Abstract
Parkinson’s disease (PD) is often associated with substantial impairment of speech respiratory and phonatory control. However, the degree to which these impairments are related to abnormal laryngeal sensory function is unknown. This study examined whether individuals with PD exhibited abnormal and more asymmetric laryngeal somatosensory function compared with healthy controls, and whether these deficits were associated with disease and voice severity. Nineteen PD participants were tested and compared with 18 healthy controls. Testing included endoscopic assessment of laryngeal somatosensory function, with aerodynamic and acoustic assessment of respiratory and phonatory control, and clinical ratings of voice and disease severity. PD participants exhibited significantly abnormal and asymmetric laryngeal somatosensory function compared with healthy controls. Sensory deficits were significantly associated with timing of phonatory onset, voice intensity, respiratory driving pressure, laryngeal resistance, lung volume expended per syllable, disease severity, and voice severity. These results suggest that respiratory and phonatory control are influenced by laryngeal somatosensory function, that speech-related deficits in PD are related to abnormal laryngeal somatosensory function, and that this function may degrade as a function of disease severity. Thus, PD may represent a model of airway sensorimotor disintegration, highlighting the important role of the basal ganglia and related neural networks in the integration of laryngeal sensory input for speech-related motor control.
Keywords: Somatosensory, Motor control, Respiratory, Voice, Speech, Larynx
Introduction
Laryngeal control is vital for airway protection, breathing, deglutition, speech, and voice. Evidence suggests that the primary somatosensory organs for encoding and guiding laryngeal movements include rapidly adapting mechanoreceptors located within the laryngeal mucosa, and innervated by the tenth cranial nerve (Andreatta et al. 2002; Sampson and Eyzaguirre 1964; Yoshida et al. 2000). Based on animal studies, impaired laryngeal somatosensory function may be associated with abnormal patterns of movement, vocalization, and deglutition, and may result in aberrant contractile properties and fiber type of the intrinsic laryngeal muscles (Jürgens 2002; Jürgens and Kirzinger 1985; McCulloch et al. 1992; Nagai et al. 2005). However, the role of somatosensation in human laryngeal control remains poorly understood.
A better understanding of the afferent mechanisms of laryngeal control in humans will be valuable in the design of assessment and intervention techniques to address illnesses related to airway protection, ventilatory regulation, deglutition, speech, and voice. Abnormal laryngeal somatosensory function in humans may result from injury or neurological disease. Such causes of abnormal laryngeal sensation may be associated with a variety of upper airway ailments, including deglutition, speech, and voice abnormalities. Previous investigators examined deglutition and laryngeal somatosensory function (Aviv et al. 1993; Sulica et al. 2002). However, the association between laryngeal somatosensory function, speech, and voice in a neurological disease such as Parkinson’s disease (PD) has not been directly explored.
Individuals with PD frequently exhibit substantial impairment of speech and voice. A hallmark feature of PD is that individuals speak with considerably reduced voice intensity and speech audibility (Ramig and Dromey 1996; Ramig et al. 2004). These deficits are associated with a more systemic decline in respiratory and laryngeal control (Baker et al. 1998; Gallena et al. 2001; Luschei et al. 1999; Solomon and Hixon 1993; Tzelepis et al. 1988). Reduced voice intensity and speech audibility in PD are typically the direct result of decreased respiratory driving pressure to propel air through the larynx, and decreased closure of the vocal folds within the larynx to effectively convert these aerodynamic events into an acoustically strong loud voice. Yet, individuals with PD are generally unaware of the severity of their voice deficits. In fact, individuals with PD often report that they feel as though they are shouting or they feel as if they are exerting great physical effort when asked to speak at a more normal audible loudness level. This clinical observation suggests that speech and voice impairments in PD may be related to impaired somatosensory function. However, this association has not been previously examined.
Another feature of speech in PD is that individuals often exhibit errors in the onset of voice (Ludlow and Bassich 1983, 1984). One example of a voice onset error in PD may be the unintended substitution of an unvoiced consonant as in the word “pie” with a voiced consonant as in the word “buy”. This type of error may lead to linguistic confusion. Voice onset results from respiratory, laryngeal, and supralaryngeal movements and aerodynamic events. Initiation of this sequence of events occurs prior to any audible acoustic signal (Larson et al. 2008), and these events are generally not visible to the speaker. Consequently, auditory and visual afferent mechanisms are insufficient to guide these movements. Rapidly adapting mechanoreceptors within the laryngeal mucosa are poised to respond to changes in respiratory air flow and air pressure, and are situated to directly encode the mucosal tissue strains associated with laryngeal movements (Andreatta et al. 2002; Yoshida et al. 2000). Accordingly, it is reasonable to suggest that somatosensory guidance is important for executing nonacoustic movements, including those preceding phonatory onset, and interacts with auditory mechanisms during voice production (Larson et al. 2008). Therefore, aberrant somatosensory function may contribute to the voice deficits associated with PD. However, whether speech respiratory and phonatory control are related to impaired laryngeal somatosensation remains unknown.
Therefore, the objective of this study was to test whether individuals with PD exhibited abnormal laryngeal somatosensory function compared with healthy controls, and whether laryngeal somatosensory function was associated with aerodynamic, acoustic, and clinical measures of voice function and disease severity. This investigation employed an endoscopic somatosensory stimulus paradigm to present pressure-calibrated bursts of air to the laryngeal mucosa to determine the threshold pressure at which each subject could perceive the air burst stimulus (Hammer 2009). Speech aerodynamic and acoustic recordings were also collected from each subject for comparison. It was hypothesized that PD subjects would require higher threshold pressures to feel the stimulus than controls, that these thresholds would be more asymmetric in PD than in controls, and that these thresholds would be associated with aerodynamic, acoustic, and clinical measures of voice function and disease severity.
