Abstract
Purpose
Donor T cells respond to minor histocompatibility antigens (mHA) resulting in both graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) after allogeneic hematopoietic stem cell transplantation (HSCT). Since relatively few mHA are known, we developed a new approach to predict and subsequently validate candidate mHA.
Experimental Design
We developed an algorithm based on genetic disparities between Y and X chromosome-encoded proteins and known requirements for binding to HLA class I molecules to predict Y-chromosome derived HLA A*0201 restricted peptides (HY) and ranked peptides based on potential immunogenicity. We evaluated T cell responses to 41 candidate peptides in 28 male recipients with female donors (FM), 22 male recipients with male donors (MM) and 26 normal individuals. All patients and donors were HLA A*0201 positive.
Results
Thirteen peptides derived from 5 proteins elicited significantly greater T cell responses in FM patients compared to MM patients and in normal females compared to normal males. Six peptides were more immunogenic than the only previously known HLA A*0201-restricted Y-encoded mHA. 27 of 28 FM patients responded to at least 1 HY peptide but despite a common Y chromosome mismatch and expression of HLA A*0201, each patient responded to a unique set of peptides.
Conclusions
Novel HLA A*0201 restricted HY epitopes can be predicted and validated in patients after allogeneic HSCT. Highly diverse patterns of T cell response against these epitopes have been identified. Prospective monitoring of responses to large panels of immunogenic peptides can facilitate the identification of clinically relevant targets of GVHD and GVL.
INTRODUCTION
In patients who undergo allogeneic hematopoietic stem cell transplantation (HSCT) with grafts from HLA-identical donors, minor histocompatibility antigens (mHA) are presumed to be the primary immunologic targets of alloreactive T cells (1,2). Minor histocompatibility antigens arise as a consequence of genetic polymorphisms which lead to the expression of unique sets of potentially immunogenic peptides in each individual (3). Following allogeneic HSCT, donor T cells recognize these epitopes as foreign antigens and develop sustained immune responses targeting multiple mHA. Immunologic targeting of mHA that are widely expressed in normal tissues results in graft versus host disease (GVHD), a common toxicity of allogeneic HSCT. In contrast, graft versus leukemia (GVL) results from expression of mHA on recipient leukemia cells and their destruction by donor T cells. The development of new approaches to enhance GVL and prevent GVHD is limited by our lack of understanding of mechanisms responsible for the immunogenicity of mHA in leukemia cells and normal tissues and by the small number of mHA that have actually been identified (4-6).
Current methods to identify mHA epitopes are dependent on the isolation and expansion of T cell clones that are most often derived from recipients with GVHD after engraftment of donor T cells. T cell clones that display selective reactivity with recipient target cells but not with donor cells are presumed to be specific for mHA (7,8). Once T cell clones are established, elegant methods have been devised to identify the target peptide and to determine the genetic basis for its immunogenicity (9-15). With these methods, over 30 human mHA epitopes presented by various HLA class I and class II molecules have been identified (16). However, it is likely that these represent only a very small fraction of the potentially immunogenic mHA that can be derived from the very large number of nucleotide polymorphisms and copy number variations that exist in the human genome (17,3). The small number of known mHA represents a major obstacle for studies of GVHD and GVL responses in large patient cohorts and for comparing T cell responses among different patients. For example, without larger panels of mHA that can be used to monitor T cell responses after HSCT, it has not been possible to determine whether acute and chronic GVHD represent immune responses to distinct epitopes or whether other factors are responsible for the distinct clinical manifestations of these syndromes. Similarly, it has not been possible to determine whether distinct sets of mHA epitopes distinguish GVHD and GVL responses.
Although genes encoding mHA exist throughout the human genome, approximately one third of the known mHA are encoded by a relatively small set of genes located on the Y-chromosome (HY). These genes are located in non-recombining regions of the Y-chromosome, have substantial disparities with their X-chromosome derived homologs and are widely expressed in normal tissues. HY peptides are recognized as foreign by T cells from female donors and elicit long-lived CD4 and CD8 T cell responses (18-28). Male recipients of stem cell grafts from female donors (MF) represent a distinct group of patients who share the identical X-Y genetic mismatch with their donors. Importantly, immunologic targeting of HY proteins results in a relatively high incidence of acute and chronic GVHD when male recipients receive hematopoietic stem cell grafts from female donors (29,30).
The clinical manifestations of GVHD and GVL likely result from the immunologic recognition of multiple mHA in each patient. However, the actual mHA targeted in individual patients are seldom known and the analysis of multiple mHA in large cohorts of patients having the same genetic mismatch with their donors has not been possible. To address this issue we developed a bioinformatic approach to predict peptides derived from Y-chromosome encoded genes with high affinity binding to HLA A*0201. Focusing on HY epitopes allowed us to validate the immunogenicity of predicted peptides in blood samples obtained from 50 HLA A*0201 positive male patients after allogeneic HSCT. Of 41 predicted peptides that were tested, a subset of 13 peptides were found to elicit significant and persistent T cell responses in male recipients with female donors (FM) compared with male recipients with male donors (MM). Twenty-seven of 28 FM patients responded to at least one of the predicted HY peptides, but responses were highly variable and each patient developed a unique pattern of T cell responses against different sets of peptides. With this approach, it is now possible to greatly expand the assessment of immune responses after allogeneic HSCT and to prospectively monitor T cell responses to novel epitopes identified primarily on the basis of genetic disparity between recipients and donors. Further prospective studies using this unbiased approach can be undertaken to identify specific patterns of response associated with distinct clinical outcomes such as GVHD and GVL and allow us to identify factors that affect immune response to mHA.
