Abstract
Objective
To determine the effect of recovery with mild hypothermia after cardiopulmonary bypass (CPB) and deep hypothermic circulatory arrest (DHCA) on the activity of selected key proteins involved in initiation (Bax, Caspase-3) or inhibition of apoptotic injury (Bcl-2, increased ratio Bcl-2/Bax) in the brain of newborn piglets.
Methods
The piglets were placed on CPB, cooled with pH-stat management to 18 °C, subjected to 30 min of DHCA followed by 1 h of low flow at 20 ml/kg/min, rewarmed to 37 °C (normothermia) or to 33 °C (hypothermia), separated from CPB, and monitored for 6 h. Expression of above proteins was measured in striatum, hippocampus and frontal cortex by Western blots. The results are mean for six experiments ± SEM.
Results
There were no significant differences in Bcl-2 level between normothermic and hypothermic groups. The Bax levels in normothermic group in cortex, hippocampus and striatum were 94 ± 9, 136 ± 22 and 125 ± 34 and decreased in the hypothermic group to 59 ± 17 ( p = 0.028), 70 ± 6 (p = 0.002) and 48 ± 8 (p = 0.01). In cortex, hippocampus and striatum Bcl-2/Bax ratio increased from 1.23, 0.79 and 0.88 in normothermia to 1.96, 1.28 and 2.92 in hypothermia. Expression of Caspase-3 was 245 ± 39, 202 ± 74 and 244 ± 31 in cortex, hippocampus and striatum in the normothermic group and this decreased to 146 ± 24 ( p = 0.018), 44 ± 16 ( p = 7 × 10−7) and 81 ± 16 ( p = 0.01) in the hypothermic group.
Conclusion
In neonatal piglet model of cardiopulmonary bypass with circulatory arrest, mild hypothermia during post bypass recovery provides significant protection from cellular apoptosis, as indicated by lower expression of Bax and Caspase-3 and an increased Bcl-2/Bax ratio. The biggest protection was observed in striatum probably by decreasing of neurotoxicity of striatal dopamine.
Keywords: Newborn, Brain, Cardiopulmonary bypass, Circulatory arrest, Hypothermia, Apoptosis
1. Introduction
Surgical repair of congenital heart disease may require cardiopulmonary bypass (CPB) and deep hypothermic circulatory arrest (DHCA). Prolonged DHCA may result in distinctive patterns of neuropsychologic dysfunction characterized by cognitive impairment, impaired executive function, expressive speech and language abnormalities, impaired visual-spatial and visual-motor skills, attention deficit/hyperactivity disorder, motor delays, and learning disabilities. The basis of these neurologic disturbances, caused mostly by brain ischemia, are changes in the cellular and molecular components that can induce apoptosis and necrosis, increased mitochondrial dysfunction and free radical production, disruptions of the blood—brain barrier, accumulation of excitatory neurotransmitters such as glutamate and prolonged excessive influx of Ca2+ into the cell, increase of inflammatory reactions and microthrombus formation, etc.
Understanding and exploring strategies for decreasing the sequelae of prolonged DHCA and protecting the brain from ischemic injury, is important and relevant to clinical management of neonates and infants following the use of CPB with DHCA.
There is substantial evidence that mild-to-moderate hypothermia is able to diminish ischemic-dependent events and is potentially a significant neuroprotective strategy in preventing ischemic injury [1—7]. In recent reviews by Polderman [8] and Nussmeier [9], the authors discuss the available evidence for use of controlled hypothermia and addressed questions regarding the timing, depth, duration, and effective management of side effects. From the currently available data in the literature, it is clear that more study is required to determine the efficacy of hypothermia after traumatic brain injury or ischemic stroke in decreasing brain injury [8,10]. Similarly, several recent reviews of cardiopulmonary and cerebral resuscitation have emphasized the potential benefit of therapeutic hypothermia after pediatric cardiac arrest [11—13].
Our present study investigates the effects of mild hypothermia on the activity of selected key regulatory proteins involved in either initiation (Caspase-3 and Bax) or inhibition (Bcl-2) of apoptotic reactions in the brain of newborn piglets following CPB and DHCA. Specifically, we hypothesized that mild hypothermia after CPB with DHCA would provide significant neuroprotection as measured by the effects on neuroregulatory proteins in the brain.
2. Methods
2.1. Animal model
Twelve newborn piglets, 3–5 days old (1.4–2.5 kg), were anesthetized with halothane, and a tracheotomy was performed. The piglets were then placed on a ventilator, and anesthesia was maintained with fentanyl, isoflurane 0.5% and pancuronium. Femoral venous and arterial cannulae were placed for the collection of blood samples and for monitoring of blood pressure. After a 1-h stabilization period, cardiopulmonary bypass was initiated. Following bypass, the animals were recovered for 6 h and then euthanized with 4 M KCl. All animal procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and have been approved by the local animal care committee.
