Abstract
Lipid accumulation in arteries induces vascular inflammation and atherosclerosis, the major cause of heart attack and stroke in humans. Extreme hyperlipidemia induced in mice and rabbits enables modeling many aspects of human atherosclerosis, but microscopic examination of plaques is possible only postmortem. Here we report that feeding adult zebrafish (Danio rerio) a high-cholesterol diet (HCD) resulted in hypercholesterolemia, remarkable lipoprotein oxidation and fatty streak formation in the arteries. Feeding an HCD supplemented with a fluorescent cholesteryl ester to optically transparent fli1:EGFP zebrafish larvae in which endothelial cells (EC) express GFP, and using confocal microscopy enabled monitoring vascular lipid accumulation and the EC layer disorganization and thickening in a live animal. The HCD feeding also increased leakage of a fluorescent dextran from the blood vessels. Administering ezetimibe significantly diminished the HCD-induced EC layer thickening and improved its barrier function. Feeding HCD to lyz:DsRed2 larvae in which macrophages and granulocytes express DsRed, resulted in the accumulation of fluorescent myeloid cells in the vascular wall. Using a fluorogenic substrate for phospholipase A2 (PLA2), we observed an increased vascular PLA2 activity in live HCD-fed larvae compared to control larvae. Furthermore, by transplanting genetically modified murine cells into HCD-fed larvae, we demonstrated that toll-like receptor-4 (TLR4) was required for efficient in vivo lipid uptake by macrophages. These results suggest that the novel zebrafish model is suitable for studying temporal characteristics of certain inflammatory processes of early atherogenesis and the in vivo function of vascular cells.
INTRODUCTION
Current experimental studies of atherosclerosis often use genetically modified mice fed high-fat, high-cholesterol diets, which rapidly induce extreme hyperlipidemia and lipid accumulation in the artery wall. One important limitation of using mice is the difficulty in studying the temporal course of pathogenic events because microscopic examination of atherosclerotic lesions can be performed only postmortem. In this regard, an advantage of using zebrafish (Danio rerio) is that their larvae are optically transparent until about the 30th day of development, which enables temporal observations of fluorescent proteins and probes in a live animal. Transgenic fli1:EGFP zebrafish, which express EGFP in the vascular endothelium, have been imaged extensively in high resolution using confocal microscopy to analyze developmental angiogenesis and tumor cell intravasation in live animals1,2. Thus, if one could induce hyperlipidemia and lipid accumulation in blood vessels in fli1:EGFP zebrafish, this will create a valuable model for in vivo monitoring of early pathologic processes of atherogenesis.
Fish are poikilothermic vertebrates that preferentially use lipids rather than carbohydrates as an energy source and would be classified, using standards applied to mammals, as mildly hyperlipidemic and hypercholesterolemic3. In 1962 Vastesaeger and Delcourt observed the presence of lipid-rich atherosclerosis-like lesions in the aorta of a tuna(Thunnus thynnus)4. More recently, Seierstad et al. demonstrated similar lesions in coronary arteries of farmed Atlantic salmon (Salmo salar)5. Lipoproteins have been studied in teleost fish, particularly in rainbow trout (Oncorhynchus mykiss)3. VLDL, LDL and HDL lipoprotein classes have been identified by analytical ultracentrifugation, with HDL dominating the lipoprotein profile. The nature and the distribution of apolipoproteins in different classes of fish lipoproteins resembles that in mammals, but plasma concentration of apolipoproteins in rainbow trout accounts for 36% of total protein, compared with only 10% in humans. Fish LDL contains more triglycerides and less cholesteryl esters than human LDL. Lipoprotein lipase, hepatic lipase and lecitin:cholesterol acyltransferase activities have been identified, and there is evidence suggesting the presence of cholesteryl ester transfer protein in rainbow trout plasma3.
During embryonic development of fish, yolk syncytial layer actively synthesizes apoE and other apolipoproteins, and forms VLDL from yolk lipids, which then enter the circulatory system and deliver nutrient lipids to the tissues6,7. As in humans, zebrafish microsomal triglyceride transfer protein is involved in VLDL assembly in the yolk and, later, in intestinal lipoprotein synthesis8,9. In addition, zebrafish have structural and functional homologs of mammalian apoAI, apoB and phospholipase A2 (PLA2)10–12. Studies of intestinal lipid metabolism in zebrafish identified annexin2-caveolin1 and fat-free as important factors in intestinal cholesterol absorption and the targets for anti-hyperlipidemic therapies13,14. Sequence and expression analyses and studies of OxLDL uptake suggest the presence of SRA, CD36, TLR4, LDLR, LRP-1, and ABCA1 in fish (Refs.15,16 and ZFIN Direct Data Submission). Taken together, these data suggest that major elements of lipid metabolism are conserved between teleost fish and mammals.