MATERIALS AND METHODS
Peptide prediction and ranking
Amino acid sequences of all known Y-chromosome encoded proteins were downloaded from the Swissprot database1. All 9 and 10 amino acid sequences from these proteins were analyzed using the HLA A*0201 binding prediction tool of the Immune Epitope Database and Analysis resource, (IEDB-AR)2. The following ranking protocol was applied to identify peptides with the greatest likelihood of being recognized as HY antigens. First, we included only peptides predicted to have very high binding affinity (≤100nM) for HLA A*0201. Peptides derived from hypothetical proteins, variants not yet proven to be expressed and proteins expressed only in male specific tissues (testis, prostate) were excluded since those tissues are rarely involved in GVHD. Peptides with 100% identical homologs in the human proteome (mostly chromosome-X derived) identified by protein-BLAST algorithm3 were also excluded. Remaining peptides were compared to their homologs and ranked based on predicted properties of specific amino acid disparities. Peptides with amino acid disparities that are predicted to change peptide conformation and alter T-cell receptor interactions were ranked high. Peptides with disparities predicted to have similar conformations (e.g. I/V, I/L, I/M, L/V, L/M, and V/M) were ranked low. For amino acid disparities that involved HLA anchor positions, we assigned a high rank when the X-encoded homolog was predicted to have a weak HLA A*0201 binding affinity and was therefore predicted not be presented in the female donor (31,32). As a result of this selection, 43 peptides were ranked as potential mHA. Forty-one peptides were synthesized (2 failed synthesis) (Proimmune, Oxford UK) at a purity of >85% and were used for subsequent in vitro functional assays (Table 1).
Table 1.
T cell response to predicted HY peptides after allogeneic HSCT in FM and MM patients.
| FM (n=28) |
MM (n=22) |
p-value |
||||
|---|---|---|---|---|---|---|
| Peptide | Sequence | Median^ | range | Median^ | range | FM vs MM |
| JARID1D311# | FIDSYICQV | 9 | 0-89 | 1 | 0-18 | 0.006 |
| JARID1D467 | NVMPVLDQSV | 7 | 0-265 | 0 | 0-37 | 0.0007 |
| JARID1D640 | KMAAFPETL | 26 | 0-518 | 0 | 0-48 | <.0001 |
| JARID1D 933* | SLVIMQGLLV | - | - | - | - | - |
| JARID1D1165 | SLMASSPTSI | 13 | 0-189 | 0 | 0-20 | <.0001 |
| JARID1D1166 | LMASSPTSI | 6 | 0-66 | 1 | 0-30 | 0.0473 |
| JARID1D1205 | HLLTSPKPSL | 11 | 0-196 | 0 | 0-17 | 0.0012 |
| UTY148 | KAFQDVLYV | 1 | 0-55 | 0 | 0-9 | 0.041 |
| UTY190 | ALIDCNPCTL | 6 | 0-79 | 0 | 0-73 | 0.0016 |
| UTY514 | KLLSSGAFSA | 13 | 0-196 | 0 | 0-28 | 0.0033 |
| UTY515 | LLSSGAFSA | 17 | 0-380 | 0 | 0-39 | 0.0014 |
| UTY558 | YLQQNTHTL | 10 | 0-342 | 0 | 0-30 | 0.0003 |
| UTY719 | LLIADNPQL | 3 | 0-273 | 0 | 0-18 | 0.0082 |
| DDX3Y428 | FLLDILGAT | 37 | 0-451 | 1 | 0-67 | 0.0014 |
| PCDH11Y23 | CLLSGTYIFA | 0 | 0-36 | 0 | 0-28 | 0.8298 |
| PCDH11Y29 | YIFAVLLVCV | 5 | 0-84 | 0 | 0-28 | 0.0711 |
| PCDH11Y437-1 | FLLENAAYL | 1 | 0-76 | 3 | 0-27 | 0.9321 |
| PCDH11Y437-2 | FLLENAAYLD | 22 | 0-118 | 0 | 0-23 | <.0001 |
| PCDH11Y591 | FTHNEYKFYV | 1 | 0-85 | 0 | 0-27 | 0.2974 |
| USP9Y271 | TLKKYFIPV | 9 | 0-169 | 0 | 0-66 | 0.054 |
| USP9Y620 | NLATYMNSI | 0 | 0-28 | 0 | 0-11 | 0.0769 |
| USP9Y624 | YMNSIRLYA | 7 | 0-135 | 1 | 0-45 | 0.0995 |
| USP9Y965 | KLTQINFNM | 0 | 0-41 | 0 | 0-44 | 0.3904 |
| USP9Y1046 | KLMPPDRTAV | 9 | 0-723 | 0 | 0-33 | 0.0669 |
| USP9Y1098 | ALLMPAGVPL | 0 | 0-42 | 0 | 0-26 | 0.2189 |
| USP9Y1099-1 | LLMPAGVPL | 3 | 0-190 | 0 | 0-24 | 0.1108 |
| USP9Y1099-2 | LLMPAGVPLT | 14 | 0-475 | 2 | 0-53 | 0.1754 |
| USP9Y1299 | ALLPTALDAL | 0 | 0-90 | 0 | 0-37 | 0.3572 |
| USP9Y1401 | LLVSEIDWL | 7 | 0-29 | 2 | 0-45 | 0.4408 |
| USP9Y1468 | FIFPASKVYL | 4 | 0-78 | 0 | 0-66 | 0.3766 |
| USP9Y1504* | FELLVALAIG | - | - | - | - | - |
| USP9Y1506 | LLVALAIGCV | 0 | 0-263 | 0 | 0-47 | 0.6012 |
| USP9Y1578 | YMIPSIRNSI | 4 | 0-84 | 0 | 0-30 | 0.0224 |
| USP9Y1785 | RLLIKKLPRV | 0 | 0-30 | 0 | 0-16 | 1 |
| USP9Y1786 | LLIKKLPRV | 2 | 0-31 | 1 | 0-48 | 0.7573 |
| USP9Y1822 | RELDMGPYTV | 0 | 0-15 | 0 | 0-8 | 1 |
| USP9Y1999 | FMHNRLQYSL | 3 | 0-31 | 1 | 0-33 | 0.1899 |
| USP9Y2006 | YSLEYFQFV | 1 | 0-65 | 0 | 0-46 | 0.1423 |
| USP9Y2033 | YLLPEAEEI | 0 | 0-38 | 0 | 0-60 | 0.6592 |
| USP9Y2156 | HLLRATLNLL | 0 | 0-18 | 0 | 0-5 | 0.9076 |
| USP9Y2196 | QLLKLNVPA | 0 | 0-22 | 0 | 0-5 | 0.9015 |
| USP9Y2337-1 | YLDLLFQIL | 0 | 0-61 | 0 | 0-79 | 0.4868 |
| USP9Y2337-2 | YLDLLFQILL | 0 | 0-28 | 0 | 0-5 | 0.1824 |
Previously known HLA A*0201 restricted HY
Failed synthesis
Number of spots/200,000 responder cells plated.
