Abstract
Transplantation of central nervous tissue has been proposed as a therapeutic intervention for age-related neurodegenerative diseases and stroke. However, survival of embryonic neuronal cells is hampered by detrimental factors in the aged host brain such as circulating inflammatory cytokines and oxidative stress. We have previously found that supplementation with 2% blueberry in the diet increases graft growth and neuronal survival in intraocular hippocampal grafts to aged hosts. In the present study we explored possible biochemical mechanisms for this increased survival, and we here report decreased microglial activation and astrogliosis in intraocular hippocampal grafts to middle-aged hosts fed a 2% blueberry diet. Markers for astrocytes and for activated microglial cells were both decreased long-term after grafting to blueberry-treated hosts compared to age-matched rats on a control diet. Similar findings were obtained in the host brain, with a reduction in OX-6 immunoreactive microglial cells in the hippocampus of those recipients treated with blueberry. In addition, immunoreactivity for the pro-inflammatory cytokine IL-6 was found to be significantly attenuated in intraocular grafts by the 2% blueberry diet. These studies demonstrate direct effects of blueberry upon microglial activation both during isolated conditions and in the aged host brain and suggest that this nutraceutical can attenuate age-induced inflammation.
Keywords: transplantation, blueberry, glial activation, oxidative stress, aging, hippocampus
Introduction
Glial activation can be detrimental to neural cell survival in a number of transplantation paradigms, including animal models of spinal cord injury (Beattie 2004), Parkinson's Disease (Duan et al., 2000; Sortwell et al., 2001), and Huntington's disease (Mazurova et al., 2006; Johann et al., 2007). The activation of inflammatory processes in transplanted neural tissue is thought to stem, in part, from the introduction of foreign cells into a damaged, diseased, or dysfunctional environment, and is also thought to be aggravated by the surgical procedure itself (Bastian et al., 2008). Another reason for poor cell survival in the aged or diseased host is that grafted cells may be subject to the same pathology affecting resident cells in the host central nervous system (CNS). Since the majority of neurodegenerative disorders occur in the aged brain, a disease state may be present along with functional perturbations associated with normal aging, further compromising transplant survival (Kordower et al., 2009). As an example of this problem, the survival rate of transplanted embryonic mesencephalic cells has been estimated to be about 5-20% in young 6-hydroxydopamine lesioned rats (Brundin et al., 1987), and a considerably reduced survival rate of grafted neurons in aged rats (Collier et al., 1999). The intraocular grafting model is a transplantation paradigm useful in the elucidation of processes involved in neural transplant survival (Olson et al., 1977). Intraocular transplants are established by grafting fetal tissue into the anterior eye chamber of adult host animals; embryonic brain tissue has been shown to attach to and become vascularized by the host iris and will remain viable for as long as the host is alive (Gerhardt et al., 1991). Intraocular transplantation allows for the separation of intrinsic versus extrinsic factors affecting graft survival since neural grafts are supported by the host blood supply but are not associated with other neural tissue. This paradigm is particularly useful when investigating graft survival in aged animals. Similar to intracranial grafts, intraocular transplants to aged hosts exhibit elevated activation of astrocytes and an abnormal vascular development (Eriksdotter-Nilsson et al., 1986; Eriksdotter-Nilsson et al., 1989), but microglial activation in intraocular grafts has not been examined previously to our knowledge.
Treatments that reduce inflammation such as the anti-inflammatory drug minocycline (Zhang et al., 2003; Johann et al., 2007) and synthetic fibronectin peptide V (Duan et al., 2000) are effective in improving neural transplant survival. Recent studies have identified a number of nutritional compounds with the ability to influence inflammation in vitro and in vivo (e.g. Lau et al., 2005), which could impact neural transplant survival, but which have not been tested using this paradigm previously. Nutritional derivatives such as epigallocatechin gallate (EGCG) in green tea (Li et al., 2004), resveratrol in grapes and red wine (Bi et al., 2005), and polyphenols in blueberries (Stromberg et al., 2005) have all been shown to influence microglial activation in vitro and in vivo. Dietary blueberry supplementation modulated microglial activation following a lesion to the striatum (Stromberg et al., 2005). Blueberries have also been shown to reduce lipopolysaccharide (LPS)-induced activation in a microglial cell line, attenuating reactive oxygen species and cytokine release (Lau et al., 2007). In the hippocampus, cytokine expression concomitant with microglial activation has been shown to inhibit neural precursor proliferation and survival during neurogenesis (Ekdahl et al., 2003), possibly through the release of soluble factors such as the cytokine interleukin-6 (IL-6) (Kempermann et al., 2003; Monje et al., 2003). IL-6 expression arising from microglial activation has been associated with disturbances in neuronal division and maturation (Ekdahl et al., 2003), and was therefore of interest in the current investigation. Our previous studies revealed a perturbed neuronal organization in grafts to aged hosts, which was ameliorated with dietary blueberry supplementation (Willis et al., 2005). The improved hippocampal graft survival and growth was subsequently shown to be largely independent of the degree of vascularization of grafted tissue (Willis et al., 2008).
Considering that blueberries have been shown to attenuate inflammation in a number of other paradigms, the present study was designed to investigate the effects of host blueberry supplementation on glial development and maintenance in intraocular hippocampal grafts. Additionally, since microglial activation has been reported to increase in the hippocampus during aging, the effects of blueberry supplementation on host microglial dynamics was also assessed.
Methods
Subjects and diet
Subjects consisted of young (5-6 months) and aged (17-18 months) Fischer 344 rats maintained on either a control or a 2% blueberry-supplemented diet for the duration of the experiment (Van Drunen Farms, Momence, IL, USA). The blueberry diet was started one week prior to grafting after the rats had accumulated to the vivarium. Both blueberry- and control-diet fed rats had access to their respective food and regular water ad libitum before and after the transplantation surgery. The blueberry-supplemented diet was prepared by homogenizing, centrifuging, and freeze-drying fresh blueberries (Vaccinium carymbosum, Van Drunen Farms). The anthocyanin content of Vaccinium Carymbosum is in the range of 6-7.5 mg/g of wet weight according to several different studies (see www.blueberry.org), and previous studies have demonstrated that freeze-drying in this manner does not affect the anthocyanin content of the fruit (Lohachoomol et al.; 2004). According to the manufacturer, only mature, ripe berries are purchased, and varieties used include Jersey Blue Crop and Duke. The raw materials for lot 33881573259 used in these experiments were generated from crop grown in the United States. Berries are grown using conventional farming methods and vendors follow Good Agricultural Practices (GAP). Freeze-dried blueberries were added to the control NIH-31 diet (Harlan Teklad) in place of cornstarch, yielding a chow consisting of control diet plus blueberries at 2% (g/kg). These diets are isocaloric and have been used by us (Willis et al., 2005; Willis et al., 2008) and others for more than a decade now (Joseph et al., 1999; Joseph et al., 2003; Shukitt-Hale et al., 2008; Williams et al., 2008). Within both diet groups, subjects were further divided into either a short term (1-2 weeks) or long term (8-12 weeks) group, yielding the following n for each group: 1-2 weeks: 6 aged control/6 aged blueberry/5 young control/5 young blueberry; 8-12 weeks: 7 aged control/7 aged blueberry/9 young control/5 young blueberry. The short- versus long-term groups represent the number of weeks post-grafting survival before sacrifice.