Methods
Participants
The local institutional ethics committee for the safety of human subjects approved the protocol. Written informed consent was obtained from all participants. Data were collected from 37 participants, including 19 with idiopathic PD (9 women, 10 men), and 18 healthy controls (10 women, 8 men). Mean age was 73 years (1.61) for PD and 76 years (1.32) for control participants (t = 1.51, P > 0.1). PD participants had moderate bilateral disease with a mean (Hoehn and Yahr 1967) score of 3 (0.16), and were a mean of 6.5 years (1.2) since PD diagnosis. Each PD participant was tested a minimum of 12 h after their most recent dose of anti-PD medication. PD participants exhibited increased voice severity based on the Consensus Auditory Perceptual Evaluation of Voice (CAPE-V, Kempster et al. 2009) with a mean score of 51.11 (3.83) compared with 7.56 (1.43) for healthy controls (t = −10.65, P < 0.001). PD participants also reported voice handicap based on the Voice Handicap Index (VHI, Jacobson et al. 1997) with mean score of 37.59 (6.22) compared with 9.15 (1.79) for controls (t = −4.39, P < 0.001). Inclusion in the PD group was limited to participants with otherwise good general health and no history of other neurological or psychiatric disease. Inclusion in the control group was limited to individuals who were in good general health, with normal breathing, speech, swallow, and voice, and no history of neurological or psychiatric disease. Exclusion criteria common across both the groups included smoking during the past 20 years, asthma, emphysema, use of inhaled medications, upper respiratory infection, recent pneumonia during the past 2 years, history of stroke or seizures, surgery of the head, neck, thorax, and abdomen, and symptoms or evidence of gastroesophageal reflux disease (Belafsky et al. 2001, 2002). Potential participants were also screened for other medications with known detrimental effects on speech and voice (National Center for Voice and Speech 2009).
Laryngeal somatosensory assessment
To assess laryngeal somatosensory function, an endoscopic stimulus delivery paradigm was employed to present pressure-calibrated bursts of air to the laryngeal mucosa to determine the threshold pressure at which each subject could perceive the stimulus (Fig. 1). The device design and experimental paradigm were described in an earlier report (Hammer 2009), and the procedures are reviewed below. While the participant sat comfortably in an exam chair, a transnasal laryngoscope (Pentax FNL-13RAP) was placed into the most patent naris. A topical decongestant (phenylephrine) was administered to each participant prior to scope placement. In order to maintain mucosal sensitivity and given the slight discomfort accompanying nasal laryngoscopy without topical anesthesia (Leder et al. 1997), the assessments were performed without anesthesia. The fiberoptic light cable of the laryngoscope was coupled to a constant halogen light source (Pentax LH-150PC) to illuminate the laryngeal structures. The eyepiece of the endoscope was coupled to a three-chip camera (Toshiba IK-C40A) connected to a video recorder with an integrated video monitor (Sony GVD-1000). The air burst port of the laryngoscope was coupled to the end of a three-foot long rigid polyethylene tube. The opposite end of the tubing was coupled to the output port of the somatosensory stimulus delivery device. This device was fabricated within a sound attenuating enclosure to prevent any potential acoustic cues of the device from imposing a bias on participant responses (Hammer 2009).
Fig. 1.
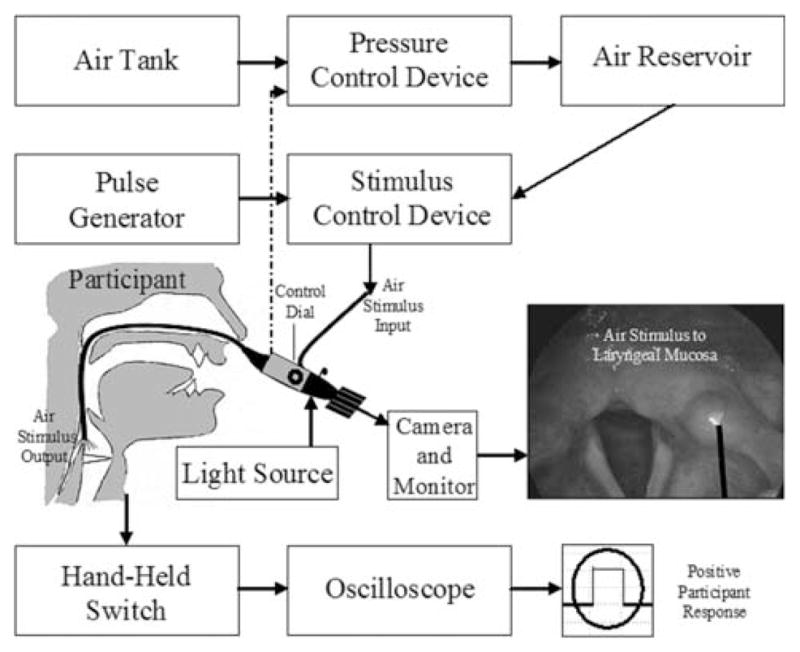
Block diagram of stimulus delivery paradigm (Hammer 2009) (reprinted with permission, ©2009 IEEE). The air stimulus is directed to the laryngeal mucosa through a port in the laryngoscope as visualized on the monitor. A +5 V signal from a hand-held switch indicates when the participant feels the laryngeal somatosensory stimulus
Previous reports indicated that the mucosa covering the arytenoid–corniculate cartilages contained the highest density of rapidly adapting mechanoreceptors and were strongly associated with vocal fold closure (Andreatta et al. 2002; Yoshida et al. 2000). Accordingly, visual guidance was used from the display monitor to direct the somatosensory stimulus through the distal end of the laryngoscope toward the superior surface of the mucosa surrounding the arytenoid–corniculate cartilage (right and left) in the posterior region of the larynx. The order of side tested was counterbalanced across participants. Great care was taken in an attempt to maintain a constant distance of 2 mm between the laryngoscope and the laryngeal mucosa. Within a scope tip to tissue distance of 2–3 mm, individual blood vessels within the vocal fold became very easy to visualize. Based on preliminary stimulus calibration testing (Hammer 2009), the air burst stimulus output from the laryngoscope was found to be reasonably laminar with little to no variation in output delivered to targets within 2–5 mm from the scope tip. In an attempt to standardize distance, at least 50% of the monitoring screen was occupied by the arytenoid unit during stimulus presentation. This approach was verified using two excised human adult cadaveric larynges. Within a scope tip to arytenoid mucosa distance of 2 mm, the arytenoid body occupied between 50 and 65% of the monitor screen. Once the target position was achieved, an index finger was positioned on the scope tube at the nasal inlet in an attempt to maintain a constant distance from scope tip to arytenoid mucosa of 2 mm during each stimulus presentation. Adjustments were made between stimulus presentations as needed.