Background values for negative control peptides are subtracted. Assays were carried out in duplicate wells. Peptides are named after the gene they are derived from and assigned a number based on the position of the first amino acid in the protein sequence. PCDH11Y437-1 and PCDH11Y437-2 represent 9 and 10mers which start at the same position.
Patients and donors
Blood samples were obtained from 50 adult male patients who had undergone allogeneic HSCT at the Dana-Farber Cancer Institute and Brigham and Women's Hospital, Boston MA. All patients provided informed consent for immunologic studies after HSCT and were enrolled on a clinical protocol for blood sample collection approved by the Human Subjects Protection Committee of the Dana-Farber/Harvard Cancer Center. The clinical characteristics of these patients are summarized in Table 2. Twenty-eight patients received grafts from female donors (FM) and 22 from male donors (MM). All patients and donors were HLA-identical and HLA A*02 positive. HLA typing was performed by serology for individuals with related sibling donors. Molecular typing for HLA A*0201 was confirmed in 10 FM and 12 MM patients with unrelated donors. The two groups of patients were similar with respect to underlying hematologic malignancy, intensity of transplant conditioning and stem cell source. A lower fraction of FM patients had unrelated donors (32%) compared to MM patients (59%) but this difference was not statistically significant. All 28 FM patients developed chronic GVHD; 82% of MM patients had chronic GVHD and the majority of patients in both groups had extensive disease. Samples were obtained >6 months post transplant for both groups although there was a wide range of times for collection of these samples. In addition, blood samples were obtained from 26 healthy HLA A*02 positive individuals (15 females and 11 males).
Table 2.
Clinical characteristics of patients evaluated for T cell response to HY epitopes.
| |
FM group n=28 |
MM group n=22 |
P value |
|---|---|---|---|
| Age (median) |
48 (19-64) |
52 (19-63) |
0.76 |
| Indication for HSCT | |||
| Leukemia/ MDS | 17/28 (60%) | 12/22 (55%) | 0.71 |
| CLL / NHL / HD | 10/28 (36%) | 8/22 (36%) | |
| Other (MM, SAA, CMML) |
1/28 (4%) |
2/22 (9%) |
|
| Conditioning Regimen | |||
| Myeloablative | 10/28 (36%) | 9/22 (40%) | 0.77 |
| Non-myeloablative |
18/28 (64%) |
13/22 (60%) |
|
| Stem Cell Source | |||
| Mobilized peripheral blood | 26/28 (93%) | 20/22 (91%) | 1 |
| Bone marrow |
2/28 (7%) |
2/22 (9%) |
|
| Donor Source: HLA-identical | |||
| Unrelated donors | 9/28 (32%) | 13/22 (59%) | 0.09 |
| Related donors |
19/28 (68%) |
9/22 (41%) |
|
| Acute GVHD (grade 2-4) |
10/28(36) |
1/22(5) |
0.01 |
| Chronic GVHD | 28/28 | 18/22 | 0.06 |
| Limited | 8/28 (29%) | 6/22 (27%) | |
| Extensive |
20/28 (71%) |
12/22 (55%) |
|
| Date of Sample (median and range) | |||
| Days post- transplant | 855 (200-2531) | 609 (189-2407) | 0.14 |
FM: male patients with female donors. MM: male patients with male donors. MDS: myelodysplastic syndrome, CLL: chronic lymphocytic leukemia, NHL: non Hodgkin lymphoma, HD: Hodgkin's disease, MM: multiple myeloma, SAA: severe aplastic anemia, CMML: chronic myelo-monocytic leukemia.
ELISPOT assay
Peripheral blood mononuclear cells (PBMC) were obtained from EDTA anti-coagulated blood samples after ficoll-hypaque density gradient centrifugation. Fresh PBMC (1-2×106) from patients and normal donors were stimulated with individual peptides (10μg/ml) in 24 well plates for one week. On day 6, T2 cells stably transfected with HLA A*0201, were pulsed with the same peptides (10μg/ml peptide with 5μg/ml ß2 microglobulin; ProImmune, Bradenton, FL) in separate wells and cultured overnight at 37C. On day 7, responding cells were counted and 200,000 PBMC were re-stimulated in duplicate with 20,000 peptide-pulsed T2 cells in ELISPOT wells that had been pre-coated with anti-IFNγ antibody. ELISPOT plates were developed with NBT/BCIP reagent and plates were scanned (S4 analyzer, Immunospot, CTL analyzer LLC). Peptides derived from HIV (KLTPLCVTL) and HCV (DLMGYIPLV) known to have high affinity binding for HLA A*0201 were used as negative controls and HLA A*0201 peptides derived from EBV (GLCTLVAML) and CMV (NLVPMVATV) as positive controls for each assay (ProImmune). The number of spots in duplicate wells was averaged.
Statistical Methods
For two-sample comparison, a two-sided Wilcoxon-Rank-Sum test was performed for continuous variables and a two-sided Fisher's exact for categorical variables. P-values for comparisons of various groups are nominal, without adjusting for multiple comparisons. To establish a cut-off point for positive ELISPOT, the recursive partitioning method and receiver operator characteristic (ROC) curve method were used. ELISPOT value of 20 spots/200,000 cells above the negative control was established as the cut-off value for determination of positive T cell reactivity for all peptides. Spearman's rank test was used for correlation analysis. Heatmap was generated using dChip software (33). Potential confounders for T cell reactivity were examined in multivariable linear and logistic regression models. All ELISPOT values were log10 transformed prior to linear modeling.