Intraocular Transplantation
Each host contained one hippocampal graft in each eye. Transplantation of embryonic day 18 hippocampal formation was performed as previously described (Olson et al., 1977). Briefly, dams were euthanized with an overdose of halothane and fetuses were removed and placed on ice until dissection. Fetal brains were removed and the hippocampal formation was dissected and cut into several pieces along the longitudinal axis as described previously (Granholm et al., 1981). Recipient hosts, which had been maintained on the respective diets for one week prior to grafting, were anesthetized with xylazine (Rompun, 12 mg/kg i.p.) and ketamine (Ketaset 80mg/kg i.p.). One drop of 1% atropine solution was applied to dilate the pupil and facilitate grafting. Fetal hippocampal formation was injected into the anterior eye chamber through a slit cut in the cornea using a modified syringe as previously described (Olson et al., 1977). Animals were kept on a standard 12:12-hr light/dark cycle. All surgical procedures were performed according to NIH guidelines for animal use and were approved by the Institutional Animal Care and Use Committee (IACUC) committee. The rats received analgesic treatment locally and in the drinking water for 48 hours following the grafting procedure. Blueberry diet was maintained throughout the experiment, and host rats were sacrificed either 1-2 weeks following grafting (short-term group) or 8-12 weeks following grafting (long-term group).
LPS Treatment
At eight weeks post-grafting, four subjects from the young control diet group were subjected to lipopolysaccharide (LPS) exposure as a positive control. Host animals were anesthetized with xylazine and ketamine as described above. LPS (2.5 mg/kg, Sigma) was administered intravenuously (i.v.) through the sublingual vein. Subjects were monitored continuously and sacrificed 24 hours post- injection, and the tissues were harvested and processed as described below.
Immunohistochemistry
Eight weeks post-grafting, host animals were anesthetized with halothane and perfused transcardially with 0.1M phosphate buffered saline (PBS) followed by 4% paraformaldehyde. Grafts and host brains were immersion fixed overnight and transferred to 30% sucrose in PBS. The transplants were sectioned on a cryostat (Zeiss-Microm) to a thickness of 10 microns and mounted on glass slides. Sections were mounted in such a way that each slide contained every 10th section throughout the entire graft. Primary antibodies consisted of: ionized calcium binding adaptor molecule 1 (Iba1, Wako Chemicals, 1:1000), OX-6 (Serotec, 1:500), glial fibrillary acidic protein (GFAP, Sigma, 1:1000), and IL-6 (Abcam, 1:100), and the fluorescent counterstain Hoechst (1ul/ml, Molecular Probes). Fluorescent secondary antibodies were obtained from Molecular Probes and included anti-mouse 594 and anti-rabbit 488 at a dilution of 1:30 in PBS. Incubation without primary or secondary antibodies revealed no staining, demonstrating specificity of the applied antibodies. Penetration of staining was confirmed by focusing through the sections in the Z-plane. For detection of activated microglia in the host hippocampus, the avidin-biotin (ABC) detection method was used. Host brains were sectioned at a thickness of 45 microns through the entire hippocampus and stained free-floating in 24 well plates according to our standard protocol. Immunohistochemistry was performed by first blocking endogenous peroxidase activity followed by incubation in 10% normal goat serum in 0.5% Triton X-100 in PBS. Sections were then placed in primary antibody solution (mouse anti-OX-6 1:1000, Serotec) for 48 hours at 4°C. After primary antibody incubation, sections were incubated in biotinylated anti-mouse secondary antibody (Vector Labs, 1:1000) for 1 hour. Sections were then incubated in avidin-biotin complex (Vectastain ABC kit, Vector Labs) for 1 hour. After ABC incubation, sections were transferred to a solution of diaminobenzedine (DAB, Sigma) and incubated for 15 minutes. Sections were washed in PBS, mounted on glass slides, and subjected to Cresyl Violet counterstain. They were dehydrated and cover slipped using Permount (Sigma Aldrich).
Quantification of Cellular Markers
Quantification of activated microglia in grafts was performed with the aid of the optical fractionator probe (StereoInvestigator, Microbrightfield) as described previously (Willis et al., 2008). Every tenth section throughout each graft was outlined under low magnification and the number of cells measured using a systematic random design of disector counting frames. A 60× objective lens with a 1.4 numerical aperture was used to count individual cells within the counting frames. The number of cells per millimeter of graft area was obtained by dividing the total number of cells per graft by the area in millimeters in which the cells had been counted (#cells/(area of counting frame × # of counting frames) = #cells/mm2). Both Ox-6 and Iba-1 positive cells were counted and data were analyzed as percentage of Iba-1 positive cells double labeled with Ox-6. GFAP and IL-6 expression in grafts was quantified through densitometry. Briefly, background fluorescence was subtracted and the LUT scale was adjusted using density slicing (using a 0-256 gray scale). This approach captured all labeled profiles above a threshold density and interactively discriminated them from density values below the threshold. Computer assisted imaging software (Scion Image) then automatically measured the mean optical density and number of pixels per area of the extracted profiles in the outlined regions. The percentage of graft area expressing the protein of interest was obtained by dividing the area of positive expression by the total graft area. Even though the fluorescence intensity was kept constant during analysis of all samples, this method must be considered semi-quantitative rather than quantitative, since the area investigated is determined by the person evaluating the sections. It should be noted that every 10th section of each graft was analyzed for each marker by an investigator blinded to the individual subject groups, after which a mean was generated for each graft and used to compare within and between groups.
Unbiased stereology was used to quantify cell number of Ox-6 positive microglia in the hippocampus of the right hemisphere of the host brain using the optical fractionator stereological method (StereoInvestigator, MicroBrightfield, Inc.). The region of interest was the anterior hippocampus from rostral to caudal coordinates of approximately 2.92mm to 4.8mm as determined from the Paxinos & Watson rat brain atlas (Paxinos et al., 2007). The hippocampus was outlined under low magnification and the number of Ox-6 positive microglia in the outlined region was obtained with a systematic random design of disector counting frames. A 40× objective lens was used to count individual cells within the counting frames. Means and standard error of the mean (SEM) of estimated cell counts were calculated from the raw data using the stereology software system.
Statistics
An omnibus two-way analysis of variance (ANOVA, Statview Version 5.0.1) was used to evaluate differences between groups at both time points followed by Fisher's PLSD posthoc test (Statview Version 5.0.1). Significance was set at p<0.05.