To explore the potential of zebrafish for atherosclerosis-related studies, we first tested lipid and lipoprotein parameters in adult zebrafish fed a high cholesterol diet. These fish were also used for histological analyses. Next, we used confocal microscopy to detect lipid and leukocyte accumulation in blood vessel walls, endothelial layer disorganization and permeability, and vascular PLA2 activity in live zebrafish larvae. In addition, to study macrophage lipid accumulation in vivo, in the environment of a fatty streak, we transplanted murine macrophages into zebrafish and measured time courses of macrophage lipid uptake in live animals.
MATERIALS AND METHODS
(abbreviated; the complete Methods section can be found in Online Data Supplement)
Zebrafish
Zebrafish maintenance and procedures were approved by the UCSD institutional animal care and use committee. A high-cholesterol diet (HCD) for adult zebrafish was made by soaking salmon starter (Aquaneering) in a diethyl ether solution of cholesterol (Sigma) to achieve a content of 4% (w/w) cholesterol in the food after ether evaporation. Similarly, larvae were fed artificial artemia (Azoo) that was enriched with 2–10% cholesterol using the above procedure. For the purposes of studying vascular lipid accumulation in larvae, both control and HCD food were supplemented with 10 μg/g of a fluorescent cholesteryl ester analog (cholesteryl BODIPY®576/589-C11 from Invitrogen).
Lipid and lipoprotein analyses
Two μl blood was drawn from the heart of adult fish. Total cholesterol (TC) and triglycerides (TG) in plasma were measured using automated enzymatic assays (Roche Diagnostics and Equal Diagnostics). Plasma lipoprotein profiles were analyzed in native agarose gel electrophoresis (Helena Laboratories). Oxidation-specific epitopes were detected in an immunoassay with EO6 monoclonal antibody17.
Histology
Ten μm thick frozen or paraffin-embedded cross-sections of adult zebrafish (trunk area) were collected. Initial morphological evaluation of non-stained sections was performed using a bright field microscope. Sections containing lesions (intimal thickening) in the dorsal aorta were stained with van Gieson's stain, LipidTOX™ Red (neutral lipid), an anti-human L-plastin antibody (macrophages) or DAPI (nuclei).
In vivo microscopy
For in vivo confocal microscopy, anaesthetized fish larvae were housed in a sealed, temperature controlled chamber in a small drop of tricaine containing water2. A Nikon C1-si confocal microscope was used in either regular or spectral acquisition modes. Images were 3D rendered and analyzed using Imaris® software (Bitplane). Detailed methods for quantifying vascular lipid and myeloid cell accumulation, apparent thickness and permeability of the EC layer, vascular PLA2 activity in larvae, and cell transplant and macrophage lipid accumulation experiments are described in the Online Supplement.
Statistics
Data in graphs are presented as mean ± standard error. Statistical differences between experimental groups were evaluated by one-way ANOVA. Values of p<0.05 were considered statistically significant.
RESULTS
Hypercholesterolemia in adult zebrafish
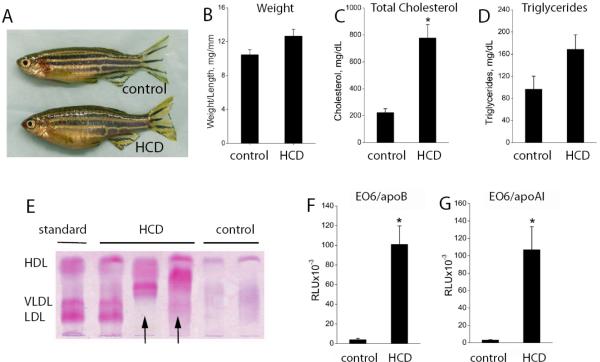
To test if zebrafish are inherently susceptible to high cholesterol feeding, HCD was fed to zebrafish starting at 5 weeks post fertilization (“adult” fish) for an additional 8–12 weeks. Compared to control animals who received normal food, HCD-fed zebrafish had an enlarged belly (Fig. 1A), but the weight gain was not statistically different (Fig. 1B). However, there was a dramatic, 4-fold increase in total plasma cholesterol (TC) levels, reaching on average 800 mg/dL (Fig. 1C), values observed in cholesterol-fed LDLR−/− mice developing atherosclerosis18. Elevated TC levels in HCD-fed fish were found as early as at 40–45 days post fertilization (dpf)(Fig. S1) and likely develop even earlier, but we were unable to collect blood from younger fish. The triglyceride (TG) levels were not statistically different (Fig. 1D) since no fat was added to the HCD. Agarose native gel electrophoresis followed by Fat Red staining demonstrated that control zebrafish plasma contained a distinct lipoprotein fraction corresponding to human HDL as well as other unresolved bands (Fig. 1E). This agrees with the reports of HDL dominating the lipoprotein profile in other teleost fish3. In contrast, plasma from the HCD-fed zebrafish had, in addition to a prominent HDL fraction, strong bands that appear to correspond to human LDL and VLDL. Interestingly, many plasma samples from HCD-fed fish contained high-mobility bands (we show three representative samples in Fig. 1E), which may correspond to electronegative, oxidized LDL19.
Figure 1. Hypercholesterolemia and oxidized plasma lipoproteins in adult zebrafish.