Stimulus presentation was triggered using the initiation of the expiratory phase of respiration as transduced using inductance plethysmography (Ambulatory Monitoring, Inc.). During the onset of expiration, a transistor–transistor logic signal from a threshold discriminator (Coulbourn LabLinkV) triggered a control pulse from a pulse generator (Berkley Nucleonics 565) resulting in event-related triggering of a 135 ms air burst stimulus. Based on results from an earlier study (Hammer 2009), the more salient 135 ms stimulus was selected to reduce the potential influence of temporal summation on sensory threshold estimates. The primary signals of interest for assessing somatosensory function were the pressure (mm Hg) of the air burst stimulus applied to the laryngeal mucosa overlying the superior surface of the arytenoid cartilage and the participant’s response to the stimulus pulse. Each subject was presented with an initial suprathreshold stimulus to orient to the response task and was instructed to press a custom designed hand-held switch as soon as they detected the stimulus. All participants found the hand-switch easy to use. Pressing the switch resulted in a +5 V signal displayed on an oscilloscope (Tektronix TDS 2004). A “positive response” was defined as a +5 V response by the subject occurring within a 2.5 s window beginning at the midpoint of the digitized control signal. The time interval between stimulus presentations was randomized, with a minimum of 5 s between stimuli. The stimulus level of the air burst source was decreased by 1.00 mm Hg (133.32 Pa) until the participant could no longer feel the stimulus. At this point, the stimulus level was increased by 0.50 mm Hg (66.66 Pa) until the participant yielded a “positive response”. Then, the stimulus was decreased by 0.10 mm Hg (13.33 Pa). This process continued until the participant reached the threshold level. The laryngeal mechanosensory detection threshold (LMDT) was defined as the level of stimulus pressure at which the subject responded 50% of the time following six crossings of the same stimulus level.
Previous reports using laryngeal sensory testing revealed a test–retest reliability of r = 0.80 when assessing stimulus detection (Aviv et al. 1993). To test reliability for the present study, five participants were randomly selected and scheduled for a second test session within a month of the original test date. Test–retest reliability of LMDT was assessed by computing a Pearson product moment correlation coefficient and the mean percent change in thresholds between test sessions.
Aerodynamic and acoustic assessment
Earlier reports described procedures for the assessment of speech aerodynamics (Barlow et al. 1999; Netsell and Hixon 1978; Smitheran and Hixon 1981; Vantipalli and Barlow 2004); the procedures are reviewed below. While the participants comfortably sat in an exam chair, each was instructed to take a full breath, then repeat the syllable [pɑ] at a rate of two syllables per second at a comfortable pitch and loudness. Air pressure in the mouth during the closed phase of the voiceless bilabial plosive [p] is equal to the air pressure below the glottis. This sampling context provides a reasonable estimate of respiratory driving pressure, also known as subglottal air pressure, for voice and speech (Netsell and Hixon 1978; Smitheran and Hixon 1981). A positive respiratory driving pressure is required for expiratory air flow through the larynx and upper airway. Air flow may be measured during speech directly through a face mask coupled to an air flow sensor placed over the mouth and nose. Analysis of the air flow signal yields important information regarding respiratory and laryngeal function. The peak in the air flow signal following release of the consonant [p] is influenced by respiratory driving pressure and the degree of vocal fold abduction. The slope of declination in the air flow signal following the peak may provide information on the relative timing of phonatory onset. Mean vocalic air flow during the vowel [ɑ] reflects vocal fold adduction and closure for voicing. The air pressure and vocalic air flow signals can be used together to estimate laryngeal airway resistance associated with vocal fold closure (Smitheran and Hixon 1981). Finally, simple time integration of the air flow signal can be used to measure the lung air volume expended for each syllable.
Air pressure in the oral cavity was measured using a polyethylene catheter (Intramedic PE 260, 1.77 mm ID, 7 cm length) placed in the mouth near the oral angle and oriented perpendicular to the breath stream during speech. The catheter was coupled to a pressure transducer (Honey-well model 164PC01D37). A 10 cm H2O pressure source (U-tube manometer) was used to calibrate the air pressure transducer. Air flow was measured using a Puritan-Bennett full-face respiratory mask (model 5253) and a Hans Rudolph pneumotachometer (model R4719) instrumented with a pressure transducer (Honeywell model 163PC01D36). A 500 cc/s flow source (Glottal Enterprises model MCU-2) was used to calibrate the air flow transducer. Bridge amplifiers (Biocommunication Electronics, model 201, LP −3 dB at 50 Hz, Butterworth 3-pole) were used to condition both air pressure and air flow signals. The speech acoustic signal was transduced using a Sony condenser microphone positioned 15 cm from the mouth.
The signals were digitized at 20 kHz per channel using a custom designed data acquisition and analysis program (Barlow et al. 1999; Vantipalli and Barlow 2004) to compute subglottal air pressure, peak air flow, mean vocalic air flow, and laryngeal airway resistance. Air flow and acoustic signals were also digitized with ADInstruments PowerLab/16sp and Chart (v5.41). Air flow data were processed using Matlab (v7.0.4) to determine the slope of declination following the peak in the air flow signal associated with phonatory onset, and the signals were integrated to determine the lung air volume expended per syllable. Acoustic measurement of voice intensity was also performed using Matlab. Acoustic measurement of voice onset time was performed for spoken syllables of [pɑ] using MultiSpeech (KayPENTAX) as previously described (Lieberman et al. 1992; Lisker and Abramson 1964; Liss et al. 1990) by measuring the time from the release of the consonant [p] to the first cycle of voicing in the vowel [ɑ]. Initial and final syllables for each test trial were excluded to eliminate utterance end-effects. To test reliability for voice onset time, 10% of the data were remeasured at least 2 weeks after the original measurements. Test–retest reliability of voice onset time was assessed by computing the Pearson product moment correlation coefficient and the mean difference in voice onset time between measurements.
