Abstract
Background and Aims
Incident self-reported physician-diagnosed clinical gallbladder disease was compared to BMI, body dimensions, physical activity (km/day run) and cardiorespiratory fitness (10 km race speed, meters per second [m/s]) in 29,110 male and 11,953 female runners.
Methods
Physician-diagnosed gallbladder disease was reported by 166 men (0.57%) and 112 women (0.94%) during (mean ± SD) 7.74 ± 1.84 and 7.42 ± 2.10 years of follow-up, respectively.
Results
There was a progressive increase in age-adjusted risk with increasing BMI that accelerated sharply above 27.5 kg/m2. Even among ostensibly healthy-weight women, the age-adjusted risk was significantly greater above 22.5 kg/m2 vis-à-vis the leanest women (P = 0.04). Age-adjusted risk declined with increasing fitness in both sexes. Compared to the least fit men and women, men who ran faster than 4.75 m/s had 83% lower risk (75% lower when adjusted for km/day and BMI) and women who ran faster than 4 m/s had 93% lower risk (85% lower adjusted for km/day and BMI). The fittest men (≥4.75 m/s) were at significantly less risk than men who ran <3.25 m/s (P <0.003) and between 3.25 and 3.75 m/s (P = 0.03), and the fittest women (≥4 m/s) were at significantly less risk than those who ran <2.8 m/s (P < 0.0001), between 2.8 and 3.2 (P = 0.0004), 3.2 and 3.6 (P = 0.002), and 3.6 and 4.0 m/s (P = 0.005). Adjustment for BMI accounted for more of the risk reduction associated with fitness in women than men. The risk for clinical gallbladder disease was also significantly related to usual running distance (men: P = 0.01; females: P = 0.008), which was attributable to the leanness of the longer-distance runners.
Conclusion
Clinical gallbladder disease risk was (a) concordantly related to BMI, (b) inversely related to usual running distance, and (c) inversely related to cardiorespiratory fitness independent of physical activity levels.
INTRODUCTION
In the United States, 10–15% of the population is affected by gallstones (cholelithiasis), with cholesterol stones being most common [1, 2]. Although the mortality associated with cholecystitis is low (0.6%), the annual US cost for the >700,000 cholecystectomies is approximately 6.5 billion dollars [2], making it the most costly and common of digestive diseases requiring hospitalization. Cholesterol stones are formed by cholesterol supersaturation, accelerated cholesterol crystal nucleation, and impaired gallbladder motility [2].
Female sex, familial history, obesity, rapid weight loss or weight cycling, high caloric intake, and diets high in fat and cholesterol are all risk factors for cholesterol stones [2]. Gallbladder disease is two to three times more likely to affect women than men when young, although after menopause the difference diminishes somewhat. Nevertheless, risk for gallstones increases with age in both sexes, particularly in conjunction with other risk factors [1, 2]. The greater risk for gallbladder disease associated with obesity appears to be due in part to increased hepatic cholesterol secretion [3].
The epidemiological evidence relating physical activity to gallbladder disease is inconsistent, and includes studies both supportive [4-10] and at variance [11-19] with their association. In part, this inconsistency may relate to the inadequate inclusion of vigorous physical activity (activities that expend at least six-fold the energy equivalent of being at rest [20]). Vigorous physical activity, in particular, reduces adiposity, hyperinsulinemia, and plasma triglyceride levels, while increasing plasma high-density lipoprotein (HDL) cholesterol levels [21], which are risk factors for cholesterol gallstone formation. Cardiorespiratory fitness and physical performance have not previously been identified as risk factors for gallbladder disease.
The National Runners’ Health Study is unique among prospective epidemiological studies in targeting vigorously active men and women to assess the dose–response relationship between vigorous exercise and health outcomes [21- 29]. Prior reports from this study show that the prevalence of hypertension, hypercholesterolemia, and diabetes are all inversely related to the weekly dose of vigorous exercise and 10-km race performance speed (a measure of cardiorespiratory fitness) [23]. They also demonstrate prospectively that vigorous exercise attenuates age-related weight gain [24], and reduces the risk of diabetes in proportion to the exercise dose [25]. This paper further demonstrates the health benefits of vigorous exercise, leanness, cardiorespiratory fitness, and physical performance in preventing symptomatic clinical gallbladder disease.
METHODS
The design and methods of the National Runners’ Health Study are described elsewhere [21-29]. Briefly, cohort recruitment was achieved by distributing two-page questionnaires nationally to runners identified through Runner’s World magazine subscription lists and among participants of foot race events. The questionnaire solicited information on demographics, running history, weight history, smoking habits, prior history of heart attacks and cancer, and medications for blood pressure, thyroid, cholesterol, and diabetes. Recruitment took place between 1991 and 1994 (primarily 1993) and follow-up between 1999 and 2002. We estimate that approximately 15% of the participants who received questionnaires responded to our baseline survey (the number is approximate because we do not know the number of survey questionnaires actually distributed and the proportion of individuals who received multiple questionnaires). The study protocol was approved by the University of California Berkeley Committee for the Protection of Human Subjects, and all participants signed committee-approved informed consents.
Follow-up questionnaires were sent by mail requesting information on current running levels, body weight, and medical condition. Multiple follow-up survey questionnaires were sent and telephone calls made until a priori determined response rate of 80% of the participants provided follow-up information or were known deceased. Participants were asked, “ Since 1991, have you been diagnosed by a physician for any of the following conditions (provide year of diagnosis if yes)” with gallbladder disease as one of the listed conditions. Self-reported cholecystectomies or physician diagnoses of gallstones have been shown by others to be 99% verifiable in the Health Professionals Follow-up Study [4]. In that study, all 441 of the self-reported symptoms and all but one self-reported diagnostic procedures were confirmed by medical record review [4]. Running distances were reported in usual miles run per week at baseline. Although other leisure-time physical activities were not recorded for this cohort, data from runners recruited after 1998 (when the question was added to the survey) showed running represented (±SD) 91.5 ± 19.1% and 85.2 ± 24.0% of all vigorously intense activity in men and women, respectively, and 73.5 ± 23.7% and 69.4 ± 25.7% of total leisure-time physical activity, respectively.