2.2. CPB technique
The circuit was primed with Plasmalyte-A and then 25% albumin was added to the circuit. Donor whole blood was added to maintain a hematocrit of 25–30%. Heparin (1000 units), fentanyl (50 mcg), pancuronium (1 mg), CaCl2 (500 mg), methylprednisolone (60 mg), cefazolin (100 mg), furosemide (2 mg), and NaHCO3 (25 meq.) were then added to the pump prime. A membrane oxygenator (Lilliput) was used as well as a roller pump system (Cobe) and arterial filter (Terumo). For CPB, a median sternotomy was performed. Prior to cannulation, 500 units of heparin was administered IV. The ascending aorta as well as the right atrial appendage were cannulated. Full CPB flow rate was set at 150 ml/kg/min. pH-stat blood gas management was used in all experiments.
2.3. Experimental protocol
All animals were placed on bypass and cooled to a nasopharyngeal temperature of 18 °C over a 30-min period. A 30-min period of deep hypothermic circulatory arrest was then followed by 1 h of low flow cardiopulmonary bypass (LF-20, at 20 ml/kg/min). All animals were then rewarmed for 30 min to 37 °C (normothermic group) or to 33 °C (hypothermic group). They were then separated from CPB and recovered for 6 h under anesthesia, and finally euthanized with 4 M KCl. After euthanasia, the frontal cortex, hippo-campus and striatum were immediately dissected from the brain and frozen for later analysis.
2.4. Western blotting
Samples of frozen tissues were homogenized in a buffer containing 2% SDS, 10 mM Tris–HCl (pH 7.4), NaF (10 mM), Na pyrophosphate (10 mM), Na3Vo4 (1 mM), Na2MoO4 (1 mM), phenylarsine oxide (1 µM), and aprotinin (10 µg/ml). The homogenate was boiled for 5 min after addition of SDS-PAGE sample buffer. Protein concentration in the homogenate aliquots was determined using BCA Protein Assay kit (Pierce, IL). Equal amounts of protein from each sample were separated by 12.5% SDS-PAGE and transferred onto a nitrocellulose membrane (Hybond C, Amersham Pharmacia Biotech.). The membrane was incubated in a blocking solution (phosphate buffered saline (PBS), pH 7.4, containing 5% non-fat milk) for 1 h at room temperature and then with specific rabbit antibodies for Bax (N-20) and Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). For determination of Caspase expression, the membrane was incubated with rabbit anti-Cleaved Caspase-3 antibodies (Cell Signaling Technology, Beverly, MA, USA). After being washed in PBS containing 0.05% Tween 20 (PBS-T; Sigma–Aldrich), the membranes were incubated for 1 h with HRP-conjugated goat anti-rabbit secondary antibodies (Jackson Immuno Research).
Beta-actin antibody (ABCAM, Cambridge, MA, USA) served as a loading control. After being washed in PBS-Tween 20, the membranes were incubated for 1 h with HRP-conjugated anti-mouse IgG (Amersham Pharmacia Biotech.).
The final reaction was visualized using enhanced chemiluminescence (ECL Western Blotting Detection Reagents, Amersham Pharmacia Biotech.), and the membranes were exposed to X-ray film.
2.5. Data analysis
Results are expressed as mean ± SEM. Autoradiographic films were analyzed using Scion Image software (NIH). Each blot contained two sets of samples, one for a normothermic group and another for the hypothermic group. The blots were scanned and changes in expressions were measured. The experiments were carried out on six animals in each group. Data for each protein expression was subjected to ANOVA with Bonferroni’s correction to test for significance of the effect of temperature.
