DN and other complications now seems likely to be well suited to expand our understanding. An example of such an unbiased approach was noted earlier. This study utilized a transcriptomic analysis of both humans with DN and mouse models of the disease to uncover a signaling pathway that may be important in DN. It found high levels of many JAK/STAT family members in humans with progressive DN but not in streptozotocin diabetic DBA/2 or BLKS db/db mice with the relatively mild kidney disease seen in these models [37]. While this pathway had been previously studied by one set of investigators as a candidate pathway, these expression changes came from of an unbiased, transcriptomic approach and opened up the new possibility that chronic changes in gene expression in both glomerular and tubulointerstitial compartments can lead to long-term signaling abnormalities important for progressive disease. Similarly, the identification of NF-κB as a key regulator of human tubulointerstitial gene-expression changes [129], while not surprising, shows how integration of unbiased gene expression profiling with promoter modeling can identify signaling abnormalities in human DN.
Five-year view
Much progress has been made in the last few years and this suggests that the next 5 years will produce major new insights into the network abnormalities that lead to progressive DN. It is already clear that there are specific alterations in pathways in DN that are not found in other chronic kidney diseases. Further elucidation of these abnormalities over the next 5 years may allow for development of specific treatments for this complication that could add materially to the current armamentarium of nonspecific therapies. A comprehensive understanding of the pathogenesis of DN via a systems biology and computational approach is ongoing, and will allow us to determine more completely how DN is similar to, and differs from, other causes of progressive chronic kidney disease. Such approaches may also reveal substantial differences among individuals with DN as one set of signaling pathways may play a critical role in some patients, while different pathways may predominate in others. Systems approaches should allow identification of protein and metabolite changes that can be measured in the blood and urine of patients with DN, and will be useful as biomarkers of pathway alterations. These will serve as indicators of prognosis but may also direct specific treatments. For example, if such biomarkers can be developed, patients who have markers of substantial renal mTOR activation may be treated with rapamycin or other mTOR inhibitors, while patients with overactive JAK/STAT signaling may respond to newly developed JAK2 or JAK3 inhibitors.
Key issues.
Virtually all cell types in the kidney participate in the progression of diabetic nephropathy (DN).
Activation of protein kinase C isoforms and TGF-β play a critical role in the pathogenesis of DN, but many other signaling abnormalities may regulate these downstream responses.
Reduced epithelial nitric oxide synthetase signaling, enhanced JAK/STAT signaling and increased mTOR activation all appear to play a role in progressive diabetic glomerulopathy.
Podocyte signaling is disrupted in DN and there are specific abnormalities in MCP-1/CCR2, Wnt/β-catenin and VEGF signaling in podocytes that appear to participate in the podocytopathy of diabetes.
TGF-β is central to the development of podocytopenia in DN by reducing attachment of the podocyte to the glomerular basement membrane and inducing activation of apoptosis.
Inflammatory pathway activation via NF-κB appears to be critical for tubulointerstitial fibrosis and progressive decline in renal function in progressive DN.
Systems biology and computational approaches will be necessary to integrate the complex set of signaling abnormalities that take place in different cells and at different times, that impact upon one another within and between cell types, and that together consort to produce the destructive process of progressive DN.
Acknowledgments
Relevant research in Frank Brosius’ laboratory was supported, in part, by NIH grant U01DK076139 and R24 R24 DK082841 and in Sheldon Chen’s laboratory by NIH grant R01DK044513 and a VA Merit Review grant.
Footnotes
Financial & competing interests disclosure. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Frank C Brosius, 3rd, Departments of Internal Medicine and Molecular and Integrative Physiology, University of Michigan Medical School, 5520 MSRB1, 1150 W. Medical Center Drive, Ann Arbor, MI 48109-0680, USA, Tel.: +1 734 764 3157, Fax: +1 734 763 4151, fbrosius@umich.edu.
Charbel C Khoury, Department of Internal Medicine, Division of Nephrology/Hypertension, Northwestern University, IL, USA.
Carolyn L Buller, Departments of Internal Medicine and Molecular and Integrative Physiology, University of Michigan Medical School, 5520 MSRB1, 1150 W. Medical Center Drive, Ann Arbor, MI 48109-0680, USA.
Sheldon Chen, Department of Internal Medicine, Division of Nephrology/Hypertension, Northwestern University, IL, USA.
References
Papers of special note have been highlighted as:
of interest
- 1.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in Type II diabetes. J Clin Invest. 1997;99(2):342–348. doi: 10.1172/JCI119163.. • First study to demonstrate that podocyte depletion was an important feature of diabetic nephropathy (DN) that predicted progressive DN.