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Self-reported waist, hip, and chest circumferences were elicited by the question, “Please provide, to the best of your ability, your body circumference in inches.” without further instruction. Bra-cup sizes were coded on a 5-point scale: 1 (A cup), 2 (B cup), 3 (C cup), 4 (D cup), and 5 (E cup or larger). Elsewhere, we have reported strong correlations between repeated questionnaires for self-reported running distances (r = 0.89) [27], between self-reported and clinically measured heights (r = 0.96) and weights (r = 0.96) [27], and for self-reported running distances versus self-reported BMIs and waist circumferences in cross-sectional analyses [27, 28]. Self-reported body circumferences were somewhat less precise, as indicated by their correlations with reported circumferences on a second questionnaire (waist: r = 0.84; hip: r = 0.79, and chest: r = 0.93) and with their clinical measurements (waist: r = 0.68; hip: r = 0.63, and chest: r = 0.77). Less than one-half of the men reported their hip circumference and therefore hip circumference was not included in the analyses.
Intakes of meat, fish and fruit were based on the questions “During an average week, how many servings of beef, lamb, or pork do you eat,” “. . .servings of fish do you eat,” and “. . .pieces of fruit do you eat.” Alcohol intake was estimated from the corresponding questions for 4-oz. (112 ml) glasses of wine, 12-oz. (336 ml) bottles of beer, and mixed drinks and liqueurs. Alcohol was computed as 10.8 g per 4-oz glass of wine 13.2 g per 12 oz. bottle of beer, and 15.1 g per mixed drink. Correlations between these responses and values obtained from 4-day diet records in 110 men were r = 0.65 for alcohol intake, r = 0.46 for red meat, r = 0.38 for fruit, and r = 0.19 for fish. These values agree favorably with published correlations between food records and more extensive food frequency questionnaires for red meat (r = 0.50), wine (r = 0.66), beer (r = 0.70), and mixed drinks (r = 0.72), somewhat less favorably for fruit intake (r = 0.50), and less favorably for fish intake (r = 0.51) [30].
For this report, baseline cardiorespiratory fitness was defined as speed in meters per second (m/s) of the participant’s best 10 km race during the previous 5 years (reported as finish time in minutes). Published data support the use of running performance to estimate maximal oxygen consumption (VO2 max) [31-33]. Balke and Ware [31] initially reported a positive correlation between walk-run endurance performance and aerobic capacity when they suggested relating laboratory-determined VO2 max to the distance covered in a given time period or to the time required to run a given distance. Hellerstein [32] accurately estimated the time in minutes to complete a marathon race from 70% of the VO2 max and published energy costs of progressive running speeds. Cooper [33] showed a correlation of r = 0.90 between VO2 max from a laboratory treadmill test and 12-min walk-run test for distance.
Statistical Analyses
Cox proportional hazard model (JMP software version 5.0, SAS Institute, Cary, NC) was used to estimate the dose–response relationships of incident gallbladder disease to baseline body weight and circumferences, average distances run per day, and cardiorespiratory fitness. Reported weekly intakes of alcohol, meat, fish, and fruit, along with age and BMI, were used as covariates, with quadratic terms for age and BMI because of their nonlinear relationships to running distance and each other [26, 28].
RESULTS
There were 29,110 men and 11,953 women who were nonsmoking, nonvegetarian, and nondiabetic at baseline who reported average weekly running distance, height, weight, and age at baseline (80% of the original cohort who were nonsmoking, nonvegetarian, nondiabetic at baseline). Relative to the original baseline nonsmoking, nonvegetarian, nondiabetic cohort, those that were excluded or lost to follow-up were younger (excluded vs. included mean ± SE, males: 42.0 ± 0.1 vs. 44.8 ± 0.1, P = 0.0005; females: 36.0 ± 0.2 vs. 38.9 ± 0.1 years, P = 0.02), heavier (males: 24.27 ± 0.03 vs. 23.87 ± 0.02, females: 21.46 ± 0.05 vs. 21.29 ± 0.02 kg/m2, both P < 0.0001), had run fewer years at baseline (males: 11.3 ± 0.1 vs. 13.0 ± 0.05; females: 8.6 ± 0.1 vs. 9.9 ± 0.1 years, both P < 0.0001) and had run longer distances if male (males: 5.5 ± 0.0 vs. 5.4 ± 0.0, P = 0.0005; females: 5.0 ± 0.1 vs. 5.1 ± 0.0 km/day, P = 0.83).
The 29,110 men and 11,953 women included 166 men (0.57%) and 112 women (0.94%) who reported incident gallbladder disease during (mean ± SD) 7.74 ± 1.84 and 7.42 ± 2.10 years of follow-up, respectively. The characteristics of the sample are presented in Table 1. Those who reported incident gallbladder diseases were significantly older and less educated if male, more overweight as measured by their BMI, bra-cup size, and circumferences of the waist, hips, and chest, and had smoked more in the past. The incident cases also reported shorter weekly running distances and slower 10 km performance times than others. Incident gallbladder disease was associated with greater weekly serving of meat in women but not men, otherwise those reporting incident events had similar reported intakes of fish, fruit, and alcohol.
Table 1.
