Abstract
As anyone who has suffered through a head cold knows, food eaten when the olfactory system is impaired tastes “wrong”–an experience that leads many to conclude that taste stimuli are processed normally only when the olfactory system is unimpaired. Evidence that taste system function influences olfactory perception, meanwhile, has been vanishingly rare. Here, we demonstrate just such an influence, showing that if taste cortex is inactivated when an odor is first presented, later presentations are properly appreciated only if taste cortex is again inactivated.
To test this, we used a putatively-olfactory “social transmission of food preference” (STFP) task: In one (training) session, a “demonstrator” rat that had just consumed chow mixed with one of four spices interacted with a “subject” rat in a neutral enclosure. One day later (testing), subjects were offered two dishes of powdered food—one flavored identically to that consumed by the demonstrator before the interaction, and one different. Subjects in this paradigm reliably prefer the food previously smelt on the breath of the demonstrator (Fig. 1a; see Supplementary Methods).
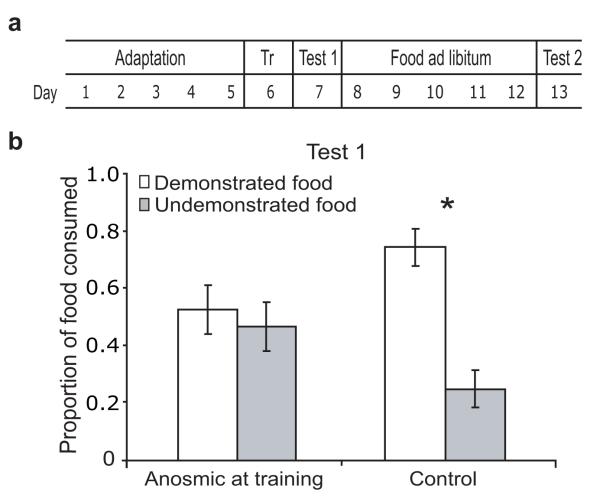
Figure 1. Food preferences are socially transmitted through odor cues.
a. Social transmission of food preference (STFP). b. Rats made anosmic before training later showed no preference for the demonstrated (white bars) food over the undemonstrated (grey bars) food. Control rats developed normal preferences. Here and in all figs, *=significant (see text).
We first directly tested olfactory involvement in STFP. One day before training, we rendered subjects temporarily anosmic using intra-nasal infusions of mild detergent. Subjects receiving control (vehicle) infusions formed the expected preferences (pcontrol<0.01; Fig. 1b); performance was similar for subjects prevented from using taste during training, either because an opaque mesh screen separated them from demonstrators, or because topical application of lingual anesthesia directly inhibited taste transduction (Fig. S1). Anosmia induced before training, meanwhile, inhibited normal preferences (F<1; Fig. 1b) despite not hindering eating (Fig. S2), even if testing was delayed until after the sense of smell had recovered (Fig. S3). We conclude, therefore, that olfactory cues are necessary and sufficient for STFP.