- 2.Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16(10):2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 3.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42(11):1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 4.Morcos M, Borcea V, Isermann B, et al. Effect of α-lipoic acid on the progression of endothelial cell damage and albuminuria in patients with diabetes mellitus: an exploratory study. Diabetes Res Clin Pract. 2001;52(3):175–183. doi: 10.1016/s0168-8227(01)00223-6. [DOI] [PubMed] [Google Scholar]
- 5.Isermann B, Vinnikov IA, Madhusudhan T, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13(11):1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 6.Zhao HJ, Wang S, Cheng H, et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17(10):2664–2669. doi: 10.1681/ASN.2006070798.. • Demonstrates robust DN that better resembles human DN than virtually any other mouse model.
- 7.Nakagawa T, Sato W, Glushakova O, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18(2):539–550. doi: 10.1681/ASN.2006050459.. • Demonstrates robust DN that better resembles human DN than virtually any other mouse model.
- 8.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human Type 1 diabetic nephropathy. Diabetes. 2007;56(8):2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 9.Oldfield MD, Bach LA, Forbes JM, et al. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108(12):1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heilig CW, Liu Y, England RL, et al. D-glucose stimulates mesangial cell GLUT1 expression and basal and IGF-I-sensitive glucose uptake in rat mesangial cells: implications for diabetic nephropathy. Diabetes. 1997;46(6):1030–1039. doi: 10.2337/diab.46.6.1030. [DOI] [PubMed] [Google Scholar]
- 11.D’Agord Schaan B, Lacchini S, Bertoluci MC, Irigoyen MC, Machado UF, Schmid H. Increased renal GLUT1 abundance and urinary TGF-β 1 in streptozotocin-induced diabetic rats: implications for the development of nephropathy complicating diabetes. Horm Metab Res. 2001;33(11):664–669. doi: 10.1055/s-2001-18683. [DOI] [PubMed] [Google Scholar]
- 12.Horie K, Miyata T, Maeda K, et al. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest. 1997;100(12):2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skolnik EY, Yang Z, Makita Z, Radoff S, Kirstein M, Vlassara H. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodelling and diabetic nephropathy. J Exp Med. 1991;174(4):931–939. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makita Z, Radoff S, Rayfield EJ, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- 15.Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43(1):1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Babazono T, Kapor-Drezgic J, Dlugosz JA, Whiteside C. Altered expression and subcellular localization of diacylglycerol-sensitive protein kinase C isoforms in diabetic rat glomerular cells. Diabetes. 1998;47(4):668–676. doi: 10.2337/diabetes.47.4.668. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor β is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90(5):1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-β. J Clin Invest. 1994;93(2):536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marre M, Bernadet P, Gallois Y, et al. Relationships between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complications. Diabetes. 1994;43(3):384–388. doi: 10.2337/diab.43.3.384. [DOI] [PubMed] [Google Scholar]
- 20.Doria A, Warram JH, Krolewski AS. Genetic predisposition to diabetic nephropathy. Evidence for a role of the angiotensin I-converting enzyme gene. Diabetes. 1994;43(5):690–695. doi: 10.2337/diab.43.5.690. [DOI] [PubMed] [Google Scholar]
- 21.Huang W, Gallois Y, Bouby N, et al. Genetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouse. Proc Natl Acad Sci USA. 2001;98(23):13330–13334. doi: 10.1073/pnas.231476798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi N, Hagaman JR, Kim HS, Smithies O. Minireview: computer simulations of blood pressure regulation by the renin–angiotensin system. Endocrinology. 2003;144(6):2184–2190. doi: 10.1210/en.2002-221045. [DOI] [PubMed] [Google Scholar]
- 23.Kakoki M, Takahashi N, Jennette JC, Smithies O. Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci USA. 2004;101(36):13302–13305. doi: 10.1073/pnas.0405449101.. • First paper to strongly suggest that reduced bradykinin signaling, independent of increased angiotensin 2 levels, accounted for much of the pathologic changes in diabetic glomerulopathy. Treatment of DN with angiotensin-converting enzyme ACE inhibitors may be beneficial by its effect on raising levels of bradykinin as well as reducing conversion of angiotensin 1 to angiotensin 2.