Baseline Characteristics of Men and Women Diagnosed with Gallbladder Disease During Follow-up
| Males | Females | |||
|---|---|---|---|---|
| Clinical Gallbladder Disease: |
Diagnosed | Not Diagnosed |
Diagnosed | Not Diagnosed |
| Sample (N) | 166 | 28,944 | 112 | 11,841 |
| Caucasian (%) | 98.2 | 95.2 | 97.3 | 94.5 |
| Age (years) | 49.5 ± 10.0§ | 44.9 ± 10.4 | 40.3 ± 10.3 | 38.9 ± 10.1 |
| Follow-up (years) | 8.0 ± 1.8* | 7.7 ± 1.8 | 8.0 ± 1.7† | 7.4 ± 2.1 |
| Running distance (km/day) | 4.6 ± 2.9† | 5.4 ± 3.2 | 4.4 ± 2.8† | 5.2 ± 3.1 |
| Cardiorespiratory fitness (m/s) |
3.7 ± 0.5§ | 3.9 ± 0.5 | 3.3 ± 0.5§ | 3.5 ± 0.5 |
| Education (years) | 16.2 ± 2.6* | 16.5 ± 2.4 | 15.7 ± 2.2 | 15.9 ± 2.4 |
| Smoking (pack years) | 10.9 ± 18.9§ | 5.9 ± 13.3 | 5.1 ± 12.0† | 3.0 ± 8.4 |
| BMI (kg/m2) | 25.2 ± 2.9§ | 23.9 ± 2.6 | 22.9 ± 3.6§ | 21.2 ± 2.4 |
| Waist circumference (cm) | 87.8 ± 7.1§ | 84.4 ± 6.2 | 71.3 ± 9.5§ | 68.7 ± 6.7 |
| Chest circumference (cm) | 104.4 ± 6.7§ | 101.8 ± 7.3 | 90.0 ± 6.4‡ | 88.1 ± 5.1 |
| Hip circumference (cm) | 93.4 ± 9.6† | 91.3 ± 6.6 | ||
| Bra cup (size) | 2.2 ± 1.0‡ | 1.9 ± 0.9 | ||
| Meat (servings/week) | 3.0 ± 2.5 | 2.8 ± 2.7 | 2.1 ± 1.9* | 1.7 ± 2.0 |
| Fish (servings/week) | 1.6 ± 1.2 | 1.5 ± 1.4 | 1.3 ± 1.3 | 1.3 ± 1.4 |
| Fruit (pieces/week) | 11.0 ± 10.1 | 11.1 ± 8.8 | 11.4 ± 7.2 | 11.5 ± 8.0 |
| Alcohol (g/week) | 64.5 ± 106.8 | 70.6 ± 99.1 | 42.4 ± 74.3 | 40.0 ± 60.2 |
| Parity | 1.33 ± 1.34 | 1.14 ± 1.36 | ||
Mean ± SD presented for all variables except Caucasian (%). Significant difference between diagnosed and nondiagnosed by t-test or Chi2 coded:
P < 0.05
P < 0.01
P < 0.001
P < 0.0001.
Table 2 shows a significant increase in cholelithiasis risk with increasing BMI, waist circumference, and chest circumference in men, and increasing BMI, bra-cup size, and circumferences of the waist, hips, and chest in women. Correction for differences in running distance had little effect on the relative risks. BMI appeared to account for all of the significant associations of circumferences and bra-cup size with incident gallbladder disease. Figure 1 displays a progressive increase in risk with increasing BMI that accelerates sharply above 27.5 kg/m2. Even among healthy-weight women, the risk for gallbladder disease was significantly increased above 22.5 kg/m2 vis-à-vis the leanest women (P = 0.04). Adjustment for physical activity diminished only slightly the increase in risk associated with BMI.
Table 2.
Relative Risk (95% Confidence Interval) for Incident Self-reported Physician-diagnosed Gallbladder Disease During Follow-up in Relationship to BMI and Body Size in 29,110 Men and 11,953 Women
| Unadjusted for km/day or BMI |
Adjusted for km/day |
Adjusted for BMI |
Adjusted for km/day and BMI |
|
|---|---|---|---|---|
| Males | ||||
| BMI, kg/m2 | 1.155§ (1.100, 1.210) |
1.143§ (1.084, 1.202) |
||
| Waist, cm | 1.059§ (1.036, 1.079) |
1.055§ (1.031, 1.077) |
1.020 (0.989, 1.051) |
1.019 (0.987, 1.051) |
| Chest, cm | 1.049§ (1.025, 1.073) |
1.046‡ (1.021, 1.070) |
1.021 (0.992, 1.051) |
1.021 (0.992, 1.051) |
| Females | ||||
| BMI, kg/m2 | 1.208§ (1.147, 1.265) |
1.196§ (1.131, 1.258) |
||
| Waist, cm | 1.044‡ (1.019, 1.066) |
1.036† (1.011, 1.060) |
1.000 (0.966, 1.032) |
0.997 (0.963, 1.029) |
| Hip, cm | 1.044† (1.014, 1.074) |
1.034* (1.003,1.065) |
0.995 (0.959,1.032) |
0.990 (0.954, 1.026) |
| Chest, cm | 1.061‡ (1.025, 1.093) |
1.053† (1.017, 1.087) |
1.003 (0.961, 1.046) |
1.002 (0.960, 1.045) |
| Bra cup, sizes | 1.368† (1.108, 1.676) |
1.317† (1.064, 1.619) |
1.118 (0.892, 1.392) |
1.108 (0.883, 1.380) |
All results adjusted for age (age and age2), race, education, pack years of cigarette use, weekly intakes of meat, fish, fruit and alcohol, and parity (women). Waist circumference was reported by 94.1% of men and 91.5% of women, hip circumference by 88.8% of women, and chest circumference by 81.5% of men and 91.5% of women, and bra cup size by 90.6% of women. Significance levels for relative risks were coded
P ≤ 0.05
P ≤ 0.01
P ≤ 0.001
P ≤ 0.0001.