Five week old zebrafish (both male and female) were fed a 4% cholesterol-enriched (HCD) or normal (control) diet, for 8–12 weeks. A, Female fish (confirmed by dissection) fed a control diet or HCD. B, The ratio of body weight to length (body mass index) (n=17 in each group, both males and females; no statistically significant differences). C and D, Total cholesterol (TC) and triglycerides (TG) in plasma of 3 month old zebrafish (n=11 in each group, both males and females). *, p<0.001. E, Native agarose gel electrophoresis of HCD and control zebrafish plasma, stained with Fat Red. “Standard” is a human plasma (36.4% alpha- (HDL), 18.4% pre-beta- (VLDL) and 45.1% beta-lipoproteins (LDL)). Each “HCD” or “control” lane shows an individual zebrafish plasma sample, representative of total 35 samples. Arrows point at high-mobility bands. F and G, The EO6 immunoassay was performed with 1:200 diluted zebrafish plasma captured on a microtiter plate coated with either anti-human apoB (F) or anti-human apoAI (G) antibody. Oxidation-specific epitopes were detected with EO6 antibody (n=8). *, p<0.05.
Lipoprotein oxidation in adult zebrafish
Our current understanding of atherogenesis considers that the oxidative modification of LDL is a leading factor in the initiation and progression of the atherosclerotic lesion20. Our laboratory has developed monoclonal antibodies that can be used in immunoassays to detect oxidation-specific epitopes on lipoproteins in plasma of different animal species and humans17. Monoclonal antibody EO6 is used to measure the amount of oxidized phospholipids bound per apoB or apoAI lipoproteins. In human epidemiological studies, a 3 to 5-fold increase in the EO6/apoB plasma levels multiplies the risk of coronary artery disease 3-fold among patients <60 years of age, and when combined with hypercholesterolemia – up to 17 fold17.
Thus, given high TC levels and evidence for elevated VLDL and LDL lipoproteins, we measured the EO6/apoB levels in zebrafish plasma. We found that a polyclonal antibody against human apoB recognized proteins in zebrafish plasma corresponding to human apoB (Fig. S2), which agrees with a similar crossreactivity of a different anti-human apoB antibody with trout apoB3. Remarkably, the EO6 reactivity in apoB lipoproteins was as much as 20–30 times higher in HCD-fed zebrafish plasma than in control plasma samples (Fig. 1F). Using an anti-human apoAI antibody (Fig. S2) to trap HDL particles from plasma revealed the equally remarkable finding that EO6 immunoreactivity on apoAI particles was also 20–30-fold higher in the HCD plasma (Fig. 1G).
Vascular lesions in adult zebrafish
Next, we examined HCD-fed and control zebrafish frozen and paraffin-embedded sections stained with van Gieson's stain, LipidTOX (a fluorescent stain for neutral lipids) and DAPI (a nuclear stain). In sections of partially perfused dorsal aorta, we found vascular lesions of enlarged intima that extended into the lumen of the dorsal aorta and were characterized by accumulation of lipid and cell infiltration (Figs. 2, S3 and Table S1). Such lesions are classified as fatty streaks, early lesions of developing atherosclerosis in mouse models and in humans. From a total of 9 HCD-fed fish (both males and females), 7 had lesions of 100–500 μm in length in a 5-mm long segment of the dorsal aorta, while only 1 out of 9 control fish had one small lesion (Table S1). The lesions were mostly found at the sites of inter-segmental arteries bifurcation, where turbulent flow can be expected (Fig. 2A). To detect macrophages in zebrafish vascular lesions, we used a polyclonal antibody against human L-plastin. Zebrafish and human L-plastin are 82% identical and 90% homologous, and in western blots the antibody stained bands of the same molecular mass in zebrafish larvae lysates and in the lysates of murine macrophages (Fig. S4). It also stained cells in murine atherosclerotic lesions (Fig. S4) and in zebrafish vascular lesions (Fig. 2E), suggesting macrophage infiltration.
Figure 2. Fatty streaks in the dorsal aorta of adult zebrafish.
A, Dorsal aorta (DA) and caudal vein (CV) of HCD-fed zebrafish; ISA, inter-segmental artery bifurcation from the DA; mln, melanocytes accumulate around zebrafish blood vessels. B and C, Dorsal aortas of HCD-fed (B) and control (C) zebrafish; van Gieson staining. D and E, Dorsal aorta of HCD-fed zebrafish stained with LipidTOX Red (neutral lipid; merged fluorescent and bright field images)(D) and an antibody against L-plastin (macrophages) counterstained with DAPI (nuclei)(E). Scale, 20 μm (A–D), 5 μm (E).