Results
Dietary blueberry supplementation attenuated microglial activation in intraocular grafts
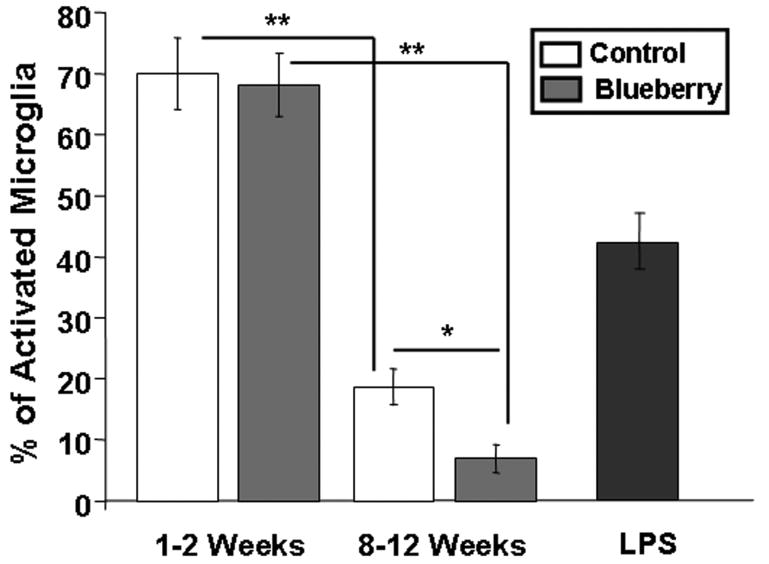
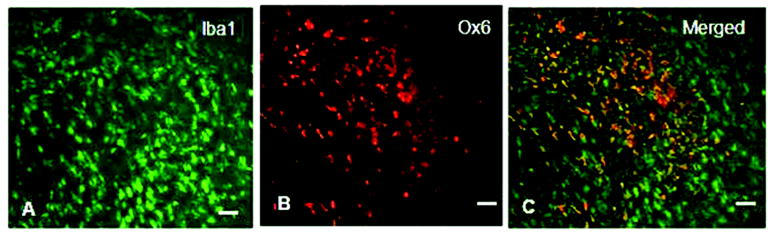
As seen in Fig. 1, microglia in hippocampal grafts to all hosts exhibited an activated morphology at 1-2 weeks post- transplantation, evidenced by large cell bodies, short processes, and co-localization of the two microglial markers Iba1 and Ox-6 (arrows, Fig. 1). However, by 8-12 weeks post-transplantation, microglia in grafts from young hosts exhibited a more resting-like morphology, with smaller cell bodies and long, thin, and branched processes, and fewer cells expressed the microglial marker OX-6 (Fig. 2 A-F). In grafts to aged hosts which were fed a control diet, however, most microglial cells continued to exhibit higher density of Ox-6 expression at 8-12 weeks post- transplantation (Fig. 2 G-L), whereas microglia in grafts from blueberry-supplemented hosts appeared to exhibit a more resting morphology, similar to those found in grafts to young recipients (Fig. 2J-L). Almost none of the microglial cells in the blueberry-treated aged hosts expressed Ox-6 immunoreactivity, as can be seen in Figure 2. Blueberry supplementation did not appear to significantly alter the number of microglia stained for the MHC class II marker (Ox-6 in young hosts (Fig. 2 A-F), and therefore quantification of Ox-6 expression by resident microglia was performed exclusively in grafts from aged hosts (not even one Ox-6 immunoreactive cell per section was found after 8-12 weeks in young grafts with our without blueberry supplementation). Quantification of the percentage of microglia expressing Ox-6 in grafts to aged hosts (see Figure 4) revealed that the number of activated microglia in grafts at 1-2 weeks was similar in both groups; 70 ± 6% of microglia expressed Ox-6 in grafts from control diet hosts, and 68 ± 5% of microglia expressed OX-6 in grafts from blueberry-supplemented recipient rats (n=12 grafts for both groups). By 8-12 weeks post-transplantation, microglial activation had declined significantly (p<0.0001) in both groups to 19 ± 3% in aged control and 7 ± 2% in aged blueberry grafts (n=14 grafts for both groups). At 8-12 weeks post-transplantation, the percentage of microglia expressing OX-6 was significantly (p<0.05) higher in grafts from control diet hosts as compared to grafts from blueberry-supplemented hosts. As a positive control for microglial activation, 4 young hosts were treated with LPS i.v 8 weeks post-transplantation. Microglia in grafts from LPS treated animals exhibited a high degree of co-localization for Iba1 and Ox-6, and an activated morphology with enlarged cell bodies and short, stubby processes (Fig. 3). The percentage of microglia expressing Ox-6 was 43 ± 5% (Fig. 4), thus representing a stronger inflammatory stimulation than was observed in the middle-aged recipient grafts, which exhibited a co-localization of the inflammatory markers of 19% 8-12 weeks post-grafting.
Fig. 1. Microglial activation at 1-2 weeks post-transplantation.
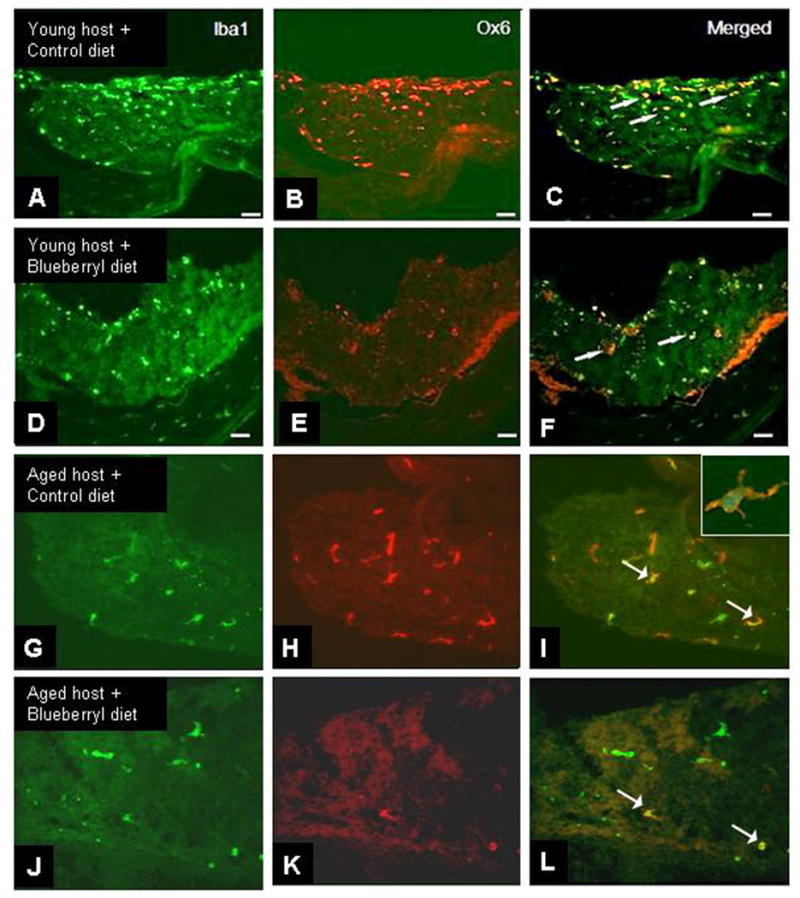
in young (A-F) and aged (G-L) hosts fed either a control (A-C, G-H) or blueberry-supplemented (D-F, J-L) diet. Expression for the pan microglial marker Iba1 (A, D, G, J, green) and the rat MHC II complex Ox-6 (B, E, H, K, red) reveals the population of microglia which are activated (arrows, C, F, I, L) in hippocampal grafts. Inset shows Ox-6 and Iba1 double labeling in a microglial cells in a hippocampal graft to a middle-aged host. Scale= 25 microns.
Fig. 2. Microglial activation at 8-12 weeks post-transplantation.