Figure 1.
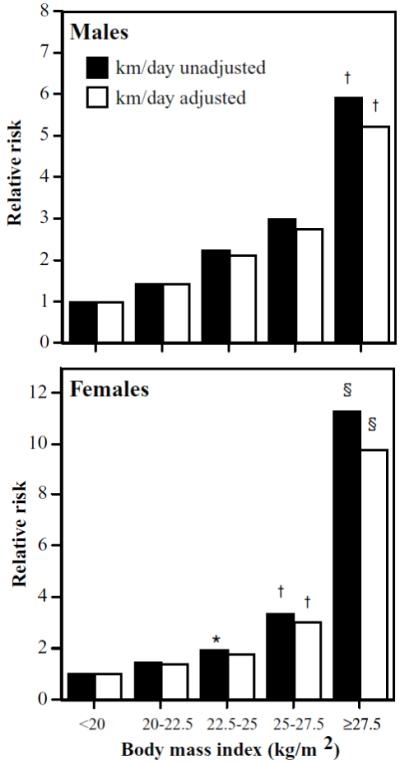
Relative risk from survival analyses of self-reported physician-diagnosed gallbladder disease by BMI in 29,110 men and 11,953 women. All results adjusted for age (age and age2), race, education, pack-years of cigarette use, weekly intakes of meat, fish, fruit and alcohol, and parity (women). Additional adjustment for physical activity (km/day) where indicated. Significant difference relative to the leanest men and women were coded *P < 0.05; †P < 0.01; ‡P < 0.001; and §P < 0.0001.
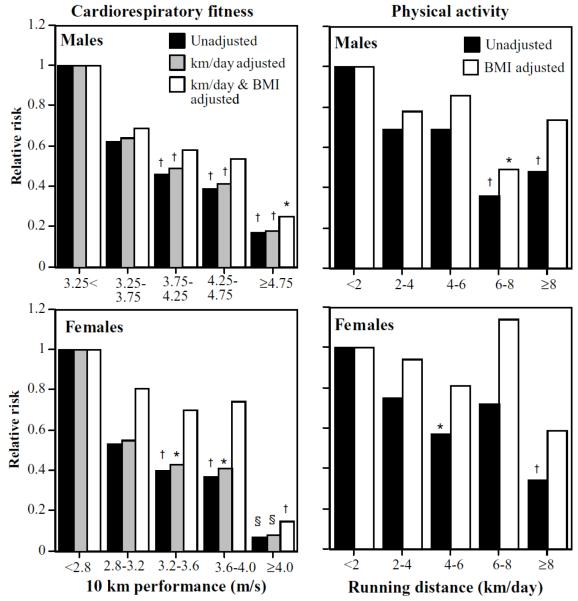
Table 3 shows that men and women of greater cardiorespiratory fitness, as measured by their 10 km performance speeds, were at significantly less risk than those who were less fit. In both sexes, adjustment for distance run per week had negligible effect on the magnitude of the risk reduction. The risk reduction remained significant when adjusted for BMI in men. Although it became nonsignificant in women (P = 0.09), the magnitude of the women’s risk reduction was entirely consistent with that of the men when BMI adjusted.
Table 3.
Relative Risk (95% Confidence Interval) for Incident Self-reported Physician-diagnosed Gallbladder Disease During Follow-up in Relation to Cardiorespiratory Fitness and Physical Activity in 29,110 Men and 11,953 Women (24,491 Men and 9,036 Women in the Fitness Subset)
| Males | Females | |||
|---|---|---|---|---|
| Model | Cardiorespiratory Fitness, m/s |
Physical Activity, km/day |
Cardiorespiratory Fitness, m/s |
Physical Activity, km/day |
| All | ||||
| Physical activity only, no BMI adjustment |
0.93† (0.87, 0.98) |
0.91† (0.84, 0.97) |
||
| Physical activity only, BMI adjusted |
0.97 (0.91, 1.02) |
0.96 (0.89, 1.03) |
||
| Fitness subset | ||||
| Cardiorespiratory fitness only, no BMI adjustment |
0.52‡ (0.36, 0.75) |
0.40§ (0.26, 0.64) |
||
| Physical activity only, no BMI adjustment |
0.94* (0.88, 1.00) |
0.92* (0.84, 0.99) |
||
| Cardiorespiratory fitness & physical activity together, no BMI adjustment |
0.54† (0.37, 0.81) |
0.98 (0.92, 1.05) |
0.44‡ (0.27, 0.72) |
0.97 (0.88, 1.05) |
| Cardiorespiratory fitness only, BMI adjusted |
0.62* (0.42, 0.92) |
0.66 (0.40, 1.06) |
||
| Physical activity only, BMI adjusted |
0.97 (0.91, 1.03) |
0.98 (0.90, 1.06) |
||
| Cardiorespiratory fitness & physical activity together, BMI adjusted |
0.63* (0.42, 0.95) |
1.00 (0.93, 1.06) |
0.65 (0.39, 1.08) |
1.00 (0.92, 1.09) |
All results adjusted for age (age and age2), race, education, pack years of cigarette use, weekly intakes of meat, fish, fruit and alcohol, and parity (women). Significance levels for risk ratios are coded
P ≤ 0.05
P ≤ 0.01
P ≤ 0.001
P ≤ 0.0001.