Vascular lipid and myeloid cell accumulation in zebrafish larvae
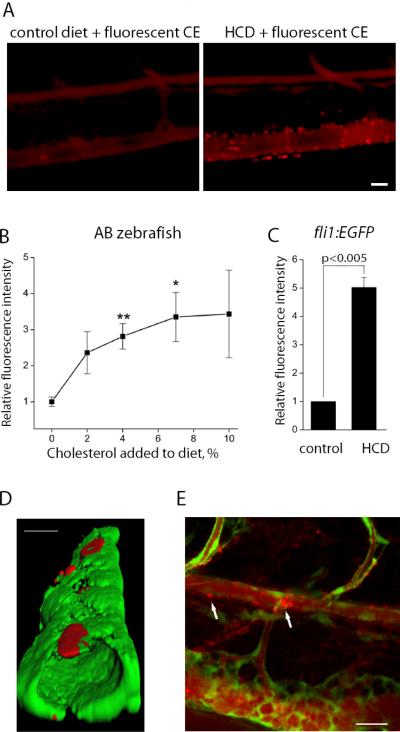
The zebrafish body is transparent during approximately 30 dpf, which would allow for a dynamic study of the processes of vascular lipid accumulation and inflammation. Thus, we explored if HCD leads to vascular lipid accumulation in larvae. On the 5th dpf, when zebrafish larvae begin free feeding, we started the HCD, supplemented with a red fluorescent cholesteryl ester analog, and continued it for 10 days. The control diet with normal cholesterol content was also supplemented with the fluorescent cholesteryl ester analog. These two diets were fed to two groups of fli1:EGFP zebrafish, constitutively expressing GFP in EC1, which enables visualization of the vasculature. Live anesthetized zebrafish larvae were imaged using a Nikon C1-si confocal microscope. We observed that vasculature of the control and HCD larvae were stained diffusely red, consistent with circulating fluorescent lipid (Fig. 3A). Remarkably, only in HCD-fed larvae there were many focal areas of bright red fluorescence in blood vessels, which we interpreted as lipid accumulation in the vessel wall (either cholesteryl ester or its hydrolyzed fatty acid chain present as a free fatty acid or re-esterified into triglycerides, phospholipids or cholesteryl esters). To further confirm that the accumulation of fluorescent lipid is indeed a consequence of HCD feeding, we fed wild type AB larvae diets with a varying concentration of cholesterol (2–10%). There was a dose-dependent increase in vascular accumulation of fluorescent lipid, with most reproducible results achieved at 4% cholesterol (Fig. 3B). Using a 4% cholesterol diet with fli1:EGFP larvae, we observed even higher levels of fluorescent lipid accumulation than in AB larvae, on average a 5-fold increase (Fig. 3C). Three-dimensional rendering demonstrated that these lipid deposits were subendothelial (Fig. 3D), which would correspond to intimal lipid accumulation in mice and humans, although lipid accumulation in adventitia cannot be excluded. Although the majority of lipid deposits were observed in the caudal vein, some deposits were found in the dorsal aorta and at sites of blood vessel bifurcation as well (Fig. 3E and Movie1 in Online Supplement). The presence of the lipid deposits in veins can be explained by the specifics of the zebrafish circulatory system at this stage of development, when large arteries and veins connect directly rather than via a capillary network. In adult zebrafish, lesions were found only in the dorsal aorta but not in the caudal vein.
Figure 3. Lipid accumulation in zebrafish larvae.
A, Five-day old zebrafish larvae were fed for 10 days a control diet or an HCD enriched with 4% cholesterol, both supplemented with 10 μg/g of red fluorescent lipid. Images of the caudal vasculature in live larvae show diffuse red fluorescence of circulating fluorescent lipid in both control and HCD-fed larvae, and bright fluorescent lipid deposits in the blood vessel wall only in HCD-fed larvae. Scale, 20 μm. B, AB larvae were fed diets supplemented with 10 μg/g of red fluorescent lipid and zero, 2, 4, 7 or 10% cholesterol for 10 days. Fluorescence intensities of red lipid in the areas shown in panel A were quantified (n=4 animals in each group). C, fli1:EGFP larvae were fed fluorescent lipid-supplemented control or 4% cholesterol diets, and fluorescence intensities of red lipid in the areas shown in panel A were quantified (n=4). D, 3D reconstruction of the caudal vein in a live fli1:EGFP zebrafish, fed HCD diet, showing green fluorescence from EC and red fluorescence from the deposits of the lipid-associated BODIPY 576/589 fluorophore, localizing beneath EC. Scale, 25 μm. E, Fluorescent lipid accumulation in the dorsal aorta. Note the larger lipid deposit at the bifurcation site. Scale, 20 μm.
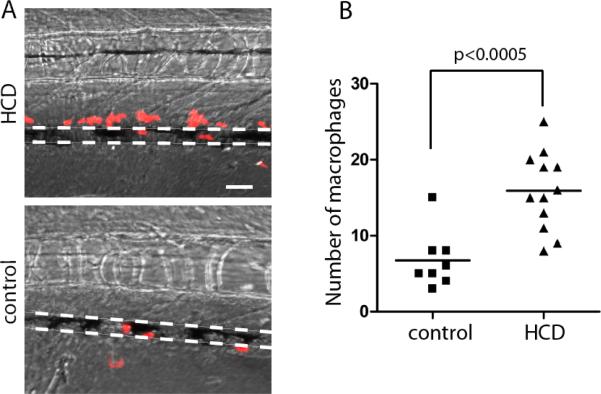
Feeding HCD to lyz:DsRed2 larvae, in which DsRed2 is expressed in monocyte/macrophages and granulocytes21, resulted in the recruitment of red fluorescent myeloid cells to the caudal vein, within a two-cell distance from the lumen (Fig. 4), suggesting accumulation of macrophages and/or neutrophils in the vascular wall. In mammalian atherosclerosis, neutrophils are notably excluded from vascular lesions20, and the absolute majority of myeloid cells are macrophages.