RESULTS
Detection of T cell response to predicted HY peptides
Based on our ranking algorithm (see methods), 43 peptides (20 -9mers and 23 -10mers) derived from 5 proteins were predicted to be potential HY epitopes (Table 1). Thirteen peptides represented 9 and 10mers of the same epitopes (marked with consecutive numbers in Table 1). The only previously known HLA A*0201 restricted HY mHA (JARID1D311) was predicted and ranked first according to our algorithm. Forty-one peptides were synthesized (two failed synthesis) and high binding affinity to HLA A*0201 was confirmed for 39 peptides using REVEAL assay (ProImmune).
Peripheral blood samples obtained from 50 male patients after allogeneic HSCT were evaluated for specific T cell responses to each of the 41 predicted HY peptides using ELISPOT (Table 2). Responses could be blocked by anti HLA Class I antibodies but not with anti HLA class II antibodies (data not shown) confirming that ELISPOT results reflect the frequency of CD8+ T cells specific for the tested peptide. HY peptides are expected to be more immunogenic in FM patients than in MM patients. The ELISPOT response to each peptide in 28 FM patients was therefore compared to responses detected in 22 MM patients. Table 1 summarizes the T cell responses to each individual peptide in FM and MM patients. Significantly greater responses in FM patients were observed for 15 peptides, including all 13 peptides derived from DDX3Y, JARID1D and UTY proteins plus one PCDH11Y and one USP9Y peptide. Responses to 2 peptides (USP9Y1578 and UTY148) were low intensity and detected in less than 20% of FM patients and were excluded from further analysis. As expected, the previously known JARID1D-derived HLA A*0201 restricted mHa was included in the group of peptides with high level T cell responses restricted to FM patients. However, six peptides elicited more frequent responses in FM patients than JARID1D311.
Quantitative assessment of T cell responses against immunogenic HY peptides
The magnitude of T cell response measured by ELISPOT to each of the 13 selected HY peptides in FM and MM patients, normal females (NF) and normal males (NM) is summarized in Figure 1. Response to negative control peptide (HIV or HCV) has been subtracted in each assay. ELISPOT values >20 spots/200,000 cells were considered positive. The magnitude of T cell response to this set of 13 HY peptides is significantly greater in the FM patient group than in MM patients, NF or NM donors (median 12, 0, 0, 0 spots/200,000 cells respectively; p<0.0001 for comparison of FM to each group separately). In 26 healthy individuals (15 NF, 11 NM), 32 positive ELISPOT responses were detected in 332 assays, 25 (78%) in NF, and 7 (22%) in NM (p=0.01). Interestingly, five females account for 22/25(88%) positive responses in NF. Long-lived T cell responses to HY have previously been reported following pregnancies with male fetuses but maternity history for the normal females we tested is not available. T cell responses to the EBV positive control peptide are not shown in Figure 1. EBV responses were similar among FM, MM, NF and NM groups (median 53, 54, 78, 24 spots/200,000 cells respectively; p=NS for all comparisons) but significantly greater than the response to HY peptides in FM patients (p=0.017) as well as in the other groups (p<0.02). Responses to both control and HY peptides were not detected in patients and donors that were HLA A*0201 negative (data not shown).
Figure 1.
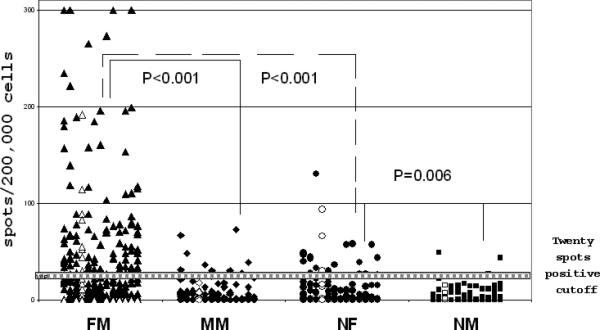
T cell responses to 13 immunogenic HY peptides in FM patients, MM patients, normal females (NF) and normal males (NM).
13 HY peptides are listed in the same order on the X axis in patient and control groups. The Y axis represents the number of spots/200,000 cells in ELISPOT assay for each peptide after subtraction of values for negative control peptides (HIV or HCV). Values >20 spots/200,000 cells are considered positive. Open symbols represent the previously known HLA A*0201 restricted HY.
Relative immunogenicty of selected HY peptides
To further characterize the immunogenicity of HY peptides, we compared the frequency and magnitude of positive responses to each peptide in FM patients (Figure 2). No correlation was found between intensity and frequency of T cell responses. For example, some peptides (JARID1D467, UTY719) elicited strong ELISPOT responses but these responses were detected in relatively few patients. Other peptides (PCDH11Y437b, DBY428 and JARID1D640) elicited weaker T cell responses but these were detected in greater numbers of FM patients. The previously reported mHA (JARID1D311) elicited a relatively strong response but this was only detected in 33.3% of FM patients.
Figure 2.

No correlation between intensity and frequency of T cell response to individual HY peptides in FM patients.
The percentage of FM patients (n=28) with a positive response to each HY peptide (n=13) is shown on the X axis. The Y axis illustrates the magnitude of response to each HY peptide (spots/200,000 cells) in the same individuals. The open square represents the previously reported HY (JARID1D311), which elicited a relatively strong response but this was only detected in 33.3% of FM patients.