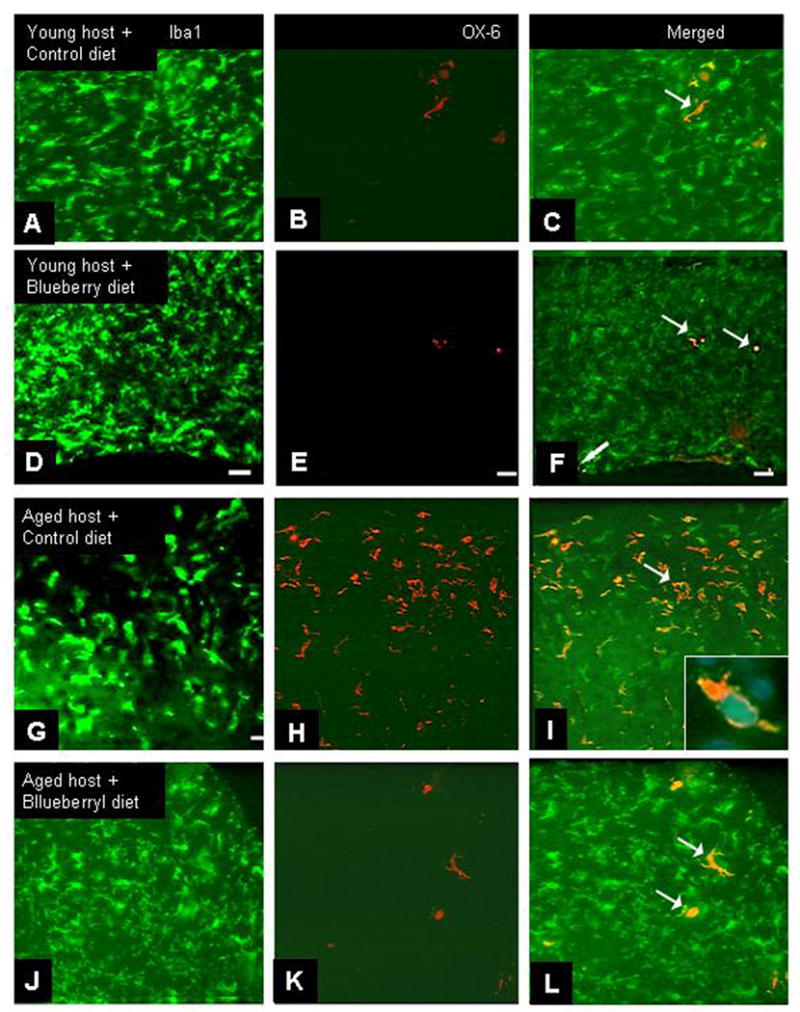
in young (A-F) and aged (G-L) hosts fed either a control (A-C, G-H) or blueberry-supplemented (D-F, J-L) diet. Co-localization of the pan microglial marker Iba1 (A, D, G, J, green) and the rat MHC II complex Ox-6 (B, E, H, K, red) reveals the presence of fewer activated microglia in grafts to aged hosts fed a blueberry-supplemented diet as compared to grafts from aged hosts fed the control diet. (arrows, C, F, I, L) in hippocampal grafts. Inset shows double labeling with Ox-6 and Iba1 within the same microglial cell in a graft to a middle-aged host. Scale= 25 microns.
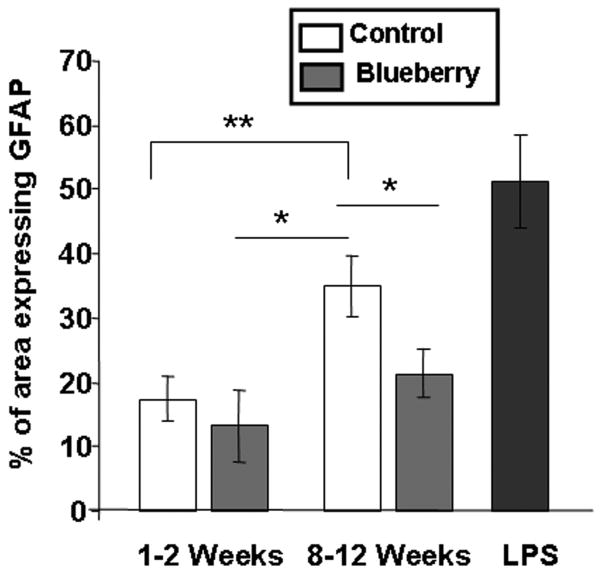
Fig. 4. Percentage of Iba1- labeled microglia expressing OX-6.
a marker of activation, in grafts from aged hosts fed either a control or a blueberry supplemented diet for 1-2 or 8-12 weeks, and grafts from hosts treated with LPS. ***p<0.0001, *p<0.05, Fisher's PLSD
Fig. 3. Microglial activation in a hippocampal graft from a young host treated with LPS i.v.
As seen in C, the proportion of Iba1 positive microglia (A) also expressing Ox-6 (B) is high. Scale= 25 microns.
Blueberry supplementation decreased GFAP expression in intraocular grafts to aged hosts
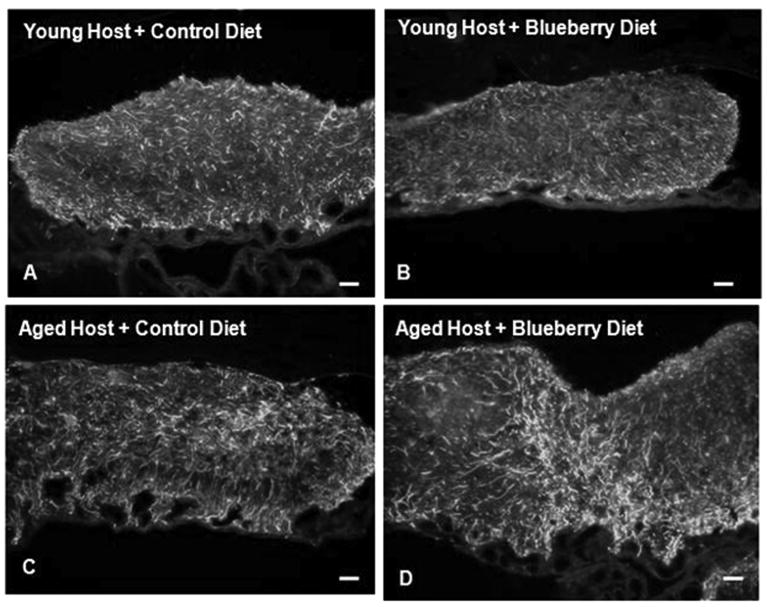
As seen in Fig. 5, GFAP immunoreactivity was similar in all groups at 1-2 weeks post-transplantation. At 8-12 weeks post-transplantation, however, all grafts in young recipients exhibited lower GFAP staining compared to that seen in aged control grafts and LPS-treated grafts (Fig. 6). Interestingly, blueberry supplementation gave rise to significantly lower GFAP expression in grafts to aged hosts, as evidenced by semi-quantitative densitometry measurements (Fig. 7). As seen in Fig. 7, the percent of graft area expressing GFAP significantly increased, from 17 ± 3% at 1-2 weeks to 35 ± 5% at 8-12 weeks in grafts from control diet hosts, suggesting a continuing astrogliosis in those grafts. In grafts from blueberry supplemented animals, GFAP expression was 13 ± 6% at 1-2 weeks post-transplantation and rose to 21 ± 4% at 8-12 weeks post-transplantation, representing significantly reduced astrogliosis compared to GFAP staining in aged hosts on a control diet. At 8-12 weeks post-transplantation, the percentage of graft area expressing GFAP was therefore significantly different between hippocampal grafts to control diet and blueberry diet fed aged hosts (p<0.05, Fig. 7).
Fig. 5. GFAP expression at 1-2 weeks post-transplantation.
from young (A, B) and aged (C, D) hosts maintained on a control (A, C) or blueberry- supplemented (B, D) diet for 1-2 weeks post-transplantation. Scale= 25 microns.