Figure 4. HCD-induced myeloid cell recruitment to the vasculature.
Five-day old lyz:DsRed2 larvae were fed HCD (n=12) or control diet (n=8) for 10 days, and red fluorescent cells accumulated within 50 μm of the caudal vein (delineated by dotted lines) were counted. Merge of fluorescent and bright field images. Scale, 50 μm.
Endothelial layer disorganization and permeability in HCD-fed larvae
Under laminar flow in non-inflamed mammalian blood vessels, EC form a regular layer with the cells oriented along the flow. At sites of turbulent flow and when the lipid deposition in the intima causes EC activation, an apparent thickness of the EC layer increases due to the loss of EC alignment, formation of large vacuole-like endothelial cell boundaries and infiltration of macrophages, as observed in early lesions in hypercholesterolemic mice22. In HCD-fed larvae we observed irregularity in endothelial layer morphology of the caudal vein (Fig. 5A) and apparent thickening of the EC layer in central and peripheral blood vessels (Fig. 5A–C). Ezetimibe, an inhibitor of intestinal cholesterol absorption, added to the fish tank water during the HCD feeding period, reduced lipid deposition in the intestine and peritoneal cavity of HCD-fed zebrafish (upper panels in Fig. 5B), which agrees with an earlier report13. Remarkably, the ezetimibe treatment attenuated HCD-induced endothelial cell disorganization in peripheral vasculature (lower panels in Fig. 5B) and reduced an apparent thickness of the EC layer in the caudal vein (Fig. 5C), which likely reflects attenuated vascular inflammation.
Figure 5. HCD-induced endothelial layer disorganization and thickening.
Experimental conditions as in Fig. 3C. A, 3D reconstruction (inner vascular surface) of the caudal vein in control and HCD-fed fli1:EGFP zebrafish. Green fluorescence is from EC. B, fli1:EGFP larvae were fed control or HC diets; one group of HCD-fed larvae was exposed to 40 μg/ml ezetimibe added into the fish tank water during the feeding period. Upper panels show lipid accumulation (red fluorescence) in the intestine and the peritoneum. Lower panels show EC morphology (green fluorescence) in peripheral vasculature. Scale, 20 μm. C, An apparent thickness of the EC layer in the caudal vein was calculated from 3D digital reconstructions of 640 μm long segments of the caudal vein, as described in Methods (n=5–7 animals per group).
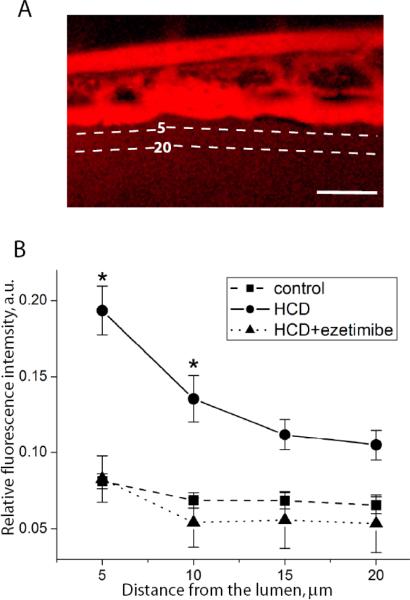
To test whether the observed EC layer disorganization resulted in the loss of its barrier function as found in mammalian atherosclerotic lesions, we injected i.v. a fluorescent dextran and observed its leakage outside the caudal vein (Figs. 6 and S5). The intensity of dextran fluorescence at 5 μm from the lumen margin was 2.5-fold higher in HCD-fed larvae compared to the control. The ezetimibe treatment prevented dextran leaking from the blood vessels.
Figure 6. HCD-induced increase in endothelial layer permeability.
Experimental conditions as in Fig. 5B, but no fluorescent lipid was added to the diet. Leakage of i.v. injected red fluorescent dextran (2×106Da) from the caudal vein was measured as described in Methods. Dashed lines at 5 and 20 μm from the lumen show where the fluorescence intensities were measured. Scale, 50 μm. *, p<0.01 (n=9–11 animals per group).