- 24.Kakoki M, Kizer CM, Yi X, et al. Senescence-associated phenotypes in Akita diabetic mice are enhanced by absence of bradykinin B2 receptors. J Clin Invest. 2006;116(5):1302–1309. doi: 10.1172/JCI26958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allard J, Buleon M, Cellier E, et al. ACE inhibitor reduces growth factor receptor expression and signaling but also albuminuria through B2-kinin glomerular receptor activation in diabetic rats. Am J Physiol. 2007;293(4):F1083–1092. doi: 10.1152/ajprenal.00401.2006. [DOI] [PubMed] [Google Scholar]
- 26.Alric C, Pecher C, Cellier E, et al. Inhibition of IGF-I-induced Erk 1 and 2 activation and mitogenesis in mesangial cells by bradykinin. Kidney Int. 2002;62(2):412–421. doi: 10.1046/j.1523-1755.2002.00475.x. [DOI] [PubMed] [Google Scholar]
- 27.Prabhakar SS. Role of nitric oxide in diabetic nephropathy. Semin Nephrol. 2004;24(4):333–344. doi: 10.1016/j.semnephrol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Mohan S, Reddick RL, Musi N, et al. Diabetic eNOS knockout mice develop distinct macro- and microvascular complications. Lab Invest. 2008;88(5):515–528. doi: 10.1038/labinvest.2008.23. [DOI] [PubMed] [Google Scholar]
- 29.Kanetsuna Y, Takahashi K, Nagata M, et al. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol. 2007;170(5):1473–1484. doi: 10.2353/ajpath.2007.060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes MS, Thornhill BA, Park MH, Chevalier RL. Lack of endothelial nitric-oxide synthase leads to progressive focal renal injury. Am J Pathol. 2007;170(1):87–99. doi: 10.2353/ajpath.2007.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dreieicher E, Beck KF, Lazaroski S, et al. Nitric oxide inhibits glomerular TGF-β signaling via SMOC-1. J Am Soc Nephrol. 2009 doi: 10.1681/ASN.2008060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amiri F, Shaw S, Wang X, et al. Angiotensin II activation of the JAK/STAT pathway in mesangial cells is altered by high glucose. Kidney Int. 2002;61(5):1605–1616. doi: 10.1046/j.1523-1755.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- 33.Banes AK, Shaw S, Jenkins J, et al. Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. Am J Physiol. 2004;286(4):F653–659. doi: 10.1152/ajprenal.00163.2003. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Shaw S, Amiri F, Eaton DC, Marrero MB. Inhibition of the Jak/STAT signaling pathway prevents the high glucose-induced increase in TGF-β and fibronectin synthesis in mesangial cells. Diabetes. 2002;51(12):3505–3509. doi: 10.2337/diabetes.51.12.3505. [DOI] [PubMed] [Google Scholar]
- 35.Banes-Berceli AK, Shaw S, Ma G, et al. Effect of simvastatin on high glucose- and angiotensin II-induced activation of the JAK/STAT pathway in mesangial cells. Am J Physiol. 2006;291(1):F116–F121. doi: 10.1152/ajprenal.00502.2005. [DOI] [PubMed] [Google Scholar]
- 36.Marrero MB, Banes-Berceli AK, Stern DM, Eaton DC. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am J Physiol. 2006;290(4):F762–768. doi: 10.1152/ajprenal.00181.2005. [DOI] [PubMed] [Google Scholar]
- 37.Berthier CC, Zhang H, Schin M, et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes. 2009;58(2):469–477. doi: 10.2337/db08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Logar CM, Brinkkoetter PT, Krofft RD, Pippin JW, Shankland SJ. Darbepoetin alfa protects podocytes from apoptosis in vitro and in vivo. Kidney Int. 2007;72(4):489–498. doi: 10.1038/sj.ki.5002362. [DOI] [PubMed] [Google Scholar]
- 39.Mori H, Inoki K, Masutani K, et al. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Comm. 2009;384(4):471–475. doi: 10.1016/j.bbrc.2009.04.136. [DOI] [PubMed] [Google Scholar]
- 40.Inoki K. Role of TSC-mTOR pathway in diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S59–S62. doi: 10.1016/j.diabres.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Lee MJ, Feliers D, Mariappan MM, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol. 2007;292(2):F617–F627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 42.Lee MN, Ha SH, Kim J, et al. Glycolytic flux signals to mTOR through glyceraldehyde-3-phosphate dehydrogenase-mediated regulation of Rheb. Mol Cell Biol. 2009;29(14):3991–4001. doi: 10.1128/MCB.00165-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choo AY, Blenis J. Not all substrates are treated equally: implications for mTOR, rapamycin-resistance and cancer therapy. Cell Cycle. 2009;8(4):567–572. doi: 10.4161/cc.8.4.7659. [DOI] [PubMed] [Google Scholar]