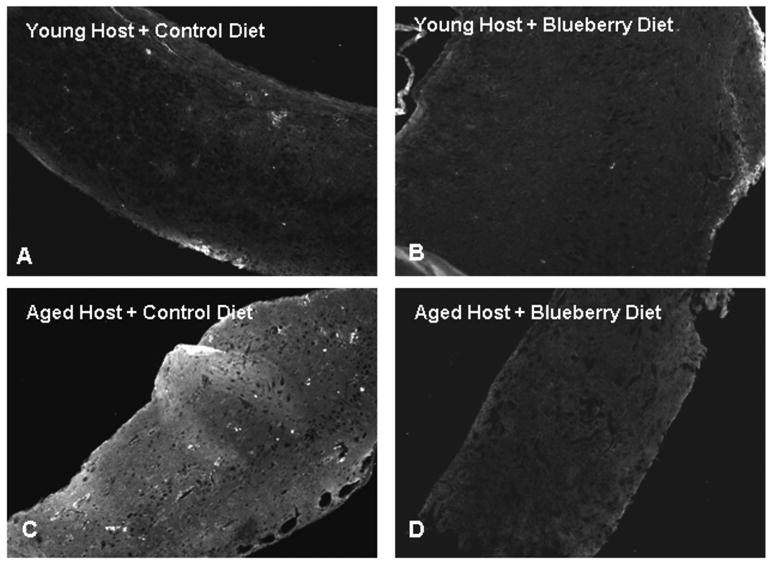
Fig. 6. GFAP expression at 8-12 weeks post-transplantation.
from young (A, B) and aged (C, D) hosts maintained on a control (A, C) or blueberry- supplemented (B, D) diet; GFAP expression in a graft from a young host exposed to LPS (E). Scale= 25 microns.
Fig. 7. Densitometry of GFAP expression in hippocampal grafts.
from aged hosts maintained on a control or blueberry- supplemented diet for 1-2 or 8-12 weeks, and GFAP expression in grafts from young hosts treated with LPS. **p<0.01, *p<0.05, Fisher's PLSD
Expression of Interleukin-6 (IL-6) was attenuated in grafts to blueberry-fed hosts
IL-6 exhibited diffuse expression in grafted tissues (Fig. 8), and was most obvious in grafts to aged host animals fed a control diet (Fig. 8C). Upon quantification of IL-6 immunoreactivity, it was found that grafts to aged hosts exhibited significantly higher overall IL-6 immunoreactivity than grafts in young hosts (Fig. 9, n=12; p<0.01). Consumption of a blueberry diet in both young and aged hosts was associated with decreased expression of IL-6, but the effect of blueberry supplementation was statistically significant only in aged hosts (p<0.05). Interestingly, although blueberry supplementation attenuated IL-6 expression in aged hosts, IL-6 expression in grafts from hosts fed a blueberry-supplemented diet was still significantly higher in aged hosts versus young hosts (p<0.05), suggesting that this cytokine may play a detrimental role in survival and organization of intraocular grafts.
Fig. 8. Interleukin-6 expression in hippocampal grafts at 8-12 weeks post-transplantation.
in young (A, B) and aged (C, D) hosts fed either a control (A, C) or blueberry-supplemented (B, D) diet.
Fig. 9. Densitometry of IL-6 expression in hippocampal grafts.
from young or aged hosts given either a control or blueberry-supplemented diet. *p<0.05, **p<0.01, Fisher's PLSD.
Dietary blueberry supplementation decreased the number of activated microglia in the aged hippocampus in situ
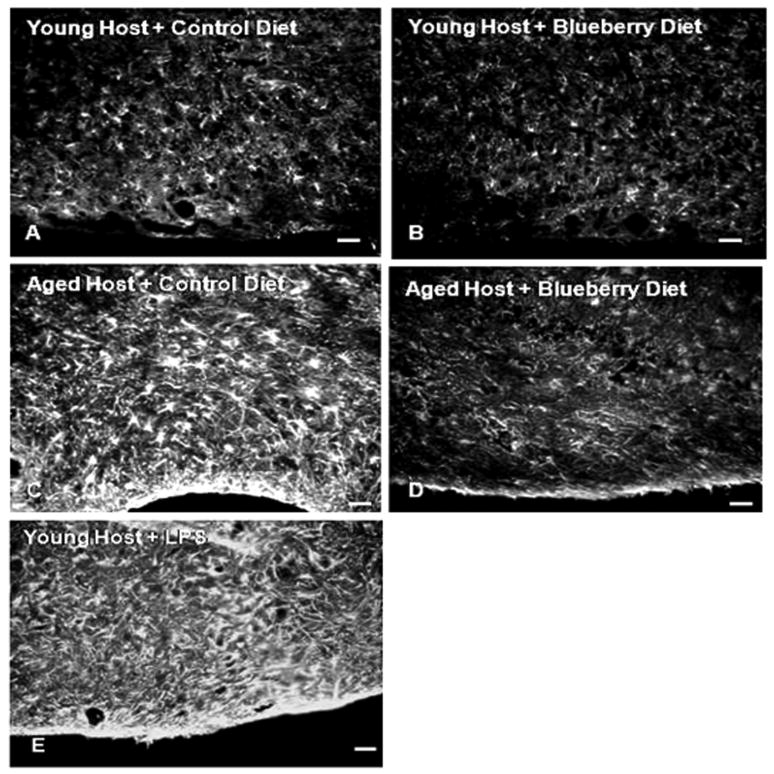
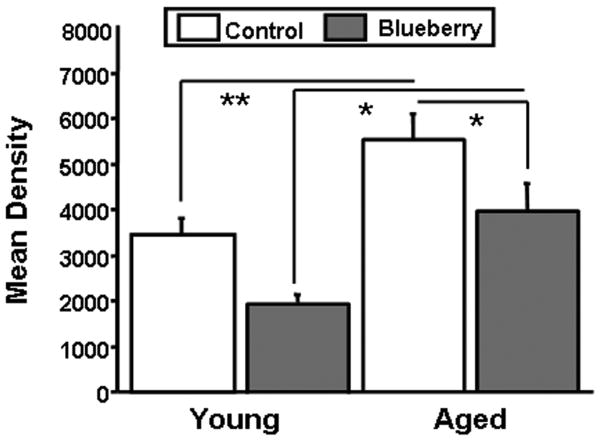
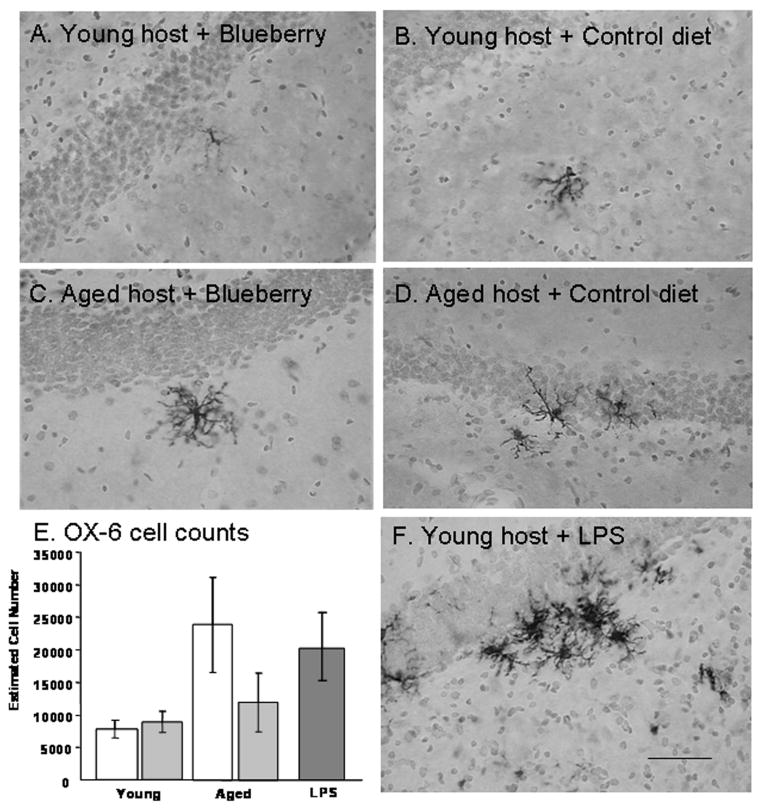
Microglial activation was quantified in the hippocampus of young and aged host rat brains maintained on either a control or blueberry supplemented diet for 8-12 weeks. As seen in Fig. 10, Ox-6 positive microglia were visible in the hippocampus of aged hosts, but few were observed in the hippocampus of young animals, and the morphology of the OX-6 stained cells was consistent with the activated form in aged hosts, with numerous processes and enlarged cell bodies. The total number of Ox-6 immunoreactive activated microglia in the hippocampal formation was assessed using unbiased stereology as described previously in our laboratory (Granholm et al., 2002). Fig. 10E shows the estimated total number of Ox-6 positive microglia in the hippocampus of young and aged animals of both dietary conditions, using unbiased stereological methods. Blueberry supplementation did not appear to affect the number of activated microglia in the hippocampus of young animals, since young hosts on a control diet had an estimated 7,776 ± 687 (S.E.M.) and young hosts on a blueberry diet had an estimated 8,845 ± 865 Ox-6 immunopositive microglia (Fig. 10E). In contrast, the number of Ox-6 immunoreactive microglia was significantly higher in the aged hippocampus. Aged hosts maintained on a control diet exhibited 23,851 ± 7,309 microglia positive for Ox-6 antibodies. In aged hosts fed a blueberry-supplemented diet, this number was decreased to 11,949 ± 4507 microglia expressing the Ox-6 marker of activation.