Vascular PLA2 activity in zebrafish larvae
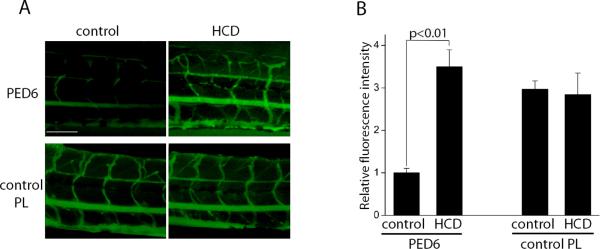
Imaging of live zebrafish permits not only morphological but also functional studies. Farber and co-workers developed a fluorescent reporter for PLA2 activity, PED6, and used it in the study of intestinal lipid metabolism in zebrafish23. We found that in addition to intestinal and gall bladder fluorescence of PLA2-hydrolyzed PED6, a bright emission was detected from zebrafish blood vessels following 10 days of HCD, but was almost absent in the vasculature of control zebrafish (Fig. 7A, upper panels, and 7B). To demonstrate that there was equal penetration of the fluorogenic substrate into vasculature of control and HCD-fed zebrafish, we used a phospholipid in which the fluorophore is unquenched and, thus, its fluorescence intensity is independent of being cleaved by PLA2. There were no differences in the intensities of the control phospholipid fluorescence in the control and HCD-fed larvae (Fig. 7A, lower panels, and 7B). These results suggest that fluorescent reporters can be used to study activities of enzymes involved in vascular inflammation in live zebrafish.
Figure 7. HCD-induced PLA2 activity.
A, AB larvae were fed a control diet or HCD for 10 days, then placed in a 1 μg/ml solution of PED6, a fluorogenic PLA2 substrate, for 2 hours. Green fluorescence (hydrolyzed PED6) indicates the PLA2 activity. In a separate set of experiments, PED6 was replaced with BODIPY®-FLC5-HPC (0.67 μg/ml), a control fluorescent phospholipid whose fluorescence is independent of PLA2 cleavage. Scale, 100 μm. B, Quantification of the data presented in panel A (n=4).
TLR4-dependent macrophage lipid uptake in zebrafish larvae
Studies of atherosclerosis in mouse models are facilitated by the availability of knockout mouse strains and engineered cell lines, but the technology for generating knockout zebrafish is not yet well established, and morpholino antisense techniques provide the gene knockdown only for first 3–5 dpf, before we initiate high-cholesterol feeding. To circumvent this problem, we transplanted genetically manipulated murine macrophages into the larvae that were fed HCD for 10 days prior to the transplantation. Because adaptive immunity in 15–20 dpf larvae is undeveloped, this technique allows for monitoring the function of mammalian macrophages in the environment of a zebrafish fatty streak. We applied this technique to investigate the function of toll-like receptor-4 (TLR4) in atherogenesis.
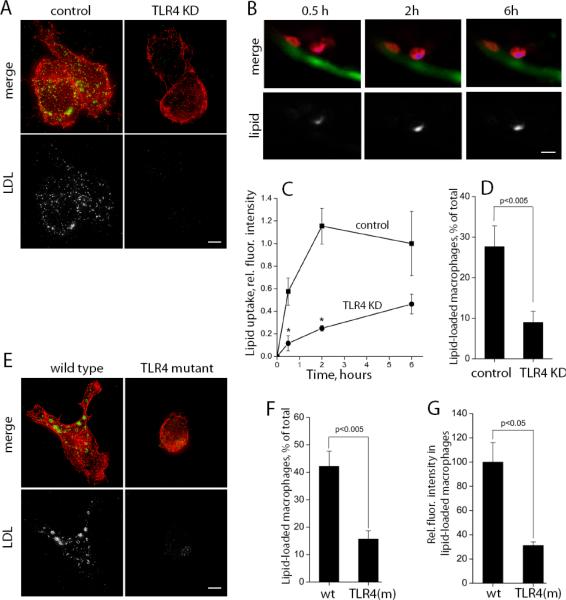
We have previously reported that minimally oxidized LDL (mmLDL) activates macrophages in a TLR4-dependent manner24,25. We noticed that in cell culture, mmLDL stimulated TLR4-competent J774 macrophages to accumulate lipid and that this effect was inhibited in TLR4-knockdown cells expressing TLR4-specific shRNA (Fig. 8A). Remarkably, when transplanted into HCD-fed zebrafish larvae, TLR4-competent macrophages, repeatedly imaged in areas of vascular lipid deposition, accumulated fluorescent lipid at a significantly higher rate than TLR4-deficient macrophages (Fig. 8B and C). At 24 hour post injection, close to 30% of TLR4-competent macrophages accumulated lipid, compared to less than 10% of TLR4-deficient macrophages (Fig. 8D). To confirm these results with primary cells, we used peritoneal macrophages and circulating mononuclear cells isolated from C3H mice; the C3H/HeJ mice carry the lps-d mutation in the TLR4 gene that makes the receptor non-functional, while the C3H/HeOuJ mice have normal functional TLR426. In a cell culture experiment, wild type, but not TLR4-mutant macrophages, stimulated with mmLDL, spread (as we reported earlier24,25) and accumulated lipid (Fig. 8E). Following transplantation into HCD-fed larvae, more than 40% of wild type macrophages accumulated endogenous (dietary) lipid, compared to only about 15% of TLR4-mutant cells (Fig. 8F). Among the transplanted cells that have accumulated lipid, the relative amount of intracellular lipid was 3-fold lower in TLR4-mutant cells compared to wild type macrophages (Fig. 8G). These in vivo experiments suggest a novel function for TLR4 in mediating lipoprotein uptake by macrophages.