Figure 2 shows the linear decline in the men’s and women’s risk with speed, independent of distance run. Compared to the least fit men and women, men who ran faster than 4.75 m/s had 83% lower risk (75% lower when adjusted for km/day and BMI) and women who ran faster than 4 m/s had 93% lower risk (85% lower adjusted for km/day and BMI). The fittest men were also at significantly less risk than men who ran between 3.25 and 3.75 m/s (P = 0.03), and the fittest women were at significantly less risk than women who ran between 2.8 and 3.2 (P = 0.0004), 3.2 and 3.6 (P = 0.002), and 3.6 and 4.0 m/s (P = 0.005, analyses not displayed). Adjustment for BMI accounted for more of the risk reduction in women than men. The risk for gallbladder disease was also significantly related to weekly running distance (males: P = 0.005; females: P = 0.004), and this was ascribed to the leanness of the longer-distance runners.
Figure 2.
Relative risk from survival analyses of self-reported physician-diagnosed gallbladder disease by cardiorespiratory fitness (10 km race performance speed) and physical activity (km/day running distance). All results adjusted for age (age and age2) race, education, pack years of cigarette use, weekly intakes of meat, fish, fruit and alcohol, and parity (women). Additional adjustment for physical activity (km/day) or BMI where indicated. Physical activity was analyzed in 29,110 men and 11,953 women, and cardiorespiratory fitness was analyzed in 24,491 men and 9,036 women. Significant difference relative to the least fit or active men and women were coded *P < 0.05; †P < 0.01; ‡P < 0.001; and §P < 0.0001.
DISCUSSION
In this paper, we have demonstrated a fundamental difference in the effects of cardiorespiratory fitness (10 km performance speed) and vigorous exercise dose on clinical gallbladder disease risk. Greater cardiorespiratory fitness was inversely related to the age-adjusted risk for gallstones, which was only slightly diminished by adjustment for usual distance run or BMI (Table 3, Fig. 2). In contrast, the inverse relationship between gallbladder disease and physical activity was eliminated by adjustment for BMI, fitness, or both (Tables 1,2). We also demonstrated clinically important declines in cholelithiasis risk that may extend through 64 km/wk.
In both men and women, physical activity had been shown by others to decrease the risk for gallbladder disease [4-10]. The Health Professionals Follow-up Study 8-year follow-up of 45,813 men found that men in the upper quintile of physical activity had a 37% risk reduction in gallbladder disease relative to the lowest quintile before adjusting for BMI, and a 28% risk reduction when adjusted for BMI [4]. They reported that most of the risk reduction was attributable to vigorous physical activity, with 2–3 h of moderate running per week reducing risk by 20%. The Nurses’ Health Study 10-year follow-up of 60,290 40–65-year old women found that the upper quintile of physical activity had a 31% risk reduction for cholecystectomies relative to the lowest quintile before adjusting for BMI, and a 21% risk reduction when adjusted for BMI [5]. In contrast to the finding for men [4], among female nurses they found that the risk reduction associated with vigorous exercise was only slightly greater than the reduction associated with moderately intense exercise (11 vs. 9 percent per 10 MET-hours per week) [5].
Our results complement the Health Professionals Follow-up and Nurses’ Health Studies by extending the range of physical activity studied, and suggest important reductions in risk for gallstones for levels of vigorous exercise that exceed current public health guidelines of 30 min brisk walking on most days of the week [20]. A two-mile brisk walk 5 days/wk is the energy equivalent of running 10.9 km/wk [34], which corresponds to the lowest or referent category of Figure 2. Thus relative to guideline levels, the analyses of Figure 2 suggests that the dose–response relationship between weekly distance run and clinical gallbladder disease risk extends through 64 km/wk (equivalent to 66 metabolic equivalents or METs), which is two-fold larger than the highest physical activity category presented by the Health Professionals Study [4], and three-fold larger than the highest category presented by the Nurses’ Health Study [5]. Our lowest physical activity category corresponds to the middle quintile of the Health Professionals Study and the middle of the fourth quintile of the Nurses’ Health Study. Taken together, the three studies suggest clinically significant reductions in cholelithiasis risk for guideline levels versus a sedentary lifestyle, and additional risk reductions of 60–70% that may accrue as high as 64 km/wk for those who exceed guideline levels.
The association between obesity and gallbladder disease is well established [2, 6]. Although overall incidence for gallbladder disease in women was twice that of men, Figure 1 shows that the proportional increase in risk associated with obesity did not especially differ between the sexes. The risk increase was linear and similar in men and women for BMI’s <27.5 kg/m2, but then increased somewhat more sharply in women then men. Higher waist and chest circumferences also increased risk in both sexes, as did greater hip circumference and bra-cup size in women, but these regional adiposity measures appeared to simply reflect the risk associated with greater BMI. Others report that increasing weight is associated with a greater increase in gallbladder disease risk in women than men [35], which may be less evident in our sample because of its overall leanness.
Both the Health Professionals Follow-up Study and the Nurses’ Health Study reported that higher doses of physical activity reduced the risk of symptomatic gallstone disease independent of body weight [4, 5]. In contrast, at the higher levels of physical activity represented by our sample, the dose–response relationship between gallbladder risk and vigorous exercise appeared to be attributable to the greater leanness of the longer-distance runners (Table 2). A reduction in gallbladder disease risk, mediated by body weight, would not diminish the public health significance promoting high-levels of vigorous activity because the leanness of the runners is largely the consequence of running. Specifically, we have previously demonstrated that self-selection accounts for only 26% of the differences in body weight between running levels in men (albeit 58% of the differences in women) [36], and that vigorous exercise prevents age-related weight gain in proportion to the exercise dose [24].