3. Results
3.1. Effect of mild hypothermia on expression of Bcl-2, Bax and Bcl-2/Bax ratio in different regions of the brain of newborn piglets
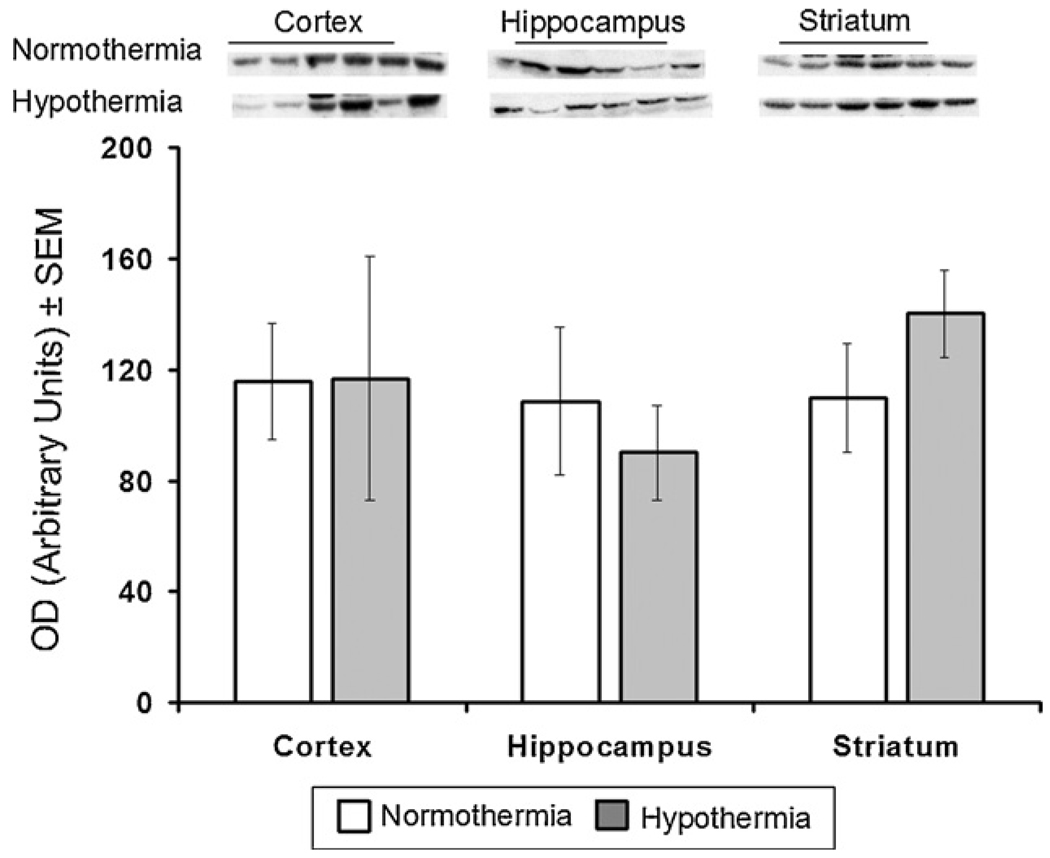
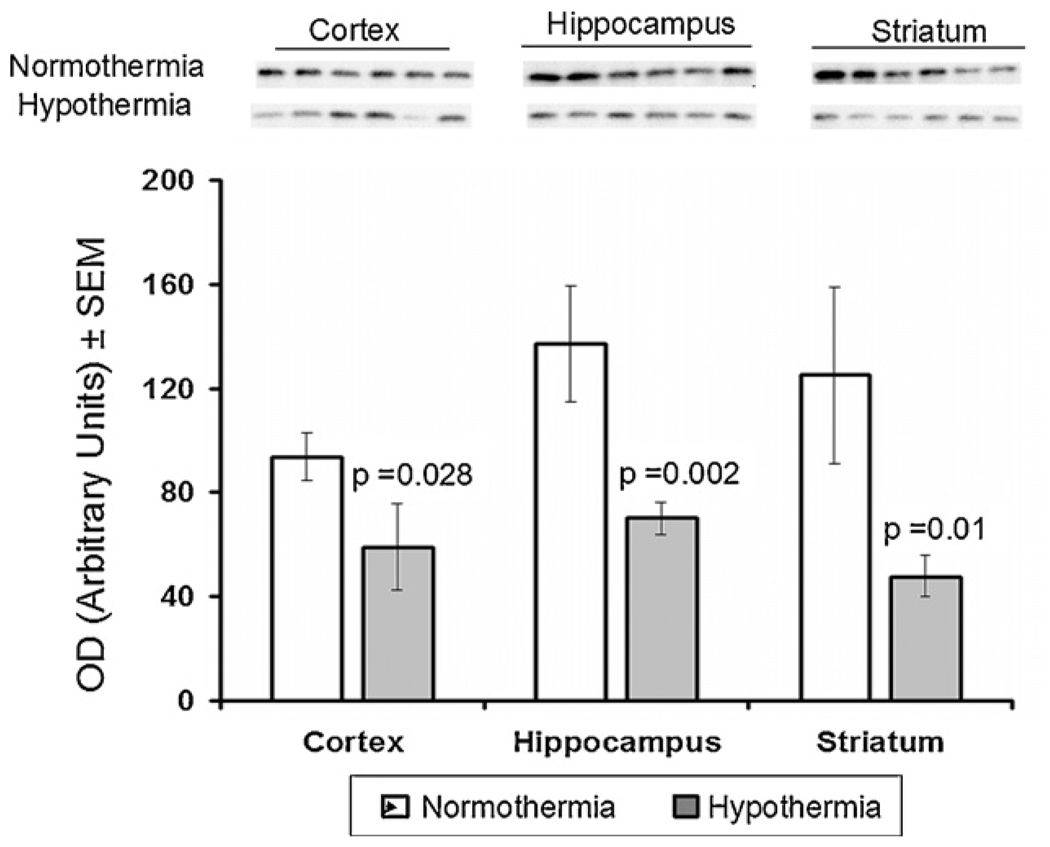
In the frontal cortex, hippocampus and striatum there were no significant differences in Bcl-2 expression between the normothermic and hypothermic groups of animals(Fig. 1). However, hypothermia did significantly decrease expression of Bax in all three regions of brain: in the frontal cortex from 94 ± 9 to 59 ± 17 ( p = 0.028), in the hippocampus from 136 ± 22 to 70 ± 6 (p = 0.002), and in the striatum from 125 ± 34 to 48 ± 8 (p = 0.01) (Fig. 2).