Figure 8. TLR4-dependent lipid uptake.
A, In a cell culture experiment, the uptake of Alexa Fluor 488-labeled native LDL (150 μg/ml) was stimulated by non-labeled mmLDL (50 μg/ml) for 1 hour. J774 macrophages were expressing scrambled shRNA (control) or TLR4-specific shRNA (TLR4 KD). Red, F-actin; green, Alexa488-LDL. The fluorescence from labeled LDL is shown in white in lower panels. Scale, 5 μm. B, CellTracker Orange-labeled control J774 macrophages were transplanted into fli1:EGFP larvae that, prior to the transplantation, was fed for 10 days a HCD supplemented with red fluorescent lipid. Repetitive images of the same cells in live fish were captured (red, macrophages; blue and white, fluorescent lipid; green, EC). Scale, 10 μm. C, From the data collected in experiments in panel B, the time courses of lipid uptake by individual transplanted macrophages were measured for control and TLR4 KD J774 cells (n=25–30 cells for each time point; total of 10 animals imaged). *, p<0.01. D, In a separate set of experiments, the percentage of transplanted macrophages that accumulated fluorescent lipid 24 hours post injection into HCD-fed larvae was determined (n=9). E, In a cell culture experiment, the uptake of Alexa Fluor 488-labeled native LDL (150 μg/ml) was stimulated by non-labeled mmLDL (50 μg/ml) for 1 hour in wild type and TLR4-mutant primary macrophages harvested from C3H mice. Red, F-actin; green and white, Alexa488-LDL. Scale, 5 μm. F, In a zebrafish transplant experiment, performed as in panel B, a percentage of transplanted primary macrophages that accumulated lipid 2 hours post injection into HCD-fed larvae was determined for wild type and TLR4-mutant primary macrophages (n=5 animals per group; total of 234 wild type and 125 TLR4-mutant cells were counted). G, Integrated intensities of intracellular fluorescent lipid in only those transplanted primary macrophages that were counted in panel F (n=26 for wild type and n=11 for TLR4-mutant cells; not all positive cells were suitable for quantification due to their position or image quality).
DISCUSSION
Many researchers and clinicians agree that the treatment of atherosclerosis must begin at the earliest possible stage – the fatty streak27. The processes that occur in the fatty streak, endothelial cell activation, monocyte recruitment and excessive lipoprotein uptake by macrophages and the formation of pro-inflammatory lipid-loaded foam cells, define the advancement of atherosclerosis and its complications. By feeding HCD to zebrafish we were able to reproduce many of the processes involved in early atherogenesis. We observed hypercholesterolemia (Fig. 1C), lipoprotein oxidation (Fig. 1F and G) and fatty streak formation (Fig. 2) in adult zebrafish. Moreover, in optically transparent zebrafish larvae, we observed vascular lipid deposition and myeloid cell accumulation (Figs. 3 and 4), EC layer disorganization and increased permeability (Figs. 5 and 6), increased PLA2 activity (Fig. 7), and lipid accumulation by transplanted macrophages (Fig. 8) – all in live animals. These findings suggest that zebrafish is suitable as a model organism for studying mechanisms of the pathologic processes important in early atherogenesis. However, as in any animal experimentation, using zebrafish enables modeling only certain aspects of the human disease.
Lipoprotein oxidation is a major pathogenic factor that accelerates atherogenesis17,20. A dramatic increase in the plasma levels of the EO6-reactive oxidation-specific epitopes that we observed in HCD-fed zebrafish (Fig. 1F and G) is very unusual for human samples or for any mammalian model of atherosclerosis. In particular, we have never observed such high levels of HDL-associated EO6 reactivity in any species. One possible explanation for these findings is that a poikilothermic fish in water at ambient temperature and at lower oxygen concentration than in the open air, has developed less sophisticated antioxidative systems28. Thus, the HCD challenge results in a higher rate of oxidation, and hence a greater accumulation of such products as oxidized phospholipids, as measured by the EO6 immunoassay. Increased PLA2 activity (Fig. 7) might be a response to the elevated levels of oxidized phospholipids found in the plasma lipoproteins (Fig. 1F and G) since such enzymes have the ability to degrade oxidized phospholipids. These data in zebrafish evocatively correlate with recent human studies showing that increasing lipoprotein-associated PLA2 activity further amplifies the risk of cardiovascular disease mediated by oxidized phospholipids29, although we do not know yet which PLA2 isoform in zebrafish was involved.