Patterns of T cell responses to HY epitopes and association with GVHD
Despite identical genetic mismatch, T cell responses to the same set of HY derived peptides were very heterogeneous among FM patients. To determine whether subsets of patients developed similar patterns of HY peptide responses we used hierarchical clustering4 to group ELISPOT results obtained in all FM patients (Figure 3). Several interesting patterns of response emerged from this analysis. Patients in Group A are characterized by high level responses to DDX3Y and UTY peptides. Responses to PCDH11Y are relatively weak and responses to most JARID1D peptides are not present. In contrast, patients in Group C are characterized by strong responses to PCDH11Y and only to some JARID1D and UTY peptides, but responses to DDX3Y are weak. Patients in Group B are very heterogeneous and responded to different JARID1D, DDX3Y and PCDH11Y peptides in contrast to weak or absent responses to UTY peptides. These groups had no distinctive clinical features. Interestingly, six out of 7 (86%) patients in Group C had myeloid leukemia compared to 11/21 (52%) in A or B, but this was not statistically significant (p=0.19).
Figure 3.
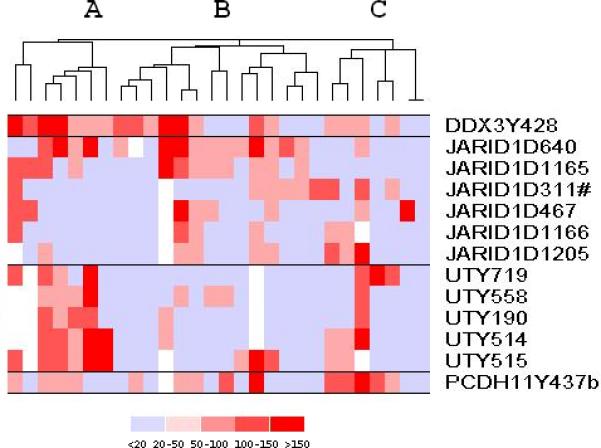
Patterns of response to 13 immunogenic HY peptides among 28 FM patients. Quantitative T cell responses to each HY peptide measured by ELISPOT are clustered using dChip software (http://biosun1.harvard.edu/complab/dchip). Each row represents one of the 13 immunogenic HY peptides and each column represents a single FM patient. The intensity of ELISPOT reactivity for each peptide is represented on a color scale. A blank represents a missing data point; light blue represents a negative response; positive responses are in red and scaled as shown. Three patient clusters (A, B, C) with similar responses emerged from this analysis.
Correlation of T cell responses with clinical course
Blood samples from patients were obtained over a wide range of times after allogeneic HSCT. Figure 4a plots all T cell responses directed against the panel of 13 HY peptides that were observed in FM patients in the first 5 years post-transplant. Responses were observed throughout this period. Although all patients in the FM group developed chronic GVHD after transplant, some patients had inactive or resolved disease at the time of analysis. Nevertheless, when all responses of all patients were assessed as a whole, the magnitude of response measured by ELISPOT did not correlate with time from transplant or with clinical grade of GVHD at the time of analysis.
Figure 4.
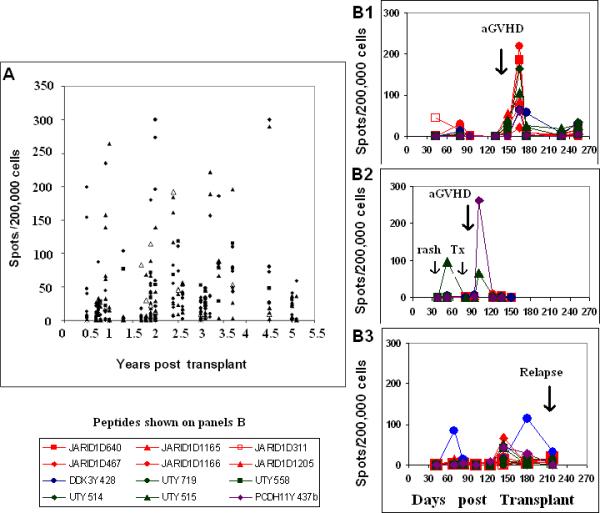
Persistence of T cell response to HY peptides in FM patients and temporal association with clinical acute GVHD.
Panel 4a depicts all HY peptide responses in 28 FM patients. The X axis represent years from HSCT to sample collection. The Y axis represents the number of spots/200,000 cells after subtraction of appropriate negative control. Values >20 spots/200,000 cells are considered positive. Responses can be detected as long as 5 years post transplant. Open triangles represent responses against the known HLA A*0201 restricted HY peptide. Panel 4b shows the prospective monitoring of three additional HLA A*0201 FM patients for 6-9 months following transplant. Samples were obtained monthly at regular follow-up visits. Patient A developed acute GVHD at approximately 3 months post-transplant. Patient B developed a rash at day 52 that responded to treatment. He subsequently developed grade 3 acute GVHD at 94 days post transplant. Patient C did not develop GVHD. ALL relapse was suspected at day 137 when a mediastinal mass was detected and immune suppression was rapidly tapered. Bone marrow relapse was confirmed at day 211. All three patients developed significant ELISPOT responses post transplant but to different HY peptides. HY responses were no longer detected after high dose steroids or other therapy was initiated.
Three additional HLA A*0201 FM patients have been monitored prospectively for at least 6 months following transplant. Two patients developed acute GVHD at approximately 3 months post-transplant. As shown in Figure 4b, Patient B1 developed high level T cell responses to DDX3Y and most JARID1D peptides including the previously known HY mHA at the time that clinical acute GVHD became evident but not to UTY and PCDH11Y peptides. Responses were not detectable prior to the diagnosis of acute GVHD or after high-dose corticosteroid treatment was initiated. The pattern of response to HY peptides was very different in Patient B2 who participated in an experimental GVHD prophylaxis protocol. Response to one UTY peptide was first detected 52 days post transplant. At that time, the patient had a mild rash that was treated with topical steroids and was given an experimental agent followed by clinical improvement. At day 94 post transplant he presented with acute GVHD of the colon and a marked response to PCDH11Y was detected at the following visit. Responses to DDX3Y and JARID1D peptides were not detected throughout this period. Patient B3 did not develop GVHD. A transient response to only 1 HY peptide (DDX3Y428) was detected in first 3 months post-HSCT. ALL relapse was first suspected on day 137 when a mediastinal mass was detected. Immune suppression was tapered rapidly and transient weak responses to several HY peptides were detected on day 148. T cell response to only 1 peptide persisted (DDX3Y428) but this response also waned when bone marrow relapse was confirmed at day 211.