The novel finding of our analyses is the demonstration of a significant dose–response relationship between 10 km performance and risk that was largely independent of weekly distance run. Race performance times have been used as one of several indirect assessments of maximum aerobic capacity (treadmill test duration, submaximal ergometer test). The relationship could relate to innate differences between individuals affecting both risk and training capacity, which may be genetic. Studies in twins and other related individuals show that genes contribute substantially to exercise performance and aerobic capacity [37-39]. Other less significant factors affecting performance include oxygen consumption at submaximal speeds (i.e., running economy), muscle fiber composition, and anaerobic capacity [40-44].
The mechanisms by which greater cardiorespiratory fitness or other attributes that contribute to 10 km performance affect the gallstone pathogenesis are not known, particularly those mechanisms that relate to performance rather than the amount of physical activity. Greater fitness or performance ability may decrease biliary cholesterol secretion, or enhance gallbladder and colonic motility, given these factors are generally known to be related to gallstone formation [45]. Physical activity may also promote cholecystokinin release [46], increase vagal tone and enhance colonic motility [47], raise HDL-cholesterol and lower triglyceride concentrations [21, 22], and improve glucose tolerance [21]. Increased intestinal throughput reduces gallbladder storage and interrupts enterohepatic circulation of bile acids without excessive caloric intake [48]. Hypomotility of the gallbladder, which is regulated by vagal cholinergic pathways and cholecystokinin [49], contributes to bile stasis and crystal formation [46]. Two studies by Utter et al. failed to show that acute or long-term exercise training increased gallbladder motility; however, the studies tended to be small and the interventions nonvigorous [50, 51]. Although an uncontrolled 4-wk training study by Sari et al. showed no significant change in gallbladder volume and motility in the early postprandial phase, it did show significantly decreased late-phase gallbladder volumes and increased late-phase gallbladder ejection fraction relative to pretraining levels [52]. Sport activity has also been associated with lower levels of biliary cholesterol, which may prevent cholesterol from precipitating in the bile [53].
Physical activity and cardiorespiratory fitness also reduce the various obesity-related metabolic risk factors known collectively as metabolic syndrome, including low HDL cholesterol, a predominance of small low-density lipoprotein cholesterol particles, elevated triglycerides, hypertension, hyperglycemia, and insulin resistance [21, 22, 54]. Activity and fitness are inversely related to abdominal adiposity [21, 26, 28, 36], a condition where excess fatty acids in tissues causes greater total cholesterol synthesis and the production of the more lithogenic bile resulting in cholesterol crystallization and gallstone formation [55]. Running also increases HDL cholesterol [21], and fitness decreases hypertension [21, 23, 29]. There is a strong association between elevated systolic blood pressure and gallbladder disease that may in part be mediated by insulin resistance and high levels of plasma insulin [56]. Both increased plasma insulin levels and decreased HDL cholesterol levels are related to a higher bile cholesterol saturation index [56].
Limitations
The primary limitation of this study is the absence of systematic gallstone screening by ultrasonography or other imaging technology. Thus, our findings are primarily germane to symptomatic clinical gallbladder disease and the incidental asymptomatic cases diagnosed by imaging for unrelated causes. Validation in the Health Professionals Study suggested only 1% of self-reported cases could not be confirmed by medical record validation [4]. Our data also do not distinguish whether vigorous physical activity lowers the prevalence of gallstone disease or its symptoms, since the majority of persons with gallstones are asymptomatic. The 1992 National Institutes of Health Consensus Conference on Gallstones estimated that 90% of patients with gallstones will not develop symptoms 5 years after diagnosis [57], and the 1995 Group for Epidemiology and Prevention of Cholelithiasis estimated that 74.2% will remain asymptomatic for over 10 years [58]. The true incidence of gallbladder disease will therefore be underestimated; however, uniform underascertainment is not expected to bias estimated risk [59].
We also caution that the cohort does not necessarily represent a random sample of all runners given that only 15% of the targeted sample was recruited. Men and women who run regularly may differ from others genetically, socio-economically, psychologically, and with respect to other health behaviors. However, despite the select nature of the sample, we expect the biological processes that relate gallbladder disease to exercise, fitness, and adiposity to be similar in runners and nonrunners.
We do not believe that our findings are due to a difference in the frequency of medical check-ups by fitness or activity level. The Health Professionals Study reported that their more vigorously active participants had more routine medical check-ups than less active men [4] and there was no difference in routine medical check-up by activity level in the Nurses’ Health Study [5]. We did not record the frequency of physician visits in our cohort, but we have no particular reason to expect that these observations would not apply to the fitter, more vigorously active men and women of our study.
Summary
These analyses show that lower cardiorespiratory fitness or other attributes associated with slower 10 km race performance are previously undescribed risk factors for cholelithiasis. This may relate to innate characteristics of the men and women studied since cardiorespiratory fitness and cholelithiasis risk are both, in part, inherited. Prior reports that have interpreted fitness as simply a more objective measure of physical activity may ignore the importance of the metabolic factors that define fitness and improve performance on disease risk.