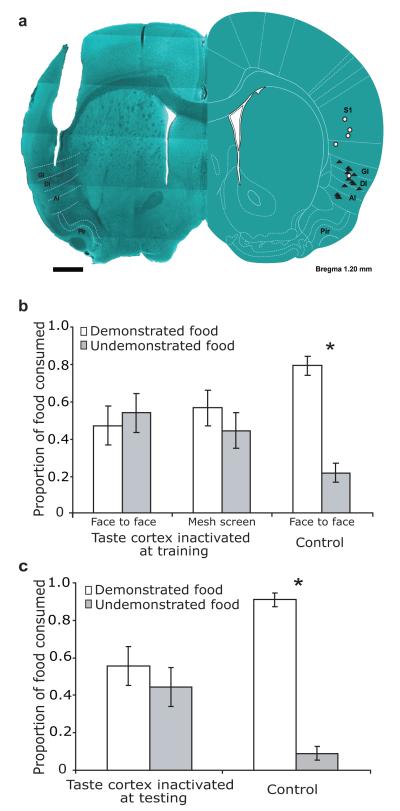
We predicted, based on the extant literature1-3, that performance in this olfactory task would be impervious to taste system perturbation. Much to our surprise, muscimol-induced inactivation of taste cortex before training inhibited STFP (pcontrol<0.03, Finactivation<1, Fig 2a-b), regardless of whether interactions were physical or across an opaque mesh. Inactivation during testing also inhibited normal performance, even though these subjects had presumably learned the preference (Fig. 2c; pcontrol<0.001, Finactivation<1). Control muscimol infusions above taste cortex, meanwhile, did not hinder learning (Fig. S4). Finally, taste-cortical inactivation impaired neither subjects’ basic food preferences nor their ability/desire to eat (Fig. S5). The most parsimonious explanation for these results (assuming that taste cortical inactivation has the same impact during training and testing) is that taste cortex in some way affects the processing of olfactory stimuli during STFP.
Figure 2. STFP depends on taste cortex.
a. Left—a coronal slice through taste cortex, showing a representative cannula track. Right—a schematic of the same slice, reprinted with permission15, showing the locations of all cannula tips. Filled triangles=muscimol infusions; open circles=control (vehicle) infusions. AI/DI/GI: agranular/dysgranular/granular insular cortex; Pir: piriform cortex; S1: somatosensory cortex. Scale bar=1mm. b. Taste cortical inactivation during STFP training inhibited learning of preference for the demonstrated food, regardless of whether training was face-to-face (left bars) or across a mesh screen (right bars). c. Cortical inactivation before testing inhibited preference learning.
Three specific possibilities present themselves: 1) taste cortex is necessary for encoding and retrieval of the odors’ incentive “value”4; 2) taste cortex is an integral part of olfactory perceptual circuitry; or 3) taste cortex modulates olfactory circuits, not coding odors per se but influencing that coding, such that inactivation fundamentally changes the percept.
This last possibility suggests a unique prediction: If taste cortical lesions impair either incentive or olfactory coding (explanations 1-2), then cortical inactivation during both interaction and testing sessions should impair STFP as much as (or more than) inactivation in either session alone. If taste cortex modulates olfactory perception (explanation 3), however, then a second inactivation should change the percept similarly to the first, and thus inactivation in both sessions should rescue normal performance—a classic state-dependency effect, previously related to systemic administration of drugs of abuse5.
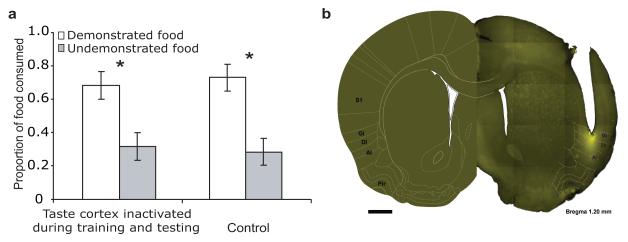
We performed this double inactivation experiment, and the results were clear: while cortical inactivation in either single session impaired STFP (Fig. 2), normal performance was rescued with double-session inactivations (Fig. 3a; Finteraction<1).
Figure 3. State-dependency of olfactory processing.
A. Double inactivation of taste cortex—once before training and again before testing—rescued normal learned preferences (compare to rats receiving control infusions). b. A coronal slice (reprinted with permission15) showing the spread of muscimol around a representative cannula tip is confined to taste cortex. Same abbreviations as in Figure 2A. Scale bar=1mm.