- 44.Lloberas N, Cruzado JM, Franquesa M, et al. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol. 2006;17(5):1395–1404. doi: 10.1681/ASN.2005050549. [DOI] [PubMed] [Google Scholar]
- 45.Hirano T, Kashiwazaki K, Moritomo Y, Nagano S, Adachi M. Albuminuria is directly associated with increased plasma PAI-1 and factor VII levels in NIDDM patients. Diabetes Res Clin Pract. 1997;36(1):11–18. doi: 10.1016/s0168-8227(97)01384-3. [DOI] [PubMed] [Google Scholar]
- 46.Eddy AA, Giachelli CM. Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int. 1995;47(6):1546–1557. doi: 10.1038/ki.1995.218. [DOI] [PubMed] [Google Scholar]
- 47.Fisher EJ, McLennan SV, Yue DK, Turtle JR. High glucose reduces generation of plasmin activity by mesangial cells. Microvasc Res. 1997;53(2):173–178. doi: 10.1006/mvre.1996.2006. [DOI] [PubMed] [Google Scholar]
- 48.McLennan SV, Fisher E, Martell SY, et al. Effects of glucose on matrix metalloproteinase and plasmin activities in mesangial cells: possible role in diabetic nephropathy. Kidney Int. 2000;77(Suppl):S81–S87. doi: 10.1046/j.1523-1755.2000.07713.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee EA, Seo JY, Jiang Z, et al. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int. 2005;67(5):1762–1771. doi: 10.1111/j.1523-1755.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 50.Sassen S, Miska EA, Caldas C. MicroRNA-implications for cancer. Virchows Arch. 2007;452(1):1–10. doi: 10.1007/s00428-007-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato M, Zhang J, Wang M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA. 2007;104(9):3432–3437. doi: 10.1073/pnas.0611192104.. • Demonstrates for the first time the critical role of miRNA-192 in regulation of TGF-β signaling in DN. This will probably start a whole field of investigation into the role of miRNAs in the progression and, perhaps, prevention of diabetic complications
- 52.Ina K, Kitamura H, Tatsukawa S, Takayama T, Fujikura Y. Glomerular podocyte endocytosis of the diabetic rat. J Electron Microsc (Tokyo) 2002;51(4):275–279. doi: 10.1093/jmicro/51.4.275. [DOI] [PubMed] [Google Scholar]
- 53.Akilesh S, Huber TB, Wu H, et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci USA. 2008;105(3):967–972. doi: 10.1073/pnas.0711515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kriz W, Hackenthal E, Nobiling R, Sakai T, Elger M, Hahnel B. A role for podocytes to counteract capillary wall distension. Kidney Int. 1994;45(2):369–376. doi: 10.1038/ki.1994.47. [DOI] [PubMed] [Google Scholar]
- 55.Durvasula RV, Petermann AT, Hiromura K, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65(1):30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 56.Iglesias-de la Cruz MC, Ziyadeh FN, Isono M, et al. Effects of high glucose and TGF-β1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 2002;62(3):901–913. doi: 10.1046/j.1523-1755.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- 57.Kitsiou PV, Tzinia AK, Stetler-Stevenson WG, et al. Glucose-induced changes in integrins and matrix-related functions in cultured human glomerular epithelial cells. Am J Physiol Renal Physiol. 2003;284(4):671–679. doi: 10.1152/ajprenal.00266.2002. [DOI] [PubMed] [Google Scholar]
- 58.Chen HC, Chen CA, Guh JY, Chang JM, Shin SJ, Lai YH. Altering expression of α3β1 integrin on podocytes of human and rats with diabetes. Life Sci. 2000;67(19):2345–2353. doi: 10.1016/s0024-3205(00)00815-8. [DOI] [PubMed] [Google Scholar]
- 59.Regoli M, Bendayan M. Alterations in the expression of the α3 β1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia. 1997;40(1):15–22. doi: 10.1007/s001250050637. [DOI] [PubMed] [Google Scholar]
- 60.Jin DK, Fish AJ, Wayner EA, et al. Distribution of integrin subunits in human diabetic kidneys. J Am Soc Nephrol. 1996;7(12):2636–2645. doi: 10.1681/ASN.V7122636. [DOI] [PubMed] [Google Scholar]