DISCUSSION
One major barrier for studying allogeneic immune response is the low number of mHA identified as targets of GVHD and GVL (34). No studies have been able to prospectively monitor responses to multiple mHA in large numbers of patients as T cell function is reconstituted after allogeneic HSCT. To address this issue we developed a bioinformatic approach to predict HY peptides with high affinity binding to HLA A*0201. By focusing on proteins encoded by genes on the Y chromosome and a relatively common HLA allele, we were able to validate our predictions in blood samples obtained from 50 HLA A*0201 positive male patients who had undergone allogeneic HSCT at our institution. Thirteen HY peptides were validated by identifying high level of responses among FM patients while responses were absent among MM patients. When tested in HLA A*0201 positive normal individuals, significant responses were also detected in some normal females but not in normal males. T cell responses to these HY peptides were not detected in patients or normal donors who were HLA A*0201 negative. Our findings are consistent with previous studies of memory T cell responses to individual HY presented by other HLA class I and class II alleles in normal females that can persist for many years after exposure to male cells during normal pregnancy (35-38).
Only one HLA A*0201 restricted HY mHA has previously been identified (19). This epitope, derived from JARID1D (SMCY), was predicted (JARID1D311) and verified as immunogenic in FM patients and normal females, supporting both the validity of our bioinformatic algorithms and the functional sensitivity of our post-transplant ELISPOT assays. However, responses were only detected in 33% of FM patients (39,36) and 6 other peptides elicited more frequent responses in FM patients. This included 2 other peptides derived from JARID1D as well as peptides from 4 other Y-encoded proteins not previously known to elicit HLA A*0201 restricted responses. These observations suggest that the relatively small number of known mHA represent only a small fraction of the mHA that actually elicit immune responses after allogeneic HSCT. Since conventional methods for identifying mHA depended on the selection and expansion of individual T cell clones, these approaches were necessarily limited by the ability of individual T cell clones to expand in vitro and by the types of donor and recipient cells used to define alloreactivity. Our approach extends previous methods by providing a comprehensive and unbiased screening for identification of multiple novel mHA. This method facilitates the identification of target antigens that reflect the broad nature of T cell responses to the large numbers of mHA that distinguish donors and recipients. It also enables the investigation of target antigens that may be selectively expressed in recipient tissues frequently involved in GVHD, such as liver, gut, mucous membrane, salivary gland, lung and skin.
All patients in our study expressed HLA A*0201 and all FM patients were genetically mismatched with donors for all Y encoded proteins. Nevertheless, the responses to Y encoded HLA A*0201 peptides were very heterogeneous. In fact, none of the 28 FM patients exhibited identical patterns of response to these 13 immunogenic HY peptides. While the reasons for this heterogeneity are not clear, one explanation may be that this is a reflection of the limited sensitivity of our ELISPOT assay. A more sensitive assay might be able to detect responses to more HY peptides in all FM patients and reveal that the heterogeneity we have observed primarily reflects quantitative rather than qualitative differences. Alternatively, differences in protein expression, antigen processing (40) or MHC presentation may also be responsible for a personalized pattern of response against mHa. Thus, within the group of only 28 FM patients, we were able to identify clusters of patients who respond similarly to subsets of HY peptides. This may reflect an interaction between patient-specific genetic and environmental factors. The repertoire of potential immunogenic peptides is determined by patient/donor mismatch, HLA type and proteasomal processing. However, the actual peptides presented in different tissues are affected by the extent of local inflammation or tissue damage. Moreover, the repertoire of donor T cells also reflects different prior exposure to these same or similar epitopes in the context of pregnancy, blood transfusion or other exposures. Previous studies in our laboratory have demonstrated that FM patients frequently develop specific antibody responses to HY proteins and that HY antibody responses are significantly associated with the development of chronic GVHD (41,42,26). The presence or absence of B cell responses to HY proteins may also influence specific T cell responses to peptide epitopes derived from the same proteins. Having identified a new panel of immunogenic HY epitopes, further studies can address these specific issues and provide us with a more comprehensive understanding of T and B cell responses in patients undergoing sex-mismatched transplant.
Although FM patients were not studied prospectively at serial time points, our results reveal a remarkable persistence of T cell responses to HY peptides for many years after transplant. Given the heterogeneity of this patient group and the variability of sampling, we were not able to correlate specific T cell responses to any clinical outcome variable. We were also not able to determine whether T cell responses to HY peptides develop in patients who do not develop GVHD. To address these issues, we have initiated prospective monitoring of T cell responses to a defined panel of HY peptides in FM patients. Results in 3 representative patients (Figure 4b) show that T cell responses increase when two of these patients develop acute GVHD but the target epitopes varied greatly. Moreover, successful treatment of acute GVHD was associated with a marked reduction in the number of circulating T cells reactive with these peptides. In one patient that did not develop GVHD, only a transient weak response to a single peptide was detected. Prospective analysis of a large cohort of FM patients will be necessary to determine whether quantitative responses to HY peptides correlate with clinical manifestations of GVHD or GVL and response to therapy and whether this may become a clinically relevant monitoring tool for patients undergoing allogeneic HSCT.
HLA A*0201 is the most common HLA allele expressed in approximately 40-50% of our patients. In future studies, our approach can be extended to other HLA class I and class II alleles. Extending this analysis to more comprehensive sets of HY peptides will be necessary to effectively capture the breadth of T cell responses to this defined set of important target antigens. Similarly, this bioinformatics-driven approach can also be extended to predict immunogenic epitopes derived from single nucleotide polymorphisms or gene deletions present throughout the genome. Unlike HY disparities, prediction of autosomal mHA must be coupled to genetic typing of patients and donors to identify potentially immunogenic disparities between each recipient/donor pair. Newer genetic platforms coupled with sophisticated bioinformatics tools have made such studies possible.