REFERENCES
- 1.Chen CY, Lu CL, Huang YS, et al. Age is one of the risk factors in developing gallstone disease in Taiwan. Age Ageing. 1998;27:437–41. doi: 10.1093/ageing/27.4.437. [DOI] [PubMed] [Google Scholar]
- 2.Schaffer EA. Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–96. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Shaffer EA, Small DM. Biliary lipid secretion in cholesterol gallstone disease. The effect of cholecystectomy and obesity. J Clin Invest. 1977;59:828–40. doi: 10.1172/JCI108705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417–25. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777–84. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]
- 6.Kato I, Nomura A, Stemmermann GN, et al. Prospective study of clinical gallbladder disease and its association with obesity, physical activity, and other factors. Dig Dis Sci. 1992;37:784–90. doi: 10.1007/BF01296440. [DOI] [PubMed] [Google Scholar]
- 7.Williams CN, Johnston JL. Prevalence of gallstones and risk factors in Caucasian women in a rural Canadian community. Can Med Assoc J. 1980;122:664–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Sarin SK, Kapur BM, Tandon RK. Cholesterol and pigment gallstones in northern India. A prospective analysis. Dig Dis Sci. 1986;31:1041–5. doi: 10.1007/BF01300256. [DOI] [PubMed] [Google Scholar]
- 9.Linos AD, Daras V, Linos DA, et al. Dietary and other risk factors in the aetiology of cholelithiasis: A case control study. HPB Surg. 1989;1:221–7. doi: 10.1155/1989/56539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortega RM, Fernandez–Azuela M, Encinas-Sotillos A, et al. Differences in diet and food habits between patients with gallstones and controls. J Am Coll Nutr. 1997;16:88–95. doi: 10.1080/07315724.1997.10718655. [DOI] [PubMed] [Google Scholar]
- 11.Kono S, Shinchi K, Todoroki I, et al. Gallstone disease among Japanese men in relation to obesity, glucose intolerance, exercise, alcohol use, and smoking. Scand J Gastroenterol. 1995;30:372–6. doi: 10.3109/00365529509093293. [DOI] [PubMed] [Google Scholar]
- 12.Sarles H, Chabert C, Pommeau Y, et al. Diet and cholesterol gallstones. A study of 101 patients with cholelithiasis compared to 101 matched controls. Am J Dig Dis. 1969;14:531–7. doi: 10.1007/BF02232927. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler M, Hills LL, Laby B. Cholelithiasis: A clinical and dietary survey. Gut. 1970;11:430–7. doi: 10.1136/gut.11.5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basso L, McCollum PT, Darling MR, et al. A descriptive study of pregnant women with gallstones. Relation to dietary and social habits, education, physical activity, height, and weight. Eur J Epidemiol. 1992;8:629–33. doi: 10.1007/BF00145375. [DOI] [PubMed] [Google Scholar]
- 15.Friedman GD, Kannel WB, Dawber TR. The epidemiology of gallbladder disease: Observations in the Framingham Study. J Chronic Dis. 1966;19:273–92. doi: 10.1016/0021-9681(66)90132-9. [DOI] [PubMed] [Google Scholar]
- 16.The Rome Group for Epidemiology and Prevention of Cholelithiasis (GREPCO) The epidemiology of gallstone disease in Rome, Italy. Part II. Factors associated with the disease. Hepatology. 1988;8:907–13. [PubMed] [Google Scholar]
- 17.Jorgensen T. Gall stones in a Danish population. Relation to weight, physical activity, smoking, coffee consumption, and diabetes mellitus. Gut. 1989;30:528–34. doi: 10.1136/gut.30.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorgensen T, Kay L, Schultz-Larsen K. The epidemiology of gallstones in a 70-year-old Danish population. Scand J Gastroenterol. 1990;25:335–40. doi: 10.3109/00365529009095495. [DOI] [PubMed] [Google Scholar]
- 19.Ostrowska L, Karczewski J, Serwin AB. Occupational social influence in the course of cholelithiasis. Med Pr. 1996;47:461–5. [PubMed] [Google Scholar]
- 20.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 21.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners. The National Runners’ Health Study. Arch Intern Med. 1997;157:191–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Williams PT. High-density lipoprotein cholesterol and other risk factors for coronary heart disease in female runners. N Engl J Med. 1996;334:1298–303. doi: 10.1056/NEJM199605163342004. [DOI] [PubMed] [Google Scholar]
- 23.Williams PT, Franklin B. Dose-response relationships between vigorous exercise and prevalence of diabetic-, hypertensive-, and hypercholesterolemia-medication use in distance runners. Med Sci Sports Exerc. 2007;39:1933–41. doi: 10.1249/mss.0b013e318145b337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams PT. Maintaining vigorous activity attenuates 7-yr weight gain in 8340 runners. Med Sci Sports Exerc. 2007;39:801–9. doi: 10.1249/mss.0b013e31803349b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams PT. Changes in vigorous physical activity and incident diabetes in male runners. Diabetes Care. 2007;30:2838–4. doi: 10.2337/dc07-1189. [DOI] [PubMed] [Google Scholar]
- 26.Williams PT, Pate RR. Cross-sectional relationships of exercise and age to adiposity in 60,617 male runners. Med Sci Sports Exerc. 2005;37:1329–37. doi: 10.1249/01.mss.0000174894.05236.45. [DOI] [PubMed] [Google Scholar]
- 27.Williams PT. Vigorous exercise and the population distribution of body weight. Int J Obes Relat Metab Disord. 2004;28:120–8. doi: 10.1038/sj.ijo.0802480. [DOI] [PubMed] [Google Scholar]
- 28.Williams PT, Satariano WA. Relationships of age and weekly running distance to BMI and circumferences in 41,582 physically active women. Obes Res. 2005;13:1370–2. doi: 10.1038/oby.2005.166. [DOI] [PubMed] [Google Scholar]