Statistical analysis
An ANCOVA design was used to test for group differences (PD vs. controls, α = 0.05) and to control for the covariates age and sex (Liss et al. 1990; Neiman et al. 1983; Ryalls et al. 1997; Swartz 1992; Sweeting and Baken 1983; Whiteside and Irving 1997, 1998). When comparing LMDT between groups (PD vs. controls), a repeated measures ANOVA was used to also account for the fact that there were two LMDT scores (right and left) for each participant. It was hypothesized that PD participants would exhibit higher and more asymmetric LMDT than controls. To examine the possible relation between LMDT and aerodynamic, acoustic, and clinical measures of voice function and disease severity, Pearson product moment correlation coefficients (α= 0.05) were calculated. It was hypothesized that LMDT would be significantly correlated with each of these measures.
Results
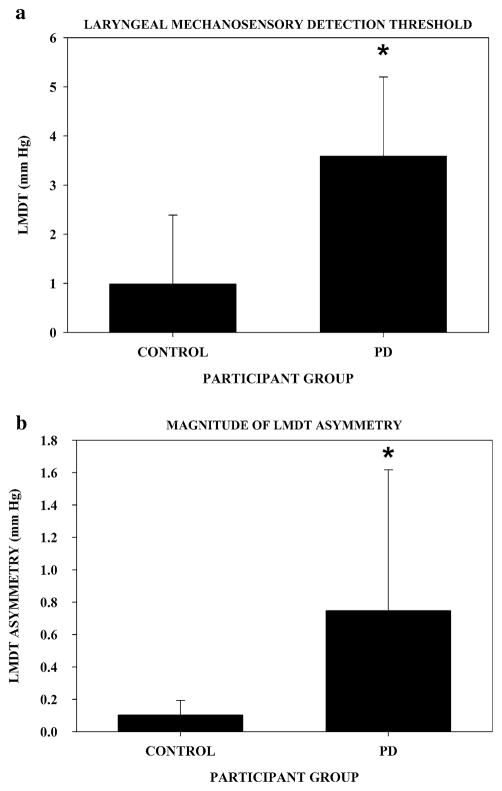
Laryngeal mechanosensory detection threshold and LMDT asymmetry results for PD and control participants are in Fig. 2. PD participants exhibited significantly higher LMDT and significantly greater asymmetry of LMDT than healthy controls. Asymmetry of PD symptoms positively, though not significantly, correlated with LMDT asymmetry (r = 0.37, P = 0.12). The predominant side affected (left vs. right) was evenly distributed across PD participants with no trend indicating that one side was more frequently affected than the other. Results for each aerodynamic measure are shown in Table 1. PD participants exhibited significantly decreased subglottal air pressure, peak air flow, laryngeal resistance, lung air volume expended for each syllable, and slope of air flow declination than healthy controls. Voice intensity and voice onset time results are also shown in Table 1. PD participants exhibited significantly decreased voice intensity and shorter voice onset time than healthy controls.
Fig. 2.
Endoscopic assessment of laryngeal somatosensory function for control and PD participants. Bar height represents mean with standard deviation for each group in mm Hg (1 mm Hg = 133.32 Pa). a Laryngeal mechanosensory detection threshold (LMDT, P < 0.001). b Absolute magnitude of LMDT asymmetry (P < 0.002)
Table 1.
Aerodynamic and acoustic measures of voice function for control and PD participants
| Measure | Control | PD |
|---|---|---|
| Subglottal air pressure (cm H2O) | 10.60 (2.08) | 6.45 (1.70)*** |
| Peak air flow (cc/s) | 1,191.10 (410.26) | 576.80 (154.74)*** |
| Vocalic air flow (cc/s) | 200.00 (60.25) | 176.30 (71.05)NS |
| Laryngeal resistance (cm H2O/l/s) | 58.04 (14.64) | 43.35 (17.87)* |
| Lung air volume expended for each syllable (cc) | 42.08 (16.59) | 24.60 (7.28)** |
| Slope of declination in air flow [(cc/s)/ms] | 9.05 (3.48) | 3.57 (1.48)*** |
| Voice intensity (dB SPL) | 72.35 (4.16) | 66.61 (4.71)*** |
| Voice onset time (ms) | 59.47 (10.44) | 39.94 (11.94)*** |
Mean value (standard deviation) is displayed for each group
P < 0.05,
P < 0.003,
P < 0.001,
P > 0.05
Correlation coefficients between LMDT and aerodynamic, acoustic, and clinical measures of voice function and disease severity are in Table 2. LMDT negatively correlated with subglottal air pressure, peak air flow, vocalic air flow (not significant), laryngeal resistance, lung air volume expended for each syllable, slope of air flow declination, voice intensity, and voice onset time and positively correlated with voice handicap, voice severity, and PD severity. In general, participants with higher LMDT values demonstrated smaller subglottal air pressure, peak air flow, vocalic air flow (not significant), laryngeal resistance, lung air volume expended for each syllable, slope in air flow declination, voice intensity, and voice onset time, and exhibited greater voice handicap, voice severity, and PD severity.
Table 2.
Correlations between laryngeal mechanosensory detection threshold (LMDT) and aerodynamic, acoustic, and clinical measures of voice function and disease severity
| Measure | r value (P value) |
|---|---|
| Subglottal air pressure | −0.67 (0.000) |
| Peak air flow | −0.52 (0.000) |
| Vocalic air flow | −0.15 (0.193) |
| Laryngeal resistance | −0.33 (0.004) |
| Lung air volume expended for each syllable | −0.46 (0.000) |
| Slope of declination in air flow | −0.55 (0.000) |
| Voice intensity | −0.40 (0.000) |
| Voice onset time | −0.42 (0.001) |
| Voice handicap | 0.45 (0.001) |
| Voice severity | 0.67 (0.000) |
| PD severity | 0.35 (0.032) |
In addition, eight PD participants asked their spouses to evaluate their voices using the Voice Handicap Index-Partner (VHI-P, Zraick et al. 2007). PD participant’s self assessment of voice handicap (VHI) was compared with the assessment given by their spouse (VHI-P). Spouse ratings of voice severity were significantly higher than the PD participant self assessments by 20.86 (t = 2.80, P < 0.027).