Fig. 1.
Effect of mild hypothermia on expression of Bcl-2 in cortex, hippo-campus and striatum of newborn brain measured after 6-h recovery following DHCA and low flow CPB. Top: Western blots for protein samples from normothermic and hypothermic groups probed with Bcl-2 antibodies. Bars represented the means ± SEM for the density of the bands for six independent experiments.
Fig. 2.
Effect of mild hypothermia on expression of Bax in cortex, hippocampus and striatum of newborn brain measured after 6-h recovery following DHCA and low flow CPB. Top: Western blots for protein samples from normothermic and hypothermic groups probed with Bax antibodies. Bars represented the means ± SEM for the density of the bands for six independent experiments. The p values for the changes from respective normothermic groups were determined by ANOVA with Bonferroni’s correction.
The calculated ratios of Bcl-2 to Bax in all three regions of brain are presented in Table 1. In the frontal cortex and hippocampus, the ratio Bcl-2/Bax increased in the hypothermia group from 1.23 to 1.96 (an increase of 59%) and from 0.79 to 1.28 (an increase of 62%), respectively. Hypothermia caused the biggest increase in Bcl-2/Bax ratio in striatum, from 0.88 to 2.92 (an increase of 230%).
Table 1.
Bcl-2/Bax ratio in frontal cortex, hippocampus and striatum measured after 6-h recovery following DHCA and low flow CPB in normothermic and hypothermic piglets.
| Region of brain | Normothermia | Hypothermia | %Increase |
|---|---|---|---|
| Cortex | 1.23 | 1.96 | 60 |
| Hippocampus | 0.79 | 1.28 | 62 |
| Striatum | 0.88 | 2.92 | 230 |
3.2. Effects of mild hypothermia on expression of Caspase-3 in the brain of newborn piglets
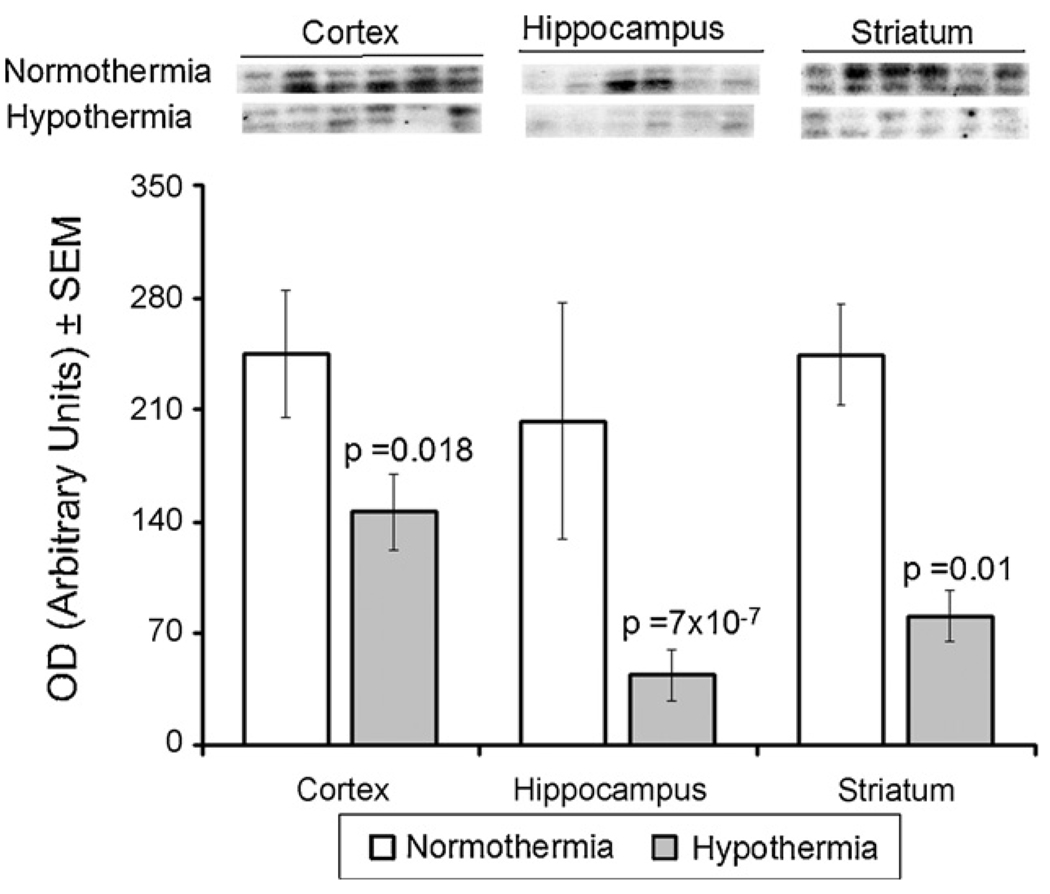
Expression of Caspase-3 in three regions of piglet brain after 6 h of recovery is shown in Fig. 3. As can be seen, hypothermia decreased Caspase-3 expression in all of the regions. In the frontal cortex, Caspase-3 expression in the hypothermia and normothermia groups was 146 ± 24 versus 245 ± 39 ( p = 0.018). In the hippocampus, expression in the hypothermia and normothermia groups was 44 ± 16 versus 202 ± 74 ( p = 7 × 10−7). Similarly, in the striatum, Caspase-3 expression in the hypothermia and normothermia groups was 81 ± 31 versus 244 ± 31 ( p = 0.01).
Fig. 3.