- 61.Yoon S, Gingras D, Bendayan M. Alterations of vitronectin and its receptor α(v) integrin in the rat renal glomerular wall during diabetes. Am J Kidney Dis. 2001;38(6):1298–1306. doi: 10.1053/ajkd.2001.29228. [DOI] [PubMed] [Google Scholar]
- 62.Wei C, Moller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 63.Munger JS, Huang X, Kawakatsu H, et al. The integrin α v β 6 binds and activates latent TGF β 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 64.Dessapt C, Baradez MO, Hayward A, et al. Mechanical forces and TGFβ1 reduce podocyte adhesion through α3β1 integrin downregulation. Nephrol Dial Transplant. 2009;124(9):2645–2655. doi: 10.1093/ndt/gfp204. [DOI] [PubMed] [Google Scholar]
- 65.Wu DT, Bitzer M, Ju W, Mundel P, Bottinger EP. TGF-β Concentration specifies differential signaling profiles of growth arrest/differentiation and apoptosis in podocytes. J Am Soc Nephrol. 2005;16(11):3211–3221. doi: 10.1681/ASN.2004121055. [DOI] [PubMed] [Google Scholar]
- 66.Lee EY, Chung CH, Khoury CC, et al. Monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-β, increases podocyte motility and albumin permeability. Am J Physiol Renal Physiol. 2009;297(1):F85–F94. doi: 10.1152/ajprenal.90642.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-β and Smad7. J Clin Invest. 2001;108(6):807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung KY, Chen K, Kretzler M, Wu C. TGF-β1 regulates the PINCH-1-integrin-linked kinase-α-parvin complex in glomerular cells. J Am Soc Nephrol. 2007;18(1):66–73. doi: 10.1681/ASN.2006050421. [DOI] [PubMed] [Google Scholar]
- 69.Niranjan T, Murea M, Susztak K. The pathogenic role of notch activation in podocytes. Nephron Exp Nephrol. 2009;111(4):e73–e79. doi: 10.1159/000209207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14(3):290–298. doi: 10.1038/nm1731.. • Shows that Notch pathways are significantly upregulated in the diabetic kidney and represent a candidate system for TGF-β-mediated podocyte apoptosis.
- 71.Wada T, Pippin JW, Terada Y, Shankland SJ. The cyclin-dependent kinase inhibitor p21 is required for TGF-β1-induced podocyte apoptosis. Kidney Int. 2005;68(4):1618–1629. doi: 10.1111/j.1523-1755.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 72.Chow FY, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in streptozotocin-induced diabetic nephropathy: potential role in renal fibrosis. Nephrol Dial Transplant. 2004;19(12):2987–2996. doi: 10.1093/ndt/gfh441. [DOI] [PubMed] [Google Scholar]
- 73.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69(1):73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- 74.Amann B, Tinzmann R, Angelkort B. ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care. 2003;26(8):2421–2425. doi: 10.2337/diacare.26.8.2421. [DOI] [PubMed] [Google Scholar]
- 75.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse Type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65(1):116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 76.Gu L, Hagiwara S, Fan Q, et al. Role of receptor for advanced glycation end-products and signalling events in advanced glycation end-product-induced monocyte chemoattractant protein-1 expression in differentiated mouse podocytes. Nephrol Dial Transplant. 2006;21(2):299–313. doi: 10.1093/ndt/gfi210. [DOI] [PubMed] [Google Scholar]
- 77.Burt D, Salvidio G, Tarabra E, et al. The monocyte chemoattractant protein-1/cognate CC chemokine receptor 2 system affects cell motility in cultured human podocytes. Am J Pathol. 2007;171(6):1789–1799. doi: 10.2353/ajpath.2007.070398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tarabra E, Giunti S, Barutta F, et al. Effect of the Mcp-1/Ccr2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes. Diabetes. 2009;58(9):2109–2118. doi: 10.2337/db08-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20(9):1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heikkila E, Ristola M, Endlich K, et al. Densin and β-catenin form a complex and co-localize in cultured podocyte cell junctions. Mol Cell Biochem. 2007;305(1–2):9–18. doi: 10.1007/s11010-007-9522-6. [DOI] [PubMed] [Google Scholar]