Reliability
To examine LMDT test–retest reliability, five randomly selected participants were scheduled for a second test session within a month of the original test date. LMDT was highly reliable, with a Pearson product moment correlation coefficient of r = 0.999 (P = 0.000) and a mean percent change in thresholds of 6.89%. To examine test–retest reliability of voice onset time, 10% of the data were remeasured at least 2 weeks after the original measurements. Voice onset time measures were also highly reliable, with a Pearson product moment correlation coefficient of r = 0.994 (P = 0.000); differences in measured voice onset time were less than 5 ms.
Discussion
This report presents the first data examining laryngeal somatosensory function in PD, its association with speech respiratory and phonatory function, and clinical measures of voice function and disease severity. To examine laryngeal somatosensory function, this study employed endoscopic somatosensory stimulus delivery to present pressure-calibrated bursts of air to the laryngeal mucosa to determine the threshold pressure (LMDT) at which each subject could perceive the air burst stimulus. Speech acoustic and aerodynamic recordings and clinical ratings were collected for each subject for comparison. The data and statistical analyses generally supported the experimental hypotheses.
Findings indicated that PD participants exhibited laryngeal somatosensory deficits compared with controls. PD participants exhibited decreased subglottal air pressure, peak air flow, laryngeal resistance, lung air volume expended for each syllable, slope of declination in air flow, voice intensity, and shorter voice onset time than healthy controls. These findings are consistent with reduced respiratory driving pressure, reduced voice intensity, and phonatory onset errors in PD (Ludlow and Bassich 1983, 1984; Ramig and Dromey 1996; Ramig et al. 2004). These speech respiratory and phonatory features were correlated with laryngeal somatosensory function. In general, more severe laryngeal somatosensory deficits were associated with greater reductions in respiratory driving pressure, more phonatory onset errors, and greater voice handicap, voice severity, and PD severity. In addition, spouse ratings of voice severity were higher than the PD participant self assessments, consistent with a decreased self-awareness of voice deficits in PD.
The fact that PD participants exhibited laryngeal somatosensory deficits and somatosensory asymmetry compared with controls is consistent with PD motor and nonmotor abnormalities observed in other studies (Bevan et al. 1994; Buchman et al. 2009; Demirci et al. 1997; Lee et al. 2005; Liberini et al. 2000; Nolano et al. 1999; Prätorius et al. 2003; Rickards and Cody 1997; Shulman et al. 2001; Snider et al. 1976; Tamburin et al. 2003; Zia et al. 2003). Asymmetric laryngeal somatosensory function in PD was not unexpected given the asymmetric manifestation of the disease, although such laryngeal asymmetry may be surprising given the pattern of bilateral cortical motor inputs for laryngeal control. For example, activation of corticofugal projections from laryngeal motor cortex results in bilateral laryngeal activity (Rodel et al. 2004). However, the laryngeal sensory asymmetry observed in PD during the present study was consistent with the predominantly contralateral representation of laryngeal afferents (Yoshida et al. 2000) and the asymmetric pattern of neurodegeneration in PD.
These findings have important implications for understanding the somatosensory mechanisms of speech respiratory and phonatory control, and the influence of PD on these mechanisms. Many speech respiratory, laryngeal, and supralaryngeal movements and aerodynamic events often occur prior to the onset of an audible acoustic signal (Larson et al. 2008), and are generally not visible to the speaker. Auditory and visual afferent mechanisms are insufficient to guide these movements. Rapidly adapting mechanoreceptors within the laryngeal mucosa are positioned to respond to changes in respiratory air flow and pressure, and to directly encode mucosal tissue strains associated with laryngeal movement (Andreatta et al. 2002; Yoshida et al. 2000). Recent evidence suggests that somatosensory and auditory mechanisms interact to provide accurate and efficient adjustments to voice pitch and intensity, and that the auditory vocal feedback system is significantly modified by alterations in laryngeal somatosensory function (Larson et al. 2008). Larson et al. (2008) demonstrated that reflexive voice pitch control was significantly altered after applying topical anesthesia to the laryngeal mucosa. In the absence of mucosal somatosensation, participants exhibited larger responses to a brief auditory perturbation in voice pitch. These findings suggest that the laryngeal somatosensorium plays a key role in phonatory control, and that alterations in somatosensory function may result in significant changes in phonatory function. The present work addresses and supports this suggestion.
Results from this study are consistent with the fact that individuals with PD are generally unaware of the severity of their voice deficits, and often report that they feel as though they are shouting or they feel as if they are exerting great physical effort when asked to speak at a more normal audible loudness level (Ramig and Dromey 1996; Ramig et al. 2004). The present results and this clinical observation suggest that speech and voice impairments in PD may be related to impaired somatosensory function. However, the specific mechanisms accounting for these impairments remain uncertain. Liu et al. (2008) demonstrated that reflexive voice intensity control was significantly altered in individuals with PD. PD participants exhibited larger responses to a brief auditory perturbation in voice intensity. These findings suggest that the vocal control system is more sensitive to vocal loudness in PD. Thus, when an individual with PD is asked to speak at a more normal audible loudness level, he may often have the impression that he is speaking too loudly, and reduce the loudness of his voice. Findings from the present report suggest that the respiratory and phonatory mechanisms contributing to reduced voice intensity in PD are associated with impaired somatosensory function. If we integrate these new findings with the vocal control system model of Larson et al. (2008), it is reasonable to suggest that reduced laryngeal somatosensory function in PD may also increase sensitivity to the auditory perception of voice intensity, resulting in reduced voice intensity in PD.