Effect of mild hypothermia on expression of Caspase-3 in cortex, hippocampus and striatum of newborn brain measured after 6-h recovery following DHCA and low flow CPB. Top: Western blots for protein samples from normothermic and hypothermic groups probed with Caspase-3 antibodies. Bars represented the means ± SEM for the density of the bands for six independent experiments. The p values for the changes from respective normothermic groups were determined by ANOVA with Bonferroni’s correction.
4 Discussion
The aim of this study was to determine if 6 h of mild hypothermia following DHCA and low flow CPB would decrease apoptotic activity (apoptotic neuronal injury) in the brain of newborn piglets and if there were regional differences in response to hypothermia. To accomplish this aim, the study of expression of selective pro- and anti-apoptotic proteins was performed. This method was chosen for two reasons. First, in neonates apoptosis is believed to be more responsible than necrosis as a cell death mechanism after hypoxic-ischemic insult. Second, apoptotic cell death occurs much more rapidly than necrosis (2–3 h). Because our measurements were done at 6 h of recovery, it was then expected that apoptosis rather then necrosis would be the major mechanism of observed neuronal injury following DHCA and low flow CPB. Yue et al. [14] reported on apoptosis and necrosis in neurons and glial cells following transient cerebral hypoxic-ischemic injury in newborn piglets. The authors suggested that immature neurons might be more prone to apoptotic death while terminally differentiated neurons would be more prone to die by necrosis.
The proteins of Bcl family are key regulatory factors in apoptotic events and can either promote cell survival (such as Bcl-2) or promote cell death (such as Bax). It is well documented that Bcl-2 heterodimerizes with Bax and their ratio determines the cellular susceptibility to apoptotic stimuli. For example, an increased ratio of Bax to Bcl-2 protein has been shown to occur in piglets following hypoxic and hypocapnic insults, demonstrating an increased susceptibility to apoptosis in the hypoxic and hypocapnic newborn brains [15]. We previously reported an increase in apoptotic events (decrease of Bcl-2 expression and low Bcl-2/Bax ratio) in striata of newborn piglets following 30 min DHCA and 60 min low flow CPB (20 ml/kg/min) with 2 h of normothermic recovery [16]. Our present study shows, in the same model but with 6 h recovery, a decrease in the Bcl-2/Bax ratio in the hippocampus and striatum.