Fig. 10. OX-6 positive microglia in the hippocampus of young and aged hosts.
The sections were stained for cresyl violet to demonstrate the morphology of the surrounding brain tissue. Note the significant increase in density of OX-6 immunoreactive cells in the aged brain with control diet, compared to much fewer cells seen with the blueberry treatment. Sections represent the typical density of OX-6 immunoreactive glial cells in respective groups. The intravenous LPS injection (Fig. 10F) resulted in a significant increase in both number and activated morphology of microglial cells, as evidenced in the hippocampal section in 10F. Scale bar in F represents 25 microns. Figure 10E: Estimated cell number of OX-6 positive microglia in the hippocampus of young and aged hosts maintained on a control or blueberry supplemented diet for 8-12 weeks, and in the hippocampus of young hosts treated with LPS i.v. The cells were counted using a systematic random design with the disector method.
Discussion
We have previously demonstrated that a 2% blueberry diet enhances survival, growth, and neuronal development in intraocular hippocampal grafts (Willis et al., 2005; 2008). In those studies, we found that a 2% blueberry diet enhanced growth and organization of grafts in both young and middle-aged recipient rats, but to a greater extent in the middle-aged versus young hosts (Willis et al., 2008). The present study is an extension of the previous work, and identified microglial activation and astrogliosis as cellular alterations persistent in hippocampal intraocular grafts to aged hosts. Blueberry supplementation of aged graft recipients for 8-12 weeks was associated with a decrease in the number of Ox-6 positive microglia, a down regulation of GFAP expression, and an attenuation of IL-6 expression in transplanted tissues. Blueberry supplementation was found to exert a similar effect on the aged hippocampus in situ, where the number of Ox-6 immunoreactive microglia was attenuated to a microglial density observed in the hippocampus of young recipients. These findings indicate that blueberry in the diet can exert direct effects on both astrocytes and microglia towards a more resting morphology and may therefore implicate microglial activation as a possible physiological culprit for the poor survival reported for hippocampal grafts in aged hosts. The findings of the current study thus reveal that one method by which blueberry supplementation improves survival of intraocular grafts to aged hosts may be through inhibition of microglial activation and astrogliosis associated with the grafting procedure and the milieu in the aged graft recipient. Further, since the density of the pro-inflammatory cytokine IL-6 was significantly reduced in blueberry-treated graft hosts, this further supports a role for blueberry diets in reducing inflammatory processes within the grafted neural tissues.
Microglial and astrocyte activation have traditionally been considered to be hallmarks of neurodegeneration, each cell type increasing the activation state of the other through release of cytokines (Dong et al., 2001), glutamate (Cotrina et al., 2002), and nitric oxide derivatives (Bal-Price et al., 2001). In an animal model of spinal cord injury, microglial activation was shown to precede astrogliosis, and inhibiting the early microglial activation resulted in reduced astroglial hyperactivity (Tian et al., 2007). Other studies have indicated that microglial activation can induce astrogliosis through the release of cytokines tumor necrosis factor (TNF)α and IL-6 (Tilleux et al., 2007). Significant microglial activation in grafts was observed at the earlier time point in our study as well, while grafting-related astrogliosis was observed at a later time point, after 8-12 weeks in oculo, suggesting that astrogliosis continued to progress long after the microglial activation wave subsided in young recipients. These findings are consistent with a recent report which indicated that activated microglia may not necessarily induce astrocyte activation, but instead may function to modulate astrocyte activation state (Little et al., 2001). In purified astrocyte cultures, application of media from LPS-treated microglia resulted in an increase in the number of astrocytes, but a decrease in the overall expression of GFAP and no effect on morphological hypertrophy (Rohl et al., 2007). These previous studies point to a relationship between microglia and astrocytes which may be more complex than simply activation of one cell type leading to activation of the other.
In the present study, both microglial activation and astrogliosis were found to be elevated in grafts from control diet hosts compared to grafts from blueberry supplemented hosts at 8-12 weeks post-transplantation. Increased GFAP expression has previously been described in intraocular grafts to aged hosts (Eriksdotter-Nilsson et al., 1989), and the degree of glial hypertrophy was correlated to a smaller size of grafts to aged hosts (Eriksdotter-Nilsson et al., 1986). Our present study corroborates these earlier studies, in that hippocampal grafts to aged control hosts were small (see Willis et al., 2008), and also presented the highest GFAP and Ox-6 immunoreactivity. The present study also extends previous work to include astrocyte and microglial activation as well as IL-6 expression in intraocular grafts to aged hosts. The results presented herein compliment a previous study by Strömberg et al demonstrating that blueberry supplementation modulated microglial activation following 6-OHDA lesion (Strömberg et al., 2005). In the aforementioned study, blueberry supplementation resulted in an increase in microglial activation 1 week post-lesion followed by a decrease in activated microglia and improved neuronal recovery 4 weeks post-lesion. Although the percentage of activated microglia was not different between the control and blueberry groups at 1-2 weeks post-transplantation in our study, blueberry supplementation was associated with a decrease in the number of activated microglia 8-12 weeks post-transplantation (Fig. 4), suggesting that long-term activation of microglial cells may be toned down by the blueberry diet. Interestingly, the study by Strömberg et al demonstrated that GFAP immunoreactivity increased in animals fed a control diet, but GFAP expression in blueberry-supplemented animals was not significantly different from 1 to 4 weeks post-lesion (Strömberg et al., 2005). The present study demonstrates similar effects of blueberry supplementation in intraocular grafts: GFAP expression increased over time in control grafts, but was not significantly different in grafts from blueberry supplemented hosts from early to late time points post-transplantation (Fig. 7). The present study, along with the findings of Strömberg et al, implies that one of the functions of blueberry supplementation may be to prevent microglial activation, either directly by for example reducing circulating pro-inflammatory cytokines, or indirectly via antioxidant capacities. In addition, blueberries could be acting exclusively on neuronal parameters of survival (Joseph et al., 1999), thus preventing the activation of microglia by degenerating neurons. However, previous studies indicate that blueberries can directly influence microglia in culture by decreasing the release of cytokines and nitric oxide from LPS-stimulated microglial cells (Lau et al., 2007). Blueberries may therefore possess a multiplicity of actions in the central nervous system, including beneficial effects on neuronal health and anti-inflammatory effects on resident microglia.