- 81.Teixeira Vde P, Blattner SM, Li M, et al. Functional consequences of integrin-linked kinase activation in podocyte damage. Kidney Int. 2005;67(2):514–523. doi: 10.1111/j.1523-1755.2005.67108.x. [DOI] [PubMed] [Google Scholar]
- 82.Guo L, Sanders PW, Woods A, Wu C. The distribution and regulation of integrin-linked kinase in normal and diabetic kidneys. Am J Pathol. 2001;159(5):1735–1742. doi: 10.1016/S0002-9440(10)63020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han SY, Kang YS, Jee YH, et al. High glucose and angiotensin II increase β1 integrin and integrin-linked kinase synthesis in cultured mouse podocytes. Cell Tissue Res. 2006;323(2):321–332. doi: 10.1007/s00441-005-0065-4. [DOI] [PubMed] [Google Scholar]
- 84.Cooper ME, Vranes D, Youssef S, et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48(11):2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- 85.Baelde HJ, Eikmans M, Lappin DW, et al. Reduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte loss. Kidney Int. 2007;71(7):637–645. doi: 10.1038/sj.ki.5002101. [DOI] [PubMed] [Google Scholar]
- 86.Hohenstein B, Hausknecht B, Boehmer K, Riess R, Brekken RA, Hugo CP. Local VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in man. Kidney Int. 2006;69(9):1654–1661. doi: 10.1038/sj.ki.5000294. [DOI] [PubMed] [Google Scholar]
- 87.Han SH, Yang S, Jung DS, et al. Gene expression patterns in glucose-stimulated podocytes. Biochem Biophys Res Commun. 2008;370(3):514–518. doi: 10.1016/j.bbrc.2008.03.121. [DOI] [PubMed] [Google Scholar]
- 88.Hoshi S, Nomoto K, Kuromitsu J, Tomari S, Nagata M. High glucose induced VEGF expression via PKC and ERK in glomerular podocytes. Biochem Biophys Res Commun. 2002;290(1):177–184. doi: 10.1006/bbrc.2001.6138. [DOI] [PubMed] [Google Scholar]
- 89.Lee E-Y, Shim MS, Kim MJ, Hong SY, Shin YG, Chung CH. Angiotensin II receptor blocker attenuates overexpression of vascular endothelial growth factor in diabetic podocytes. Exp Mol Med. 2004;36(1):65–70. doi: 10.1038/emm.2004.9. [DOI] [PubMed] [Google Scholar]
- 90.Chen S, Lee JS, Iglesias-de la Cruz MC, et al. Angiotensin II stimulates {alpha}3(IV) collagen production in mouse podocytes via TGF-β and VEGF signalling: implications for diabetic glomerulopathy. Nephrol Dial Transplant. 2005;20(7):1320–1328. doi: 10.1093/ndt/gfh837. [DOI] [PubMed] [Google Scholar]
- 91.Chuang PY, He JC. Signaling in regulation of podocyte phenotypes. Nephron Physiol. 2009;111(2):p9–p15. doi: 10.1159/000191075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen S, Kasama Y, Lee JS, Jim B, Marin M, Ziyadeh FN. Podocyte-derived vascular endothelial growth factor mediates the stimulation of alpha3(IV) collagen production by transforming growth factor-β1 in mouse podocytes. Diabetes. 2004;53(11):2939–2949. doi: 10.2337/diabetes.53.11.2939. [DOI] [PubMed] [Google Scholar]
- 93.Ichinose K, Maeshima Y, Yamamoto Y, et al. 2-(8-hydroxy-6-methoxy-1-oxo-1h-2-benzopyran-3-yl) propionic acid, an inhibitor of angiogenesis, ameliorates renal alterations in obese Type 2 diabetic mice. Diabetes. 2006;55(5):1232–1242. [PubMed] [Google Scholar]
- 94.Yamamoto Y, Maeshima Y, Kitayama H, et al. Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes. 2004;53(7):1831–1840. doi: 10.2337/diabetes.53.7.1831. [DOI] [PubMed] [Google Scholar]
- 95.Ichinose K, Maeshima Y, Yamamoto Y, et al. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a Type 1 diabetic nephropathy model. Diabetes. 2005;54(10):2891–2903. doi: 10.2337/diabetes.54.10.2891. [DOI] [PubMed] [Google Scholar]
- 96.Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol. 2006;17(11):3093–3104. doi: 10.1681/ASN.2006010064. [DOI] [PubMed] [Google Scholar]
- 97.Foster RR, Saleem MA, Mathieson PW, Bates DO, Harper SJ. Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes. Am J Physiol Renal Physiol. 2005;288(1):F48–F57. doi: 10.1152/ajprenal.00146.2004. [DOI] [PubMed] [Google Scholar]
- 98.Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol. 2006;291(2):F422–F428. doi: 10.1152/ajprenal.00448.2005. [DOI] [PubMed] [Google Scholar]
- 99.Futrakul N, Butthep P, Vongthavarawat V, et al. Early detection of endothelial injury and dysfunction in conjunction with correction of hemodynamic maladjustment can effectively restore renal function in Type 2 diabetic nephropathy. Clin Hemorheol Microcirc. 2006;34(3):373–381. [PubMed] [Google Scholar]