Acknowledgments
Y.O- designed research, performed research and wrote the paper. H.K- analyzed data V.B- preformed Epitope predictions. L.B and M.M- performed research. C.W, S.S, R.B and D.K- Contributed to Study design and performance. R.S and J.A- A senior clinical researcher. J.R -designed research, and wrote the paper. Supported by NIH grants CA142106 and AI29530 and the Ted and Eileen Pasquarello Research Fund and the American Physicians Fellowship for Medicine in Israel.
Footnotes
Translational Relevance
Minor histocompatibility antigens (mHA) are the primary targets for GVHD and GVL. All mHA arise from genetic disparities between recipients and stem cell donors but relatively few mHA have been identified. To address this issue we developed a new algorithm for predicting mHA based on known genetic disparities between recipient and donor. The immunogenicity of candidate peptides was subsequently validated in patients undergoing allogeneic HSCT and normal donors. Using this approach we identified 13 novel HLA A*0201 restricted peptides derived from Y chromosome-encoded proteins. These peptides elicited T cell responses in male recipients of stem cells from female donors but not in male patients with male donors. To identify additional mHA, this approach can be extended to other HLA alleles and genetic disparities. This new approach will enable prospective monitoring of responses to panels of immunogenic peptides to determine which epitopes may be clinically relevant targets of GVHD and GVL.
dChip available at http://biosun1.harvard.edu/complab/dchip
References
- 1.Bleakley M, Riddell SR. Molecules and mechanisms of the graft versus leukemia effect. Nat Rev Cancer. 2004;4:371–80. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 2.Shlomchik WD. Graft versus Host disease. Nat Rev Immunol. 2007;7:340–52. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 3.Mullally A, Ritz J. Beyond HLA: the significance of genomic variation for allogeneic hematopoietic stem cell transplantation. Blood. 2007;109:1355–62. doi: 10.1182/blood-2006-06-030858. [DOI] [PubMed] [Google Scholar]
- 4.Goulmy E. Minor histocompatibility antigens: from transplantation problems to therapy of cancer. Hum Immunol. 2006;67:433–8. doi: 10.1016/j.humimm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Spaapen RM, Lokhorst HM, Van den Oudenalder K, et al. Toward targeting B cell cancers with CD4+ CTLs: identification of a CD19-encoded minor histocompatibility antigen using a novel genome-wide analysis. J Exp Med. 2008;205:2863–72. doi: 10.1084/jem.20080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norde WJ, Overes IM, Maas F, et al. Myeloid leukemic progenitor cells can be specifically targeted by minor histocompatibility antigen LRH-1–reactive cytotoxic T cells. Blood. 2009;113:2312–23. doi: 10.1182/blood-2008-04-153825. [DOI] [PubMed] [Google Scholar]
- 7.den Haan JM, Sherman NE, Blokland E, et al. Identification of a graft versus host disease-associated human minor histocompatibility antigen. Science. 1995;268:1476–80. doi: 10.1126/science.7539551. [DOI] [PubMed] [Google Scholar]
- 8.Wallny HJ, Rammensee HG. Identification of classical minor histocompatibility antigen as cell-derived peptide. Nature. 1990;343:275–8. doi: 10.1038/343275a0. [DOI] [PubMed] [Google Scholar]
- 9.de Rijke B, van Horssen-Zoetbrood A, Veenbergen S, et al. Refinement of molecular approaches to improve the chance of identification of hematopoietic-restricted minor histocompatibility antigens. J Immunol Methods. 2008;329:125–37. doi: 10.1016/j.jim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 10.van de Corput L, Chaux P, Van der Meijden ED, et al. A novel approach to identify antigens recognized by CD4 T cells using complement-opsonized bacteria expressing a cDNA library. Leukemia. 2005;19:279–85. doi: 10.1038/sj.leu.2403583. [DOI] [PubMed] [Google Scholar]
- 11.Akatsuka Y, Nishida T, Kondo E, et al. Identification of a polymorphic gene, BCL2A1, encoding two novel hematopoietic lineage-specific minor histocompatibility antigens. J Exp Med. 2003;197:1489–1500. doi: 10.1084/jem.20021925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawase T, Akatsuka Y, Torikai H, et al. Alternative splicing due to an intronic SNP in HMSD generates a novel minor histocompatibility antigen. Blood. 2007;110:1055–63. doi: 10.1182/blood-2007-02-075911. [DOI] [PubMed] [Google Scholar]
- 13.Brickner AG, Warren EH, Caldwell JA, et al. The immunogenicity of a new human minor histocompatibility antigen results from differential antigen processing. J Exp Med. 2001;193:195–206. doi: 10.1084/jem.193.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamei M, Nannya Y, Torikai H, et al. HapMap scanning of novel human minor histocompatibility antigens. Blood. 2009;113:5041–8. doi: 10.1182/blood-2008-07-171678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spierings E, Brickner AG, Caldwell JA, et al. The minor histocompatibility antigen HA-3 arises from differential proteasome-mediated cleavage of the lymphoid blast crisis (Lbc) oncoprotein. Blood. 2003;102:621–9. doi: 10.1182/blood-2003-01-0260. [DOI] [PubMed] [Google Scholar]
- 16.Akatsuka Y, Morishima Y, Kuzushima K, Kodera Y, Takahashi T. Minor histocompatibility antigens as targets for immunotherapy using allogeneic immune reactions. Cancer Sci. 2007;98:1139–46. doi: 10.1111/j.1349-7006.2007.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The International HapMap Consortium A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–61. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Meadows LR, den Haan JM, et al. Human H-Y: a male-specific histocompatibility antigen derived from the SMCY protein. Science. 1995;269:1588–90. doi: 10.1126/science.7667640. [DOI] [PubMed] [Google Scholar]