Results from this study are also consistent with other proposed mechanisms of movement abnormalities in PD, including sensory gating deficits. Clinical symptoms of PD may be due in part to abnormally excessive sensory gating, or reduced input of somatosensory information required for normal movement execution (Bischoff-Grethe et al. 2002; Kaji 2001; Kaji et al. 2005; Lee 1989; Lee and Tatton 1975; Schneider 1987; Schneider et al. 1986, 1987). Excessive sensory gating in PD may reflect impaired integration of sensory inputs with the planning and execution of movement, resulting in impaired goal-related movements. For example, PD participants in the present report exhibited reduced respiratory driving pressure. The inability of the sensorimotor regions of the basal ganglia, cerebral cortex, and other associated regions to receive accurate afferent input may account for errors in the initiation, timing, and range of movement. The fact that individuals with PD often exhibit abnormally increased muscle tone and rigidity may reflect a tendency of the central nervous system to remain in a state of movement preparation, while awaiting the arrival of the afferent signal, resulting in a delayed or aberrant transition to movement execution (Bischoff-Grethe et al. 2002). Aberrant afferent transmission may also coincide with aberrant movement patterns. For example, phonatory onset errors and abnormal muscle activity have been reported for the respiratory and laryngeal muscles in PD (Baker et al. 1998; Gallena et al. 2001; Ludlow and Bassich 1983, 1984; Luschei et al. 1999; Tzelepis et al. 1988). The new data from the present report suggest that respiratory and phonatory control deficits in PD may be influenced by abnormal laryngeal somatosensory afferent function.
One final issue concerns the dissociation between limb-and larynx-related measures. This study found that asymmetry of PD limb symptoms positively, though not significantly, correlated with LMDT asymmetry, and that PD severity was only weakly associated with LMDT. These findings may reflect fundamental differences in the somatotopy and/or pathophysiology of limb versus speech-related cerebral regions in PD. For example, Braak et al. (2003a, b, 2004) conducted a series of neuropathological examinations and reported that PD affects multiple cerebral regions in addition to, and even prior to, exerting an insult to the basal ganglia, beginning caudally in the brainstem and extending rostrally toward the cerebral cortex. Therefore, it is possible that the laryngeal somatosensory findings, and the speech respiratory and phonatory control deficits observed in PD, reflect a more caudal and axial neuropathology including the brain stem and thalamus, rather than a confined neuropathy of the nigrostriatal pathway. Other regions, including sensorimotor cortex, may also be involved. These types of clinical to neuropathological correlations and limb versus speech distinctions will be interesting areas to consider for future investigation.
In summary, the present study found that changes in laryngeal somatosensory function were significantly related to clinical and instrumental measures of voice function. These results suggest that speech respiratory and phonatory control are influenced by laryngeal somatosensory function, that speech-related deficits in PD are related to abnormal laryngeal somatosensory function, and that this function may degrade as a function of disease severity. Thus, PD may represent a model of airway sensorimotor disintegration, highlighting the important role of the basal ganglia and related neural networks in the integration of laryngeal sensory input for speech-related motor control.
Acknowledgments
Dr. Hammer is supported through National Institutes of Health grants DC007260, RR025012, RR023268, and the American Speech Language Hearing Foundation. Dr. Barlow is supported through National Institutes of Health grants DC003311, DC005803, and HD02528.
Contributor Information
Michael J. Hammer, Email: hammer@surgery.wisc.edu, Division of Otolaryngology, Department of Surgery, University of Wisconsin, 600 Highland Avenue K4/769, Madison, WI 53792, USA
Steven M. Barlow, Department of Speech-Language-Hearing: Sciences and Disorders, Program in Neuroscience, University of Kansas, Lawrence, KS, USA
References
- Andreatta RD, Mann EA, Poletto CJ, Ludlow CL. Mucosal afferents mediate laryngeal adductor responses in the cat. J Appl Physiol. 2002;93:1622–1629. doi: 10.1152/japplphysiol.00417.2002. [DOI] [PubMed] [Google Scholar]
- Aviv JE, Martin JH, Keen MS, Debell M, Blitzer A. Air pulse quantification of supraglottic and pharyngeal sensation: a new technique. Ann Otol Rhinol Laryngol. 1993;102:777–780. doi: 10.1177/000348949310201007. [DOI] [PubMed] [Google Scholar]
- Baker KK, Ramig LO, Luschei ES, Smith ME. Thyroarytenoid muscle activity associated with hypophonia in Parkinson disease and aging. Neurology. 1998;51:1592–1598. doi: 10.1212/wnl.51.6.1592. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Suing G, Andreatta RD. Speech aerodynamics using AEROWIN. In: Barlow SM, editor. Handbook of clinical speech physiology. Singular Publishing Group; San Diego: 1999. [Google Scholar]
- Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS) Laryngoscope. 2001;111:1313–1317. doi: 10.1097/00005537-200108000-00001. [DOI] [PubMed] [Google Scholar]
- Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI) J Voice. 2002;16:274–277. doi: 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- Bevan L, Cordo P, Carlton L, Carlton M. Proprioceptive coordination of movement sequences: discrimination of joint angle versus angular distance. J Neurophys. 1994;71:1862–1872. doi: 10.1152/jn.1994.71.5.1862. [DOI] [PubMed] [Google Scholar]