- 100.Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009;58(7):1471–1478. doi: 10.2337/db09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thomas S, Vanuystel J, Gruden G, et al. Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular disease. J Am Soc Nephrol. 2000;11(7):1236–1243. doi: 10.1681/ASN.V1171236. [DOI] [PubMed] [Google Scholar]
- 102.Nakagawa T. Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. Am J Physiol. 2007;292(6):F1665–F1672. doi: 10.1152/ajprenal.00495.2006. [DOI] [PubMed] [Google Scholar]
- 103.Chen S, Ziyadeh FN. Vascular endothelial growth factor and diabetic nephropathy. Curr Diab Rep. 2008;8(6):470–476. doi: 10.1007/s11892-008-0081-3. [DOI] [PubMed] [Google Scholar]
- 104.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312(5):549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 105.Rizkalla B, Forbes JM, Cao Z, Boner G, Cooper ME. Temporal renal expression of angiogenic growth factors and their receptors in experimental diabetes: role of the renin–angiotensin system. J Hypertens. 2005;23(1):153–164. doi: 10.1097/00004872-200501000-00026. [DOI] [PubMed] [Google Scholar]
- 106.Lim HS, Lip GY, Blann AD. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis. 2005;180(1):113–118. doi: 10.1016/j.atherosclerosis.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 107.Davis B, Dei Cas A, Long DA, et al. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol. 2007;18(8):2320–2329. doi: 10.1681/ASN.2006101093. [DOI] [PubMed] [Google Scholar]
- 108.Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004;15(3):566–574. doi: 10.1097/01.asn.0000115397.22519.03. [DOI] [PubMed] [Google Scholar]
- 109.Tsukada T, Yokoyama K, Arai T, et al. Evidence of association of the ecNOS gene polymorphism with plasma NO metabolite levels in humans. Biochem Biophys Res Comm. 1998;245(1):190–193. doi: 10.1006/bbrc.1998.8267. [DOI] [PubMed] [Google Scholar]
- 110.Veldman BA, Spiering W, Doevendans PA, et al. The Glu298Asp polymorphism of the NOS 3 gene as a determinant of the baseline production of nitric oxide. J Hypertens. 2002;20(10):2023–2027. doi: 10.1097/00004872-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 111.Nakayama M, Yasue H, Yoshimura M, et al. T-786–>C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation. 1999;99(22):2864–2870. doi: 10.1161/01.cir.99.22.2864. [DOI] [PubMed] [Google Scholar]
- 112.Ksiazek P, Wojewoda P, Muc K, Buraczynska M. Endothelial nitric oxide synthase gene intron 4 polymorphism in Type 2 diabetes mellitus. Mol Diagn. 2003;7(2):119–123. doi: 10.1007/BF03260027. [DOI] [PubMed] [Google Scholar]
- 113.Ezzidi I, Mtiraoui N, Mohamed MB, Mahjoub T, Kacem M, Almawi WY. Association of endothelial nitric oxide synthase Glu298Asp, 4b/a, and -786T>C gene variants with diabetic nephropathy. J Diabetes Complications. 2008;22(5):331–338. doi: 10.1016/j.jdiacomp.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 114.Zanchi A, Moczulski DK, Hanna LS, Wantman M, Warram JH, Krolewski AS. Risk of advanced diabetic nephropathy in Type 1 diabetes is associated with endothelial nitric oxide synthase gene polymorphism. Kidney Int. 2000;57(2):405–413. doi: 10.1046/j.1523-1755.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 115.Hohenstein B, Hugo CP, Hausknecht B, Boehmer KP, Riess RH, Schmieder RE. Analysis of NO-synthase expression and clinical risk factors in human diabetic nephropathy. Nephrol Dial Transplant. 2007;23(4):1346–1354. doi: 10.1093/ndt/gfm797. [DOI] [PubMed] [Google Scholar]
- 116.Quaggin SE, Coffman TM. Toward a mouse model of diabetic nephropathy: is endothelial nitric oxide synthase the missing link? J Am Soc Nephrol. 2007;18(2):364–366. doi: 10.1681/ASN.2006121396. [DOI] [PubMed] [Google Scholar]
- 117.Najafian B, Kim Y, Crosson JT, Mauer M. Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. J Am Soc Nephrol. 2003;14(4):908–917. doi: 10.1097/01.asn.0000057854.32413.81. [DOI] [PubMed] [Google Scholar]