- 19.Meadows L, Wang W, den Haan JM, et al. The HLA-A*0201-restricted H-Y antigen contains a posttranslationally modified cysteine that significantly affects T cell recognition. Immunity. 1997;6:273–81. doi: 10.1016/s1074-7613(00)80330-1. [DOI] [PubMed] [Google Scholar]
- 20.Pierce RA, Field ED, den Haan JM, et al. Cutting edge: the HLA-A*0101-restricted HY minor histocompatibility antigen originates from DFFRY and contains a cysteinylated cysteine residue as identified by a novel mass spectrometric technique. J Immunol. 1999;163:6360–4. [PubMed] [Google Scholar]
- 21.Vogt MH, de Paus RA, Voogt PJ, Willemze R, Falkenburg JH. DFFRY codes for a new human male-specific minor transplantation antigen involved in bone marrow graft rejection. Blood. 2000;95:1100–5. [PubMed] [Google Scholar]
- 22.Vogt MH, Goulmy E, Kloosterboer FM, et al. UTY gene codes for an HLA-B60-restricted human male-specific minor histocompatibility antigen involved in stem cell graft rejection: characterization of the critical polymorphic amino acid residues for T-cell recognition. Blood. 2000;96:3126–32. [PubMed] [Google Scholar]
- 23.Warren EH, Gavin MA, Simpson E, et al. The human UTY gene encodes a novel HLA-B8-restricted H-Y antigen. J Immunol. 2000;164:2807–14. doi: 10.4049/jimmunol.164.5.2807. [DOI] [PubMed] [Google Scholar]
- 24.Torikai H, Akatsuka Y, Miyazaki M, et al. A novel HLA-A*3303-restricted minor histocompatibility antigen encoded by an unconventional open reading frame of human TMSB4Y gene. J Immunol. 2004;173:7046–54. doi: 10.4049/jimmunol.173.11.7046. [DOI] [PubMed] [Google Scholar]
- 25.Rosinski KV, Fujii N, Mito JK, et al. DDX3Y encodes a class I MHC-restricted HY antigen that is expressed in leukemic stem cells. Blood. 2008;111:4817–26. doi: 10.1182/blood-2007-06-096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorn E, Miklos DB, Floyd BH, et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med. 2004;199:1133–42. doi: 10.1084/jem.20031560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogt MH, van den Muijsenberg JW, Goulmy E, et al. The DBY gene codes for an HLA-DQ5-restricted human male-specific minor histocompatibility antigen involved in graft-versus-host disease. Blood. 2002;99:3027–32. doi: 10.1182/blood.v99.8.3027. [DOI] [PubMed] [Google Scholar]
- 28.Spierings E, Vermeulen CJ, Vogt MH, et al. Identification of HLA class II-restricted H-Y-specific T-helper epitope evoking CD4+ T-helper cells in H-Y-mismatched transplantation. Lancet. 2003;362:610–15. doi: 10.1016/S0140-6736(03)14191-8. [DOI] [PubMed] [Google Scholar]
- 29.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103:347–52. doi: 10.1182/blood-2003-07-2603. [DOI] [PubMed] [Google Scholar]
- 30.Stern M, Passweg JR, Locasciulli A, et al. Influence of donor/recipient sex matching on outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia. Transplantation. 2006;82:218–26. doi: 10.1097/01.tp.0000226156.99206.d1. [DOI] [PubMed] [Google Scholar]
- 31.Nicholls S, Piper KP, Mohammed F, et al. Secondary anchor polymorphism in the HA-1 minor histocompatibility antigen critically affects MHC stability and TCR recognition. PNAS. 2009;106:3889–94. doi: 10.1073/pnas.0900411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spierings E, Gras S, Reiser JB, et al. Steric Hindrance and Fast Dissociation Explain the Lack of Immunogenicity of the Minor Histocompatibility HA-1Arg Null Allele. J Immunol. 2009;182:4809–16. doi: 10.4049/jimmunol.0803911. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc. Natl. Acad. Sci. 2001;98:31–6. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen JA. Genomic and Proteomic Analysis of Allogeneic Hematopoietic Cell Transplant Outcome. Seeking Greater Understanding the Pathogenesis of GVHD and Mortality. Biol Blood Marrow Transplant. 2008;15(1 Suppl):e1–7. doi: 10.1016/j.bbmt.2008.12.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piper KP, McLarnon A, Arrazi J, et al. Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol Reprod. 2007;76:96–101. doi: 10.1095/biolreprod.106.055426. [DOI] [PubMed] [Google Scholar]
- 36.Kollgaard T, Hadrup SR, Petersen SL. Natural T-cell responses against minor histocompatibility antigen (mHag) HY following HLA-matched hematopoietic cell transplantation: what are the requirements for a ‘good’ mHag? Leukemia. 2008;22:1948–51. doi: 10.1038/leu.2008.75. [DOI] [PubMed] [Google Scholar]
- 37.James E, Chai JG, Dewchand H, Macchiarulo E, Dazzi F, Simpson E. Multiparity induces priming to male-specific minor histocompatibility antigen, HY, in mice and humans. Blood. 2003;102:388–93. doi: 10.1182/blood-2002-10-3170. [DOI] [PubMed] [Google Scholar]
- 38.Verdijk RM, Kloosterman A, Pool J, et al. Pregnancy induces minor histocompatibility antigen–specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961–4. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 39.van Halteren AG, Jankowska-Gan E, Joosten A, Blokland E, Pool J, et al. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114:2263–72. doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warren EH, Vigneron NJ, Gavin MA, et al. An antigen produced by splicing of noncontiguous peptides in the reverse order. Science. 2006;313:1444–7. doi: 10.1126/science.1130660. [DOI] [PubMed] [Google Scholar]
- 41.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–8. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miklos DB, Kim HT, Zorn E, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–9. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]