- Bischoff-Grethe A, Crowley MG, Arbib MA. Movement inhibition and next sensory state prediction in the basal ganglia. In: Graybiel AM, DeLong MR, Kitai ST, editors. The Basal Ganglia VI. Kluwer Academic/Plenum Publishers; New York: 2002. [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RAI, Steur ENHJ, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003a;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Rev. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici KD. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003b;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Leurgans S, Bennett DA. Vibratory thresholds and mobility in older persons. Muscle Nerve. 2009;39:754–760. doi: 10.1002/mus.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci M, Grill S, McShane L, Hallett M. A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann Neurol. 1997;41:781–788. doi: 10.1002/ana.410410614. [DOI] [PubMed] [Google Scholar]
- Gallena S, Smith PL, Zeffiro T, Ludlow CL. Effects of levodopa on laryngeal muscle activity for voice onset and offset in Parkinson disease. J Speech Lang Hear Res. 2001;44:1284–1299. doi: 10.1044/1092-4388(2001/100). [DOI] [PubMed] [Google Scholar]
- Hammer MJ. Design of a new somatosensory stimulus delivery device for measuring laryngeal mechanosensory detection thresholds in humans. IEEE Trans Biomed Eng. 2009;56:1154–1159. doi: 10.1109/TBME.2008.2007968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Jacobson BH, Johnson A, Grywalski C, Jacobson G, Benninger MS, Newman CW. The voice handicap index (VHI): development and validation. Am J Speech Lang Path. 1997;6:66–70. [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Jürgens U, Kirzinger A. The laryngeal sensory pathway and its role in phonation: a brain lesioning study in the squirrel monkey. Exp Br Res. 1985;59:118–124. doi: 10.1007/BF00237672. [DOI] [PubMed] [Google Scholar]
- Kaji R. Basal ganglia as a sensory gating devise for motor control. J Med Invest. 2001;47:142–146. [PubMed] [Google Scholar]
- Kaji R, Urushihara R, Murase N, Shimazu H, Goto S. Abnormal sensory gating in basal ganglia disorders. J Neurol. 2005;252:IV/13–IV/16. doi: 10.1007/s00415-005-4004-9. [DOI] [PubMed] [Google Scholar]
- Kempster GB, Gerratt BR, Verdolini-Abbott K, Barkmeier-Kraemer J, Hillman RE. Consensus auditory-perceptual evaluation of voice: development of a standardized clinical protocol. Am J Speech Lang Path. 2009;18:124–132. doi: 10.1044/1058-0360(2008/08-0017). [DOI] [PubMed] [Google Scholar]
- Larson CR, Altman KW, Liu H, Hain TC. Interactions between auditory and somatosensory feedback for voice F0 control. Exp Brain Res. 2008;187:613–621. doi: 10.1007/s00221-008-1330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder SB, Ross DA, Briskin KB, Sasaki CT. A prospective, double-blind, randomized study on the use of topical anesthetic, vasoconstrictor, and placebo during transnasal flexible fiberoptic endoscopy. J Speech Lang Hear Res. 1997;40:1352–1357. doi: 10.1044/jslhr.4006.1352. [DOI] [PubMed] [Google Scholar]
- Lee RG. Pathophysiology of rigidity and akinesia in Parkinson’s disease. Eur Neurol. 1989;29(Suppl 1):13–18. doi: 10.1159/000116448. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kim HS, Lyoo CH. “Off” gait freezing and temporal discrimination threshold in patients with Parkinson disease”. Neurology. 2005;64:670–674. doi: 10.1212/01.WNL.0000151961.14861.BA. [DOI] [PubMed] [Google Scholar]
- Lee RG, Tatton WG. Motor responses to sudden limb displacements in primates with specific CNS lesions and in human patients with motor system disorders. Can J Neurol Sci. 1975;2:285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- Liberini P, Parola S, Spano PF, Antonini L. Olfaction in Parkinson’s disease: methods of assessment and clinical relevance. J Neurol. 2000;247:88–96. doi: 10.1007/pl00007803. [DOI] [PubMed] [Google Scholar]
- Lieberman P, Kako E, Friedman J, Tajchman G, Feldman LS, Jiminez EB. Speech production, syntax comprehension, and cognitive deficits in Parkinson’s disease. Brain Lang. 1992;43:169–189. doi: 10.1016/0093-934x(92)90127-z. [DOI] [PubMed] [Google Scholar]
- Lisker L, Abramson AS. A cross-language study of voicing in initial stops: acoustical measurements. Word. 1964;20:384–422. [Google Scholar]
- Liss JM, Weismer G, Rosenbek JC. Selected acoustic characteristics of speech production in very old males. J Gerontol. 1990;45:35–45. doi: 10.1093/geronj/45.2.p35. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang EQ, Metman LV, Larson CR. Vocal responses to loudness- and pitch-shift perturbations in individuals with Parkinson’s disease. Abstracts from the 14th biennial speech motor control conference; Monterey. 2008. [Google Scholar]
- Ludlow CL, Bassich CJ. The results of acoustic and perceptual assessment of two types of dysarthria. In: Berry W, editor. Clinical dysarthria. College Hill; San Diego: 1983. [Google Scholar]
- Ludlow CL, Bassich CJ. Relationships between perceptual ratings and acoustic measures of hypokinetic speech. In: McNeil MR, Rosenbek JC, Aronson AE, editors. The dysarthrias. College Hill; San Diego: 1984. [Google Scholar]
- Luschei ES, Ramig LO, Baker KL, Smith ME. Discharge characteristics of laryngeal single motor units during phonation in young and older adults and in persons with Parkinson disease. J Neurophysiol. 1999;81:2131–2139. doi: 10.1152/jn.1999.81.5.2131. [DOI] [PubMed] [Google Scholar]
- McCulloch TM, Flint PW, Richardson MA, Bishop MJ. Lidocaine effects on the laryngeal chemoreflex, mechanoreflex, and afferent electrical stimulation reflex. Ann Otol Rhinol Laryngol. 1992;101:583–589. doi: 10.1177/000348949210100707. [DOI] [PubMed] [Google Scholar]
- Nagai H, Ota F, Connor NP. Effect of deficits in laryngeal sensation on laryngeal muscle biochemistry. Ann Otol Rhinol Laryngol. 2005;114:352–360. doi: 10.1177/000348940511400504. [DOI] [PubMed] [Google Scholar]
- National Center for Voice and Speech. [Accessed 11 August 2009];Frequently prescribed medications and effects on voice and speech. 2009 (online). http://www.ncvs.org/e-learning/rx2.html.