- 118.Du XL, Edelstein D, Rossetti L, et al. Hyperglycemi-induced mitochondrial superoxide overproduction activates hexosamine pathway and induces plasminogen-activator inhibitor-1 expression by increasing Sp-1 glycosylation. Proc Natl Acad Sci USA. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 120.Gilbert RE, Marsden PA. Activated protein C and diabetic nephropathy. N Engl J Med. 2008;358(15):1628–1630. doi: 10.1056/NEJMcibr0801042. [DOI] [PubMed] [Google Scholar]
- 121.Fujiwara Y, Tagami S, Kawakami Y. Circulating thrombomodulin and hematological alterations in Type 2 diabetic patients with retinopathy. J Atheroscler Thromb. 1998;5(1):21–28. doi: 10.5551/jat1994.5.21. [DOI] [PubMed] [Google Scholar]
- 122.Bader R, Bader H, Grund KE, Mackensen-Haen S, Christ H, Bohle A. Structure and function of the kidney in diabetic glomerulosclerosis. Correlations between morphological and functional parameters. Pathol Res Pract. 1980;167(2–4):204–216. doi: 10.1016/S0344-0338(80)80051-3. [DOI] [PubMed] [Google Scholar]
- 123.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural–functional relationships in diabetic nephropathy. J Clin Invest. 1984;74(4):1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol. 2007;27(2):195–207. doi: 10.1016/j.semnephrol.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zoja C, Donadelli R, Colleoni S, et al. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-κB activation. Kidney Int. 1998;53(6):1608–1615. doi: 10.1046/j.1523-1755.1998.00905.x. [DOI] [PubMed] [Google Scholar]
- 126.Tang S, Leung JC, Abe K, et al. Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest. 2003;111(4):515–527. doi: 10.1172/JCI16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eddy AA, Kim H, Lopez-Guisa J, Oda T, Soloway PD. Interstitial fibrosis in mice with overload proteinuria: deficiency of TIMP-1 is not protective. Kidney Int. 2000;58(2):618–628. doi: 10.1046/j.1523-1755.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y, Chen J, Chen L, Tay YC, Rangan GK, Harris DC. Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J Am Soc Nephrol. 1997;8(10):1537–1545. doi: 10.1681/ASN.V8101537. [DOI] [PubMed] [Google Scholar]
- 129.Schmid H, Boucherot A, Yasuda Y, et al. Modular activation of nuclear factor-κB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55(11):2993–3003. doi: 10.2337/db06-0477.. • Documents the central role of NF-κB and a specific set of downstream target genes in the tubulointerstitial progression of human DN.
- 130.Navarro JF, Milena FJ, Mora C, Leon C, Garcia J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol. 2006;26(6):562–570. doi: 10.1159/000098004. [DOI] [PubMed] [Google Scholar]
- 131.Tesch GH. Role of macrophages in complications of Type 2 diabetes. Clin Exp Pharmacol Physiol. 2007;34(10):1016–1019. doi: 10.1111/j.1440-1681.2007.04729.x. [DOI] [PubMed] [Google Scholar]
- 132.Morcos M, Sayed AA, Bierhaus A, et al. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes. 2002;51(12):3532–3544. doi: 10.2337/diabetes.51.12.3532. [DOI] [PubMed] [Google Scholar]
- 133.Mizuno M, Sada T, Kato M, Fukushima Y, Terashima H, Koike H. The effect of angiotensin II receptor blockade on an end-stage renal failure model of Type 2 diabetes. J Cardiovasc Pharmacol. 2006;48(4):135–142. doi: 10.1097/01.fjc.0000245241.79959.d6. [DOI] [PubMed] [Google Scholar]
- 134.Agarwal R. Anti-inflammatory effects of short-term pioglitazone therapy in men with advanced diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290(3):F600–F605. doi: 10.1152/ajprenal.00289.2005. [DOI] [PubMed] [Google Scholar]
- 135.Yozai K, Shikata K, Sasaki M, et al. Methotrexate prevents renal injury in experimental diabetic rats via anti-inflammatory actions. J Am Soc Nephrol. 2005;16(11):3326–3338. doi: 10.1681/ASN.2004111011. [DOI] [PubMed] [Google Scholar]
- 136.Usui HK, Shikata K, Sasaki M, et al. Macrophage scavenger receptor-a-deficient mice are resistant against diabetic nephropathy through amelioration of microinflammation. Diabetes. 2007;56(2):363–372. doi: 10.2337/db06-0359. [DOI] [PubMed] [Google Scholar]
- 137.Chander PN, Gealekman O, Brodsky SV, et al. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen. J Am Soc Nephrol. 2004;15(9):2391–2403. doi: 10.1097/01.ASN.0000135971.88164.2C. [DOI] [PubMed] [Google Scholar]