Three experiments confirmed that the source of this effect is taste cortex proper: 1) infusions that impaired STFP also impaired conditioned taste aversion (Fig. S6a), the paradigmatic example of taste-cortex-dependent learning; 2) muscimol did not diffuse beyond the localized region in which neurons respond to tastes (Fig. 3b)6; and 3) inactivations near olfactory cortex (which is just ventral to taste cortex) impaired learning no more than inactivation of more dorsal parts of taste cortex (Fig. S6b). The double-inactivation experiment can therefore be interpreted as evidence that if taste cortex is inactivated when a rat first smells an odor (or at least a food odor), then that rat will subsequently only respond appropriately to the food associated with that odor when cortex is again inactivated—the only example of state-dependency in neural circuit function of which we are aware.
At first blush this appears to contradict previous literature (save one recent study demonstrating that unilateral taste-cortical lesions change olfactory intensity perception7), but closer inspection reveals an easy accord. Most studies2, 3 permanently lesioned taste cortex before training, and are in fact comparable to our double inactivation experiment, in which normal performance was rescued. Functional recovery across the week(s) between surgery and training further complicate interpretation of these studies—even the accepted impact of anosmia on gustatory perception is eliminated when the anosmia is caused by permanent lesion8. The only study to suggest that a form of conditioned odor aversion can be acquired with inactivated taste cortex1, meanwhile, involved odors delivered retronasally (i. e., via the back of the throat9) whereas STFP cues are orthonasal (through the nostrils). These two delivery methods activate very different neural circuits10, and thus these two tasks likely require different circuits.
Despite obvious differences between various receptor surfaces, and the fact that each such surface projects to a distinct sensory cortex, the intrinsic multimodality of perceptual processing is increasingly recognized11. Here, we show that that flavor, long known to reflect an influence of smell on taste, also reflects the opposite: as predicted by studies suggesting reciprocal interactions between gustatory and olfactory cortices12-14, neither smell nor taste unilaterally “controls” the multimodal perception of food.
Supplementary Material
References
- 1.Bertrand D, et al. Eur J Neurosci. 2009;29:1654–1662. doi: 10.1111/j.1460-9568.2009.06711.x. [DOI] [PubMed] [Google Scholar]
- 2.Kiefer SW, Morrow NS. Behav Neurosci. 1991;105:25–32. doi: 10.1037//0735-7044.105.1.25. [DOI] [PubMed] [Google Scholar]
- 3.Roman C, Nebieridze N, Sastre A, Reilly S. Behav Neurosci. 2006;120:1257–1267. doi: 10.1037/0735-7044.120.6.1257. [DOI] [PubMed] [Google Scholar]
- 4.Balleine BW, Dickinson A. J Neurosci. 2000;20:8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jafari MR, Zarrindast MR, Djahanguiri B. Physiol Behav. 2006;88:146–151. doi: 10.1016/j.physbeh.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Katz DB, Simon SA, Nicolelis MA. J Neurosci. 2001;21:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mak YE, Simmons KB, Gitelman DR, Small DM. Behav Neurosci. 2005;119:1693–1700. doi: 10.1037/0735-7044.119.6.1693. [DOI] [PubMed] [Google Scholar]
- 8.Hasan KS, Reddy SS, Barsony N. Endocr Pract. 2007;13:716–720. doi: 10.4158/EP.13.7.716. [DOI] [PubMed] [Google Scholar]
- 9.Hummel T, Welge-Luessen A. Adv Otorhinolaryngol. 2006;63:84–98. doi: 10.1159/000093752. [DOI] [PubMed] [Google Scholar]
- 10.Small DM, Gerber JC, Mak YE, Hummel T. Neuron. 2005;47:593–605. doi: 10.1016/j.neuron.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Ghazanfar AA, Schroeder CE. Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Azuma S, Kawamura Y. Exp Brain Res. 1984;56:23–31. doi: 10.1007/BF00237438. [DOI] [PubMed] [Google Scholar]
- 13.Fu W, Sugai T, Yoshimura H, Onoda N. Neuroscience. 2004;126:1033–1041. doi: 10.1016/j.neuroscience.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 14.McDonald AJ, Jackson TR. J Comp Neurol. 1987;262:59–77. doi: 10.1002/cne.902620106. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.