- Neiman GS, Klich RJ, Shuey EM. Voice onset time in young and 70-year-old women. J Speech Hear Res. 1983;26:118–123. doi: 10.1044/jshr.2601.118. [DOI] [PubMed] [Google Scholar]
- Netsell R, Hixon TJ. A noninvasive method for clinically estimating subglottal pressure. J Speech Hear Disord. 1978;43:326–330. doi: 10.1044/jshd.4303.326. [DOI] [PubMed] [Google Scholar]
- Nolano M, Provitera V, Estraneo A, Selim MM, Caporaso G, Stancanelli A, Saltalamacchia AM, Lanzillo B, Santoro L. Sensory deficit in Parkinson’s disease: evidence of cutaneous denervation. Brain. 1999;131:1903–1911. doi: 10.1093/brain/awn102. [DOI] [PubMed] [Google Scholar]
- Prätorius B, Kimmeskamp S, Milani TL. The sensitivity of the sole of the foot in patients with Morbus Parkinson. Neurosci Lett. 2003;346:173–176. doi: 10.1016/s0304-3940(03)00582-2. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Dromey C. Aerodynamic mechanisms underlying treatment-related changes in vocal intensity in patients with Parkinson’s disease. J Speech Hear Res. 1996;39:798–807. doi: 10.1044/jshr.3904.798. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Fox C, Sapir S. Parkinson’s disease: speech and voice disorders and their treatment with the Lee Silverman voice treatment. Semin Speech Lang. 2004;25:169–180. doi: 10.1055/s-2004-825653. [DOI] [PubMed] [Google Scholar]
- Rickards C, Cody FWJ. Proprioceptive control of wrist movements in Parkinson’s disease: reduced muscle vibration-induced errors. Brain. 1997;120:977–990. doi: 10.1093/brain/120.6.977. [DOI] [PubMed] [Google Scholar]
- Rodel R, Olthoff A, Tergau F, Simonyan K, Kraemer D, Markus H, Kruse E. Human cortical motor representation of the larynx as assessed by transcranial magnetic stimulation (TMS) Laryngoscope. 2004;114:918–922. doi: 10.1097/00005537-200405000-00026. [DOI] [PubMed] [Google Scholar]
- Ryalls J, Zipprer A, Baldauff P. A preliminary investigation of the effects of gender and race on voice onset time. J Speech Lang Hear Res. 1997;40:642–645. doi: 10.1044/jslhr.4003.642. [DOI] [PubMed] [Google Scholar]
- Sampson S, Eyzaguirre C. Some functional characteristics of mechanoreceptors in the larynx of the cat. J Neurophysiol. 1964;27:464–480. doi: 10.1152/jn.1964.27.3.464. [DOI] [PubMed] [Google Scholar]
- Schneider JS. Basal ganglia-motor influences: role of sensory gating. In: Schneider JS, Lidsky TI, editors. Basal ganglia and behavior: sensory aspects of motor functioning. Hans Huber Publishers; Toronto: 1987. [Google Scholar]
- Schneider JS, Diamond SG, Markham CH. Deficits in orofacial sensorimotor function in Parkinson’s disease. Ann Neurol. 1986;19:275–282. doi: 10.1002/ana.410190309. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Diamond SG, Markham CH. Parkinson’s disease: sensory and motor problems in arms and hands. Neurology. 1987;37:951–956. doi: 10.1212/wnl.37.6.951. [DOI] [PubMed] [Google Scholar]
- Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord. 2001;16:507–510. doi: 10.1002/mds.1099. [DOI] [PubMed] [Google Scholar]
- Smitheran J, Hixon T. Clinical method for estimating laryngeal airway resistance during vowel production. J Speech Hear Disord. 1981;46:138–146. doi: 10.1044/jshd.4602.138. [DOI] [PubMed] [Google Scholar]
- Snider SR, Fahn S, Isgreen WP, Cote LJ. Primary sensory symptoms in parkinsonism. Neurology. 1976;26:423–429. doi: 10.1212/wnl.26.5.423. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Hixon TJ. Speech breathing in Parkinson’s disease. J Speech Hear Res. 1993;36:294–310. doi: 10.1044/jshr.3602.294. [DOI] [PubMed] [Google Scholar]
- Sulica L, Hembree A, Blitzer A. Swallowing and sensation: evaluation of deglutition in the anesthetized larynx. Ann Otol Rhinol Laryngol. 2002;111:291–294. doi: 10.1177/000348940211100402. [DOI] [PubMed] [Google Scholar]
- Swartz BL. Gender differences in voice onset time. Percept Mot Skills. 1992;75:983–992. doi: 10.2466/pms.1992.75.2.415. [DOI] [PubMed] [Google Scholar]
- Sweeting PM, Baken RJ. Voice onset time in a normal-aged population. J Speech Hear Res. 1983;25:129–134. doi: 10.1044/jshr.2501.129. [DOI] [PubMed] [Google Scholar]
- Tamburin S, Fiaschi A, Idone D, Lochner P, Manganotti P, Zanette G. Abnormal sensorimotor integration is related to disease severity in Parkinson’s disease: a TMS study. Mov Disord. 2003;18:1316–1324. doi: 10.1002/mds.10515. [DOI] [PubMed] [Google Scholar]
- Tzelepis GE, McCool DF, Friedman JH, Hoppin FG. Respiratory muscle dysfunction in Parkinson’s disease. Am Rev Resp Disord. 1988;138:266–271. doi: 10.1164/ajrccm/138.2.266. [DOI] [PubMed] [Google Scholar]
- Vantipalli R, Barlow SM. AEROWIN RT clinical application for motor speech disorders. Abstracts from the 12th biennial speech motor control conference; Albuquerque. 2004. [Google Scholar]
- Whiteside SP, Irving CJ. Speakers’ sex differences in voice onset time: some preliminary findings. Percept Mot Skills. 1997;85:459–463. doi: 10.2466/pms.1997.85.2.459. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Irving CJ. Speakers’ sex differences in voice onset time: a study of isolated word production. Percept Mot Skills. 1998;86:651–654. doi: 10.2466/pms.1998.86.2.651. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Tanaka Y, Hirano M, Nakashima T. Sensory innervation of the pharynx and larynx. Am J Med. 2000;108:51S–61S. doi: 10.1016/s0002-9343(99)00342-3. [DOI] [PubMed] [Google Scholar]
- Zia S, Cody FWJ, O’Boyle DJ. Discrimination of bilateral differences in the loci of tactile stimulation is impaired in subjects with Parkinson’s disease. Clin Anat. 2003;16:241–247. doi: 10.1002/ca.10100. [DOI] [PubMed] [Google Scholar]
- Zraick R, Risner BY, Smith-Olinde L, Gregg BA, Johnson FL, McWeeny EK. Patient versus partner perception of voice handicap. J Voice. 2007;21:485–494. doi: 10.1016/j.jvoice.2006.06.006. [DOI] [PubMed] [Google Scholar]