The use of mild hypothermia following low flow CPB with DHCA caused a significant decrease in the level of Bax and an increase in the Bcl-2/Bax ratio in all three regions of piglet brain. This strongly suggests that mild hypothermia following CPB with DHCA decreases the induction of apoptotic activity in newborn piglets. The use of mild hypothermia appears to provide some protective effect in all of the regions of the brain that were examined. However, there was a regional difference in response to mild hypothermia with significantly greater increase in Bcl-2/Bax ratio in the striatum compared to the hippocampus or frontal cortex. The striatum (caudate putamen and nucleus accumbens) is the main input site to the basal ganglia, and one of the most important subcortical structures in the motor circuit. The observation that the greatest protection by hypothermia from DHCA-low flow CPB-dependent apoptotic injury was in the striatum, as compared to frontal cortex and hippocampus, indicates that hypothermia may play a significant protective role against motor function related injury observed clinically in newborns and infants following cardiac surgery using DHCA with low flow CPB.
The exact mechanism of this greater protection of the striatum by hypothermia needs further investigation. However, we postulate that it may occur by decreasing the neurotoxicity of dopamine. Dopamine is widely believed to play a critical role in the pathophysiology of brain function. Its excessive release during different hypoxic-ischemic conditions has been implicated in mediating neuronal damage, particularly in the striatum. Dopamine can potentiate neuronal damage through several mechanisms, particularly by increasing the production of free radicals and by activating apoptotic pathways. Dopamine-dependent increase in oxygen radicals is one of the major mechanisms of striatal injury following hypoxia-ischemia in the newborn. Oxidation of excess dopamine released during hypoxiaischemia and oxidation of cytosolic dopamine taken up during reperfusion results in formation of free radicals. Our previous studies have shown a direct relationship between dopamine levels and the increase in hydroxyl radicals in the striatum of newborn piglets during recovery after hypoxia (FiO2 = 6%) [17]. In those studies, the maximal increase in hydroxyl radicals occurred after 100 min of reoxygenation.
During DHCA with low flow CPB, just as in our hypoxia model, there is an increase in the extracellular dopamine in striatum of newborn piglets. This process may be responsible for an increase of free radical levels during reperfusion, which may be attenuated by hypothermia. Studies published by others have indicated that hypothermia decreases the levels of free radicals in the brain following hypoxia and ischemia in different experimental animal models.
It is also possible that DHCA with low flow CPB causes secondary release of dopamine during post-bypass recovery. This mechanism could be inhibited by hypothermia. Our earlier studies have shown that during hypoxia there was a large increase in the level of striatal extracellular dopamine, followed by a decrease of dopamine almost to the control level during 1 h of post-hypoxic reoxygenation. However, at about 2 h into the post-hypoxia period there was a secondary increase in extracellular dopamine indicating substantial release of dopamine from the neurons [18]. Horiguchi et al. [19] reported that hypothermia (32 °C) significantly inhibited the ischemia-induced increase in extracellular dopamine in the striatum of rats. This may be a protective mechanism, since it is known that increased levels of dopamine can lead to apoptosis [20—22].
In the light of these results, we postulate that hypothermia could provide substantially more protection for the striatum than for the other regions of the brain by suppression of extracellular dopamine levels in the striatum and, consequently, decreased levels of free radicals. This role of dopamine is consistent with the greater vulnerability of the striatum to hypoxic/ischemic injury as well as with the greater increase in Bcl-2/Bax ratio, in response to hypothermia, observed in the striatum compared to other regions of the brain. Further studies are required to test this hypothesis.
The present study also has shown that hypothermia led to a decrease in Caspase-3 expression. Activation of caspases and cysteine proteases is an essential component of the process of apoptosis. Caspase-3 is especially important in the brain, where it plays a central role in the initiation of the apoptotic pathway and is thought to be responsible for a number of cytological changes associated with neuronal apoptosis. Thus, Caspase-3 is considered an early marker of the apoptotic pathway activation.
The evidence for a protective effect of mild hypothermia in our model of CPB with DHCA in newborn piglets is consistent with the reported potential benefit of therapeutic hypothermia after pediatric cardiac arrest [11—13]. Animal studies have also shown that mild hypothermia implemented within hours following ischemia improved both histologic and functional outcomes [23]. Mild hypothermia has been reported to protect against neuronal loss in the hippocampus and improve neurobehavioral outcomes following global cerebral ischemia in the gerbil [23,24]. In an ischemic rat brain model, hypothermia decreased Bax protein expression, which is consistent with a reduction of pro-apoptotic events [25].
In conclusion, our results suggest that mild hypothermia, when applied following CPB with DHCA, can provide significant protection against neuronal apoptosis in newborn piglets. The greatest protection is observed in the striatum, which may be partly due to hypothermia-induced decrease in the level of extracellular dopamine and in the levels of free radicals.