- 29.Williams PT. Vigorous exercise, fitness and incident hypertension, high cholesterol, and diabetes. Med Sci Sports Exerc. 2008;40:998–1006. doi: 10.1249/MSS.0b013e31816722a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food frequency questionnaire. Am J Clin Nutr. 1999;69:243–9. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 31.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med J. 1959;10:875–88. [PubMed] [Google Scholar]
- 32.Hellerstein HK. Limitations of marathon running in the rehabilitation of coronary patients: Anatomic and physiologic determinants. Ann NY Acad Sci. 1977;301:484–94. doi: 10.1111/j.1749-6632.1977.tb38224.x. [DOI] [PubMed] [Google Scholar]
- 33.Cooper KH. A means of assessing maximal oxygen intake: Correlation between field and treadmill testing. JAMA. 1968;203:201–4. [PubMed] [Google Scholar]
- 34.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 35.Sahi T, Puffenbarger RS, Hseih C, et al. Body mass index, cigarette smoking and other characteristics as predictors of self-reported, physician-diagnosed gallbladder disease in male college alumni. Am J Epidemiol. 1998;147:644–51. doi: 10.1093/oxfordjournals.aje.a009505. [DOI] [PubMed] [Google Scholar]
- 36.Williams PT. Self-selection accounts for inverse association between weight and cardiorespiratory fitness. Obes Res. 2008;16:102–6. doi: 10.1038/oby.2007.5. [DOI] [PubMed] [Google Scholar]
- 37.Klissouras V. Heritability of adaptive variation. J Appl Physiol. 1971;31:338–44. doi: 10.1152/jappl.1971.31.3.338. [DOI] [PubMed] [Google Scholar]
- 38.Bouchard C, Lesage R, Lortie G, et al. Aerobic performance in brothers, dizygotic and monozygotic twins. Med Sci Sports Exerc. 1986;18:639–46. [PubMed] [Google Scholar]
- 39.An P, Rice T, Gagnon J, et al. Familial aggregation of stroke volume and cardiac output during submaximal exercise: The HERITAGE Family Study. Int J Sports Med. 2000;21:566–72. doi: 10.1055/s-2000-12983. [DOI] [PubMed] [Google Scholar]
- 40.Bouchard C, An P, Rice T, et al. Familial aggregation of VO2 max response to exercise training: Results from the Heritage family study. J Appl Physiol. 1999;87:1003–8. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 41.Evans SL, Davy KP, Stevenson ET, et al. Physiological determinants of 10-km performance in highly trained female runners of different ages. J Appl Physiol. 1995;78:1931–41. doi: 10.1152/jappl.1995.78.5.1931. [DOI] [PubMed] [Google Scholar]
- 42.Baumann H, Jaggi M, Soland F, et al. Exercise training induces transitions of myosin isoform subunits within histochemically typed human muscle fibres. Pflugers Arch. 1987;409:349–60. doi: 10.1007/BF00583788. [DOI] [PubMed] [Google Scholar]
- 43.Glenmark B. Skeletal muscle fibre types, physical performance, physical activity and attitude to physical activity in women and men: A follow-up from age 16 to 27. Acta Physiol Scand Suppl. 1994;623:1–47. [PubMed] [Google Scholar]
- 44.Scrimgeour AG, Noakes TD, Adams B, et al. The influence of weekly training distance on fractional utilization of maximum aerobic capacity. Eur J Appl Physiol Occup Physiol. 1986;55:202–9. doi: 10.1007/BF00715006. [DOI] [PubMed] [Google Scholar]
- 45.Erpecum Van K, Van Berge-Henegouwen GP. Gallstones: An intestinal disease? Gut. 1999;44:435–8. doi: 10.1136/gut.44.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oettle GJ. Effect of moderate exercise on bowel habit. Gut. 1991:941–4. doi: 10.1136/gut.32.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philipp E, Wilckens T, Friess E, et al. Cholecystokinin, gastrin and stress hormone responses in marathon runners. Peptides. 13:125–8. doi: 10.1016/0196-9781(92)90150-2. [DOI] [PubMed] [Google Scholar]
- 48.Bowen JC. Gallstone disease. Med Clin North Am. 1992;76:1143–57. doi: 10.1016/s0025-7125(16)30313-3. [DOI] [PubMed] [Google Scholar]
- 49.O’Donnell JL. Gall stones and gall bladder motility. Gut. 1993;34:440–3. doi: 10.1136/gut.34.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Utter AC, Whitcomb DC, Nieman DC, et al. Effects of exercise training on gallbladder function in an obese female population. Med Sci Sports Exerc. 2000;32:41–45. doi: 10.1097/00005768-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Utter AC, Goss FL, Whitcomb DC, et al. The effects of acute exercise on gallbladder function in an adult female population. Med Sci Sports Exerc. 1996;28:280–4. doi: 10.1097/00005768-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Sari R, Balci N, Balci MK. Effects of exercise on gallbladder volume and motility in obese women. J Clin Ultrasound. 2005;33:218–22. doi: 10.1002/jcu.20117. [DOI] [PubMed] [Google Scholar]
- 53.Chuang CZ, Martin LF, LeGardeur BY, et al. Physical activity, biliary lipids, and gallstones in obese subjects. Am J Gastroenterol. 2001;96:1860–5. doi: 10.1111/j.1572-0241.2001.03884.x. [DOI] [PubMed] [Google Scholar]
- 54.LaMonte MJ, Barlow CE, Jurca R, et al. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: A prospective study of men and women. Circulation. 2005;112:505–12. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 55.Grundy SM. Cholesterol gallstone: A fellow traveler with metabolic syndrome? Am J Clin Nutr. 2004;80:1–2. doi: 10.1093/ajcn/80.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Méndez-Sánchez N, Chavez-Tapia NC, Motola-Kuba D, et al. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol. 2005;11:1653–7. doi: 10.3748/wjg.v11.i11.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Am J Surg. Vol. 165. 1993. National Institutes of Health Consensus Development Conference statement on gallstones and laparoscopic cholecystectomy; pp. 390–8. [DOI] [PubMed] [Google Scholar]
- 58.Attili AF, De Santis A, Capri R, et al. The GREPCO Group The natural history of gallstones: The GREPCO experience. Hepatology. 1995;21:655–60. doi: 10.1002/hep.1840210309. [DOI] [PubMed] [Google Scholar]
- 59.Rothman KJ. Modern epidemiology. Little, Brown; Boston: 1986. p. 87. [Google Scholar]