The blueberry diet also had remarkable and previously unreported effects on microglial cells in the middle-aged rat brain. We found that the hippocampal region contained double the number of Ox-6 immunoreactive microglia in aged hosts compared to young hosts, and that the Ox-6 immunoreactive glial cells were reduced to young levels with the 2% blueberry diet. Previous studies have indicated that microglial activation increases in the aged brain, possibly as a reaction to age-related alterations in neuronal functioning (Perry et al., 1993) or increased circulating cytokine levels, although other authors have not been able to detect increased microglial activation in the aged rodent brain (Virgili et al., 2001). These contrary findings may be due to different antibodies used or strain-specific alterations with aging, since it has been reported that different rat strains exhibit significant differences in the aging process (see Sheridan KM 2007; Kelly et al., 2006). Microglial activation in the hippocampus of aged rats has also been linked to a reduced rate of neurogenesis and the reduced survival of newly generated neurons that occurs as part of the normal aging process (Kuhn et al., 1996; Ekdahl et al., 2003; Heine et al., 2004). Interestingly, dietary blueberry supplementation has been shown to increase the number of newly generated cells in the hippocampus, possibly through influencing levels of insulin-like growth factor-1 (Casadesus et al., 2004).Based on the documented influence of microglial activation upon neurogenesis, it is therefore possible that blueberry supplementation could influence hippocampal neurogenesis indirectly by decreasing microglial activation in the aged hippocampus.
A physiological mechanism for microglial impact on neurogenesis has been proposed through release of the cytokine IL-6. IL-6 has been shown to increase in the brain (Ye et al., 1999) and serum of aged animals and humans (Ershler 1993; Spaulding et al., 1997), and the level of circulating IL-6 in humans correlated with worse performance on memory tasks (Krabbe et al., 2004), one behavioral parameter that has been shown to be affected by a decline in neurogenesis (Kempermann et al., 2004). In the present study we therefore examined IL-6 protein distribution in hippocampal grafts by means of immunohistochemistry. Given the well-established role that IL-6 can play in disrupting new neuron development, migration, and maturation in the hippocampus (Nakashima et al., 2002; Nakanishi et al., 2007), increases in IL-6 in hippocampal grafts could account for the neuronal disorganization previously reported in grafts to aged hosts (Willis et al., 2005), even though further studies are needed to confirm this proposed mechanism. Other studies have also highlighted the consequences of increased IL-6 expression in reduced survival of stem cell transplants to the injured spinal cord (Okano et al., 2005) and to the brain (Ideguchi et al., 2008). The precise mechanism of action of blueberries on microglial IL-6 remains to be delineated, and will be a focus for future research studies.
Data from the current study demonstrated that dietary blueberry supplementation decreased microglial activation and astrogliosis in hippocampal grafts to aged hosts. In addition, dietary blueberry supplementation was associated with an approximately 50% decrease in the estimated cell number of activated microglia in the hippocampus of aged animals. Neural tissue transplantation represents one possible therapeutic option to treat spinal cord injury and neurodegenerative diseases. Unfortunately, the reduced survival and maturation of transplanted cells into aged hosts has presented a hurdle to the use of neural tissue transplantation. We have presented herein that dietary supplementation of graft recipients with blueberries may in fact improve the long-term viability of grafted tissues by inhibiting inflammatory processes significantly and for a long time period. These results may be relevant for transplant survival in other organ systems in which inflammatory processes can compromise transplant survival, since the blueberry diet gave rise to a robust increase in graft growth, neuronal survival (Willis et al., 2008), and a significant decrease in both astrogliosis and microglial activation in the grafted tissue, even though blueberry diets have not been attempted after peripheral organ transplantation, to our knowledge. Although other studies have demonstrated the efficacy of antioxidants to improve survival and function of transplanted liver (Evans et al., 2008), kidney (Fassett et al., 2008), and neural tissue (Bagga et al., 2008), the present study is the first to demonstrate that dietary blueberry supplementation has direct effects on anti-inflammatory processes occurring in grafted tissues.
Acknowledgments
This work was made possible by a program project grant from the National Institutes on Aging (AG04418).
References
- Bagga V, Dunnett SB, Fricker-Gates RA. Ascorbic acid increases the number of dopamine neurons in vitro and in transplants to the 6-OHDA-lesioned rat brain. Cell Transplant. 2008;17:763–73. doi: 10.3727/096368908786516774. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480–91. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian D, Tamburstuen MV, Lyngstadaas SP, Reikeras O. Systemic and local cytokine kinetics after total hip replacement surgery. Eur Surg Res. 2008;41:334–40. doi: 10.1159/000157176. [DOI] [PubMed] [Google Scholar]
- Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004;10:580–3. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Bi XL, Yang JY, Dong YX, Wang JM, Cui YH, Ikeshima T, Zhao YQ, Wu CF. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int Immunopharmacol. 2005;5:185–93. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Brundin P, Bjorklund A. Survival, growth and function of dopaminergic neurons grafted to the brain. Prog Brain Res. 1987;71:293–308. doi: 10.1016/s0079-6123(08)61832-4. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Shukitt-Hale B, Stellwagen HM, Zhu X, Lee HG, Smith MA, Joseph JA. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7:309–16. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Sortwell CE, Daley BF. Diminished viability, growth, and behavioral efficacy of fetal dopamine neuron grafts in aging rats with long-term dopamine depletion: an argument for neurotrophic supplementation. J Neurosci. 1999;19:5563–73. doi: 10.1523/JNEUROSCI.19-13-05563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M. Astrocytes in the aging brain. J Neurosci Res. 2002;67:1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Duan WM, Zhao LR, Westerman M, Lovick D, Furcht LT, McCarthy JB, Low WC. Enhancement of nigral graft survival in rat brain with the systemic administration of synthetic fibronectin peptide V. Neuroscience. 2000;100:521–30. doi: 10.1016/s0306-4522(00)00299-2. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–7. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksdotter-Nilsson M, Bjorklund H, Dahl D, Olson L. Growth and development of intraocular fetal cortex cerebri grafts in rats of different ages. Brain Res. 1986;393:75–84. doi: 10.1016/0165-3806(86)90067-2. [DOI] [PubMed] [Google Scholar]
- Eriksdotter-Nilsson M, Olson L. Growth of brain tissue grafts is dependent upon host age. Mech Ageing Dev. 1989;49:1–22. doi: 10.1016/0047-6374(89)90064-x. [DOI] [PubMed] [Google Scholar]
- Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–81. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- Evans ZP, Ellett JD, Fariss MW, Schnellmann RG, Schmidt MG, Chavin K. Vitamin e succinate reduces ischemia/reperfusion injury in steatotic livers. Transplant Proc. 2008;40:3327–9. doi: 10.1016/j.transproceed.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassett RG, Healy H, Driver R, Robertson IK, Geraghty DP, Sharman JE, Coombes JS. Astaxanthin vs placebo on arterial stiffness, oxidative stress and inflammation in renal transplant patients (Xanthin): a randomised controlled trial. BMC Nephrol. 2008;9:17. doi: 10.1186/1471-2369-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt GA, Palmer MR, Granholm AC. Age-induced changes in single locus coeruleus brain transplants grown in oculo: an in vivo electrochemical study. Neurobiol Aging. 1991;12:487–94. doi: 10.1016/0197-4580(91)90078-x. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Seiger A. Thyroid hormone dependency in immature but not mature grafted locus coeruleus neurons. Evidence from intraocular innervation of iris transplants Med Biol. 1981;59:51–7. [PubMed] [Google Scholar]
- Granholm ACh, Ford KA, Hyde LA, Bimonte HA, Hunter CL, Nelson M, Albeck D, Sanders LA, Mufson EJ, Crnic LS. Estrogen restores cognition and cholinergic phenotype in an animal model of Downs Syndrome. Physiology and Behavior. 2002;77:371–385. doi: 10.1016/s0031-9384(02)00884-3. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–75. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Ideguchi M, Shinoyama M, Gomi M, Hayashi H, Hashimoto N, Takahashi J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell-derived neural precursor cells. J Neurosci Res. 2008;86:1936–43. doi: 10.1002/jnr.21652. [DOI] [PubMed] [Google Scholar]
- Johann V, Schiefer J, Sass C, Mey J, Brook G, Kruttgen A, Schlangen C, Bernreuther C, Schachner M, Dihne M, Kosinski CM. Time of transplantation and cell preparation determine neural stem cell survival in a mouse model of Huntington's disease. Exp Brain Res. 2007;177:458–70. doi: 10.1007/s00221-006-0689-y. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Arendash G, Gordon M, Diamond D, Shukitt-Hale B, Morgan D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–62. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–21. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM, Nadon NL, Morrison JH, Thibault O, Barnes CA, Blalock EM. The neurobiology of aging. Epilepsy Res. 2006;68 1:S5–20. doi: 10.1016/j.eplepsyres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Neumann H. Neuroscience. Microglia: the enemy within? Science. 2003;302:1689–90. doi: 10.1126/science.1092864. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–91. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Brundin P. Lewy body pathology in long-term fetal nigral transplants: is Parkinson's disease transmitted from one neural system to another? Neuropsychopharmacology. 2009;34:254. doi: 10.1038/npp.2008.161. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–99. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau FC, Bielinski DF, Joseph JA. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J Neurosci Res. 2007;85:1010–7. doi: 10.1002/jnr.21205. [DOI] [PubMed] [Google Scholar]
- Lau FC, Shukitt-Hale B, Joseph JA. The beneficial effects of fruit polyphenols on brain aging. Neurobiol Aging. 2005;26 1:128–32. doi: 10.1016/j.neurobiolaging.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Li R, Huang YG, Fang D, Le WD. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J Neurosci Res. 2004;78:723–31. doi: 10.1002/jnr.20315. [DOI] [PubMed] [Google Scholar]
- Little AR, O'Callagha JP. Astrogliosis in the adult and developing CNS: is there a role for proinflammatory cytokines? Neurotoxicology. 2001;22:607–18. doi: 10.1016/s0161-813x(01)00032-8. [DOI] [PubMed] [Google Scholar]
- Lohachoompol V, Srzednicki George, Craske John. The Change of Total Anthocyanins in blueberries and their antioxidant effect after drying and freezing. Journal of Biomedicine and Biotechnology Volume. 2004;2004(5):248–252. doi: 10.1155/S1110724304406123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurova Y, Latr I, Osterreicher J, Guncova I. Progressive reparative gliosis in aged hosts and interferences with neural grafts in an animal model of Huntington's disease. Cell Mol Neurobiol. 2006;26:1423–41. doi: 10.1007/s10571-006-9051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–58. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Taga T. Mechanisms underlying cytokine-mediated cell-fate regulation in the nervous system. Mol Neurobiol. 2002;25:233–44. doi: 10.1385/MN:25:3:233. [DOI] [PubMed] [Google Scholar]
- Okano H, Okada S, Nakamura M, Toyama Y. Neural stem cells and regeneration of injured spinal cord. Kidney Int. 2005;68:1927–31. doi: 10.1111/j.1523-1755.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- Olson L, Freedman R, Seiger A, Hoffer B. Electrophysiology and cytology of hippocampal formation transplants in the anterior chamber of the eye. I. Intrinsic organization. Brain Res. 1977;119:87–106. doi: 10.1016/0006-8993(77)90093-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–7. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Rohl C, Lucius R, Sievers J. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res. 2007;1129:43–52. doi: 10.1016/j.brainres.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Sheridan KM, F M, Distasi MR, Witzmann FA, Dalsing MC, Miller SJ, Unthank JL. Impact of genetic background and aging on mesenteric collateral growth capacity in Fische 344, Brown Norway, and Fisched 344 × Brown Norway hybrid rats. American Journal of Physiology. 2007;293:H3498–H3505. doi: 10.1152/ajpheart.00040.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, Lau FC, Carey AC, Galli RL, Spangler EL, Ingram DK, Joseph JA. Blueberry polyphenols prevent kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr Neurosci. 2008;11:172–182. doi: 10.1179/147683008X301487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sortwell CE, Camargo MD, Pitzer MR, Gyawali S, Collier TJ. Diminished survival of mesencephalic dopamine neurons grafted into aged hosts occurs during the immediate postgrafting interval. Exp Neurol. 2001;169:23–9. doi: 10.1006/exnr.2001.7644. [DOI] [PubMed] [Google Scholar]
- Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Gemma C, Vila J, Bickford PC. Blueberry- and spirulina-enriched diets enhance striatal dopamine recovery and induce a rapid, transient microglia activation after injury of the rat nigrostriatal dopamine system. Exp Neurol. 2005;196:298–307. doi: 10.1016/j.expneurol.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Tian DS, Dong Q, Pan DJ, He Y, Yu ZY, Xie MJ, Wang W. Attenuation of astrogliosis by suppressing of microglial proliferation with the cell cycle inhibitor olomoucine in rat spinal cord injury model. Brain Res. 2007;1154:206–14. doi: 10.1016/j.brainres.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Berger J, Hermans E. Induction of astrogliosis by activated microglia is associated with a down-regulation of metabotropic glutamate receptor 5. J Neuroimmunol. 2007;189:23–30. doi: 10.1016/j.jneuroim.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Virgili M, Monti B, Polazzi E, Angiolini G, Contestabile A. Topography of neurochemical alterations in the CNS of aged rats. Int J Dev Neurosci. 2001;19:109–16. doi: 10.1016/s0736-5748(00)00057-5. [DOI] [PubMed] [Google Scholar]
- Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JP. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Willis L, Bickford P, Zaman V, Moore A, Granholm AC. Blueberry extract enhances survival of intraocular hippocampal transplants. Cell Transplant. 2005;14:213–23. doi: 10.3727/000000005783983142. [DOI] [PubMed] [Google Scholar]
- Willis LM, Small BJ, Bickford PC, Umphlet CD, Moore AB, Granholm AC. Dietary blueberry supplementation affects growth but not vascularization of neural transplants. J Cereb Blood Flow Metab. 2008;28:1150–64. doi: 10.1038/jcbfm.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Goetz BD, Duncan ID. Suppression of activated microglia promotes survival and function of transplanted oligodendroglial progenitors. Glia. 2003;41:191–8. doi: 10.1002/glia.10172. [DOI] [PubMed] [Google Scholar]