Appendix A. Conference discussion
Dr H. Lindberg (Oslo, Norway): I think there are a lot of people who prefer ‘medium rare to well done’, as we have done in our department for many years, although we haven’t your sophisticated way of checking our results. I have some questions on that.
Your priming solution was whole blood and albumin. And have you any thoughts or measured at any time the oncotic pressure in that solution? And also the size of the heart–lung machine, the ratio between the blood volume and the prime volume to see what effect that could have? And especially I think during resuscitation of the brain, that the ionized calcium, management is very crucial. So do you do normal ionized calcium such as you did during your bypass period? And if you do low ionic calcium, which happens if you don’t do something to it, when do you correct the calcium levels?
And the same goes for the glucose levels, as you put the steroids in the pump, do you do any pharmacological treatment? Do you use insulin? Or do you just let the blood glucose level raise as it does?
I would also like to know, but you said that in your conclusion, that the period of hypothermia, you just use 6 h, if you think that’s long enough, or if you should continue it for days, as cerebral edema is usually at its max at 48 h or something like that.
Dr Pastuszko: To answer your question regarding the glucose and calcium and the pump management, we obtained blood gases at regular intervals throughout the entire process of cardiopulmonary bypass. We correct calcium. To my knowledge, we don’t give insulin. I think we haven’t noticed very high levels of glucose in the piglets.
As far as the priming of the pump is concerned, we use furosemide along with albumin and whole blood. I cannot tell you about the oncotic pressure. I can tell you that most of our underlying parameters are consistent throughout both groups, but I cannot tell you about the oncotic pressure.
And your last question was?
Dr Lindberg: How large is your prime volume, your circuit, compared to the piglet’s blood volume, is to 2 to 1 or 1 to 1?
Dr Pastuszko: It’s about 2 to 1.
Footnotes
Presented at the 22nd Annual Meeting of the European Association for Cardio-thoracic Surgery, Lisbon, Portugal, September 14—17, 2008.
Supported by HL058669 and NS031465.
References
- 1.Marion DW, Penrod LE, Kelsey SF, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 2.Smith SL, Hall ED. Mild pre- and posttraumatic hypothermia attenuates blood-brain barrier damage following controlled cortical impact injury in the rat. J Neurotrauma. 1996;13:1–9. doi: 10.1089/neu.1996.13.1. [DOI] [PubMed] [Google Scholar]
- 3.Busto R, Dietrich WD, Globus MY, Ginsberg MD. The importance of brain temperature in cerebral ischemic injury. Stroke. 1989;20:1113–1114. doi: 10.1161/01.str.20.8.1113. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Asai S, Kanematsu K, Kunimatsu T, Kohno T, Ishikawa K. Real-time monitoring of the effects of normothermia and hypothermia on extracellular glutamate re-uptake in the rat following global brain ischemia. Neuroreport. 1997;8:2389–2393. doi: 10.1097/00001756-199707070-00057. [DOI] [PubMed] [Google Scholar]
- 5.Lei B, Tan X, Cai H, Xu Q, Guo Q. Effect of moderate hypothermia on lipid peroxidation in canine brain tissue after cardiac arrest and resuscitation. Stroke. 1994;25:147–152. doi: 10.1161/01.str.25.1.147. [DOI] [PubMed] [Google Scholar]
- 6.Hicks SD, DeFranco DB, Callaway CW. Hypothermia during reperfusion after asphyxial cardiac arrest improves functional recovery and selectively alters stress-induced protein expression. J Cereb Blood Flow Metab. 2000;20:520–530. doi: 10.1097/00004647-200003000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Hicks SD, Parmele KT, DeFranco DB, Klann E, Callaway CW. Hypothermia differentially increases extracellular signal-regulated kinase and stress-activated protein kinase/c-Jun terminal kinase activation in the hippocampus during reperfusion after asphyxial cardiac arrest. Neuroscience. 2000;98:677–685. doi: 10.1016/s0306-4522(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 8.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 9.Nussmeier NA. Management of temperature during and after cardiac surgery. Tex Heart Inst J. 2005;32:472–476. [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkpatrick AW, Chun R, Brown R, Simons RK. Hypothermia and the trauma patient. Can J Surgery. 1999;42(5):333–343. [PMC free article] [PubMed] [Google Scholar]
- 11.Kochanek PM, Clark RS, Ruppel RA, Dixon CE. Cerebral resuscitation after traumatic brain injury and cardiopulmonary arrest in infants and children in the new millennium. Pediatr Clin North Am. 2001;48:661–681. doi: 10.1016/s0031-3955(05)70333-3. [DOI] [PubMed] [Google Scholar]
- 12.Marion DW, Leonov Y, Ginsberg M, Katz LM, Kochanek PM, Lechleuthner A, Nemoto EM, Obrist W, Safar P, Sterz F, Tisherman SA, White RJ, Xiao F, Zar H. Resuscitative hypothermia. Crit Care Med. 1996;24:S81–S89. [PubMed] [Google Scholar]
- 13.Schleien CL, Osmond MH, Hickey R, Hutchison J, Buunk G, Douglas IS, Gervais HW, Wenzel V. Postresuscitation management. Ann Emerg Med. 2001;37:S182–S195. doi: 10.1067/mem.2001.114170. [DOI] [PubMed] [Google Scholar]
- 14.Yue X, Mehmet H, Penrice J, Cooper C, Cady E, Wyatt JS, Reynolds EO, Edwards AD, Squier MV. Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischaemia. Neuropathol Appl Neurobiol. 1997;23:16–25. [PubMed] [Google Scholar]
- 15.Fritz KI, Zubrow AB, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. The effect of hypocapnia (PaCO2 27 mmHg) on CaM kinase IV activity. Bax/Bcl-2 protein expression and DNA fragmentation in the cerebral cortex of newborn piglets. Neurosci Lett. 2003;352:211–215. doi: 10.1016/j.neulet.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 16.Pastuszko P, Liu H, Mendoza A, Schultz S, Markowitz S, Greeley W, Wilson DF, Pastuszko A. Regulatory pathways to neuronal injury or survival are dependent on the rate of low flow cardiopulmonary bypass following circulatory arrest in newborn piglets. Eur J Cardiothorac Surg. 2007;31:899–905. doi: 10.1016/j.ejcts.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 17.Olano M, Song D, Murphy S, Wilson DF, Pastuszko A. Relationships of dopamine, cortical oxygen pressure and hydroxyl radicals in brain of newborn piglets during hypoxia and posthypoxic recovery. J Neurochem. 1995;65:1205–1212. doi: 10.1046/j.1471-4159.1995.65031205.x. [DOI] [PubMed] [Google Scholar]
- 18.Huang Ch-Ch, Yonetani M, Lajevardi N, Delivoria-Papadopoulos M, Pastuszko A, Wilson DF. Comparison of post-asphyxial resuscitation with 100% and 21% oxygen on striatal dopamine metabolism in newborn piglets. J Neurochem. 1995;64:92–298. doi: 10.1046/j.1471-4159.1995.64010292.x. [DOI] [PubMed] [Google Scholar]
- 19.Horiguchi T, Shimizu K, Ogino M, Yamaguchi N, Suga S, Inamasu J, Kawase T. Neuroprotection role of adenosine under hypothermia in the rat global ischemia involves inhibition of not dopamine release but delayed post-ischemic hypoperfusion. Brain Res. 2002;952:222–231. doi: 10.1016/s0006-8993(02)03242-0. [DOI] [PubMed] [Google Scholar]
- 20.Hoyt KR, Reynolds IJ, Hastings TG. Mechanisms of dopamine-induced cell death in cultured rat forebrain neurons: interactions with and differences from glutamate-induced cell death. Exp Neurol. 1997;143(2):269–281. doi: 10.1006/exnr.1996.6374. [DOI] [PubMed] [Google Scholar]
- 21.Porat S, Simantov R. Bcl-2 and p53: role in dopamine-induced apoptosis and differentiation. Ann N Y Acad Sci. 1999;893:372–375. doi: 10.1111/j.1749-6632.1999.tb07858.x. [DOI] [PubMed] [Google Scholar]
- 22.Noh JS, Kim EY, Kang JS, Kim HR, Oh YJ, Gwag BJ. Neurotoxic and neuroprotective actions of catecholamines in cortical neurons. Exp Neurol. 1999;159(1):217–224. doi: 10.1006/exnr.1999.7144. [DOI] [PubMed] [Google Scholar]
- 23.Colburne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;654:265–272. doi: 10.1016/0006-8993(94)90488-x. [DOI] [PubMed] [Google Scholar]
- 24.Corbett D, Nurse S, Colbourne F. Hypothermic neuroprotection. A global ischemia study using 18- to 20-month-old gerbils. Stroke. 1997;28:2238–2242. doi: 10.1161/01.str.28.11.2238. [DOI] [PubMed] [Google Scholar]
- 25.Eberspacher E, Werner C, Engelhard K, Pape M, Gelb A, Hutzler P, Henke J, Kochs E. The effect of hypothermia on the expression of the apoptosis-regulating protein Bax after incomplete cerebral ischemia and reperfusion in rats. J Neurosurg Anesthesiol. 2003;15:200–208. doi: 10.1097/00008506-200307000-00007. [DOI] [PubMed] [Google Scholar]