Studies with atherosclerosis-prone apoE−/− mice in which either TLR4 or MyD88 (a critical downstream molecule in TLRs signaling) was knocked out, demonstrated reduced atherosclerosis in the animals fed a high fat diet30,31. Although these studies suggested a role for TLR4 in atherogenesis, the mechanisms remain obscure. Earlier, we observed that a putative endogenous ligand for TLR4, mmLDL, induced extensive membrane ruffling in macrophages and cell spreading, associated with intracellular vacuolization24,32,33. Based on these findings, we hypothesized that the TLR4-mediated cytoskeletal rearrangements and liquid phase uptake may quantitatively increase the rate of lipoprotein uptake by macrophages and thus accelerate foam cell formation. We tested this hypothesis using the zebrafish model. Because lipoprotein oxidation occurs in vivo in HCD-fed zebrafish (Fig. 1F and G), we expected that an in vivo generated zebrafish analog of mmLDL may induce TLR4-dependent lipid uptake. Indeed, we observed that murine macrophages transplanted into zebrafish that was fed HCD prior to transplantation, accumulated endogenous (dietary) lipid, and that this effect was significantly attenuated in TLR4-deficient macrophages (Fig. 8). Moreover, we were able, for the first time, to monitor time-dependent macrophage lipid uptake in a fatty streak in live animals. This technique will be useful in calculating rates of in vivo lipid uptake by genetically modified macrophages and, thus, quantitatively comparing different mechanisms of foam cell formation.
Based on these results, we propose that HCD-fed larvae and adult zebrafish make a useful and highly informative experimental model of certain aspects of vascular inflammation and atherosclerosis, which can be further developed using the knowledge of zebrafish intestinal lipid metabolism, angiogenesis and innate immunity9,13,14,23,34,35. The optical transparency of zebrafish larvae provides the opportunity to observe specific processes in the vascular wall repeatedly over time in a live animal. Adult zebrafish can be used to study in vivo lipoprotein oxidation and its attendant biological responses. Economic colony maintenance, ease of genetic manipulation, fast maturation and short feeding periods, and simple method of drug administration make the zebrafish model particularly attractive for studies of vascular lipid accumulation and inflammation.
Supplementary Material
References
- (1).Lawson ND, Weinstein BM. In Vivo Imaging of Embryonic Vascular Development Using Transgenic Zebrafish. Developmental Biology. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- (2).Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. PNAS. 2007;104:17406–17411. doi: 10.1073/pnas.0703446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Babin PJ, Vernier JM. Plasma lipoproteins in fish. J Lipid Res. 1989;30:467–489. [PubMed] [Google Scholar]
- (4).Vastesaeger MM, Delcourt R. The Natural History of Atherosclerosis. Circulation. 1962;26:841–855. doi: 10.1161/01.cir.26.5.841. [DOI] [PubMed] [Google Scholar]
- (5).Seierstad SL, Poppe TT, Koppang EO, Svindland A, Rosenlund G, Froyland L, Larsen S. Influence of dietary lipid composition on cardiac pathology in farmed Atlantic salmon, Salmo salar L. Journal of Fish Diseases. 2005;28:677–690. doi: 10.1111/j.1365-2761.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- (6).Poupard G, Andre M, Durliat M, Ballagny C, Boeuf G, Babin PJ. Apolipoprotein E gene expression correlates with endogenous lipid nutrition and yolk syncytial layer lipoprotein synthesis during fish development. Cell and Tissue Research. 2000;300:251–261. doi: 10.1007/s004419900158. [DOI] [PubMed] [Google Scholar]
- (7).Durliat M, Andre M, Babin PJ. Conserved protein motifs and structural organization of a fish gene homologous to mammalian apolipoprotein E. Eur J Biochem. 2000;267:549–559. doi: 10.1046/j.1432-1327.2000.01033.x. [DOI] [PubMed] [Google Scholar]
- (8).Marza E, Barthe C, Andre M, Villeneuve L, Helou C, Babin PJ. Developmental expression and nutritional regulation of a zebrafish gene homologous to mammalian microsomal triglyceride transfer protein large subunit. Dev Dyn. 2005;232:506–518. doi: 10.1002/dvdy.20251. [DOI] [PubMed] [Google Scholar]
- (9).Schlegel A, Stainier DYR. Microsomal Triglyceride Transfer Protein Is Required for Yolk Lipid Utilization and Absorption of Dietary Lipids in Zebrafish Larvae. Biochemistry. 2006;45:15179–15187. doi: 10.1021/bi0619268. [DOI] [PubMed] [Google Scholar]
- (10).Babin PJ, Thisse C, Durliat M, Andre M, Akimenko MA, Thisse B. Both apolipoprotein E and A-I genes are present in a nonmammalian vertebrate and are highly expressed during embryonic development. PNAS. 1997;94:8622–8627. doi: 10.1073/pnas.94.16.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Farber SA, Olson ES, Clark JD, Halpern ME. Characterization of Ca2+-dependent phospholipase A2 activity during zebrafish embryogenesis. J Biol Chem. 1999;274:19338–19346. doi: 10.1074/jbc.274.27.19338. [DOI] [PubMed] [Google Scholar]
- (12).Rawls JF, Samuel BS, Gordon JI. From The Cover: Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. PNAS. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Smart EJ, De Rose RA, Farber SA. Annexin 2-caveolin 1 complex is a target of ezetimibe and regulates intestinal cholesterol transport. PNAS. 2004;101:3450–3455. doi: 10.1073/pnas.0400441101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (14).Ho SY, Lorent K, Pack M, Farber SA. Zebrafish fat-free is required for intestinal lipid absorption and Golgi apparatus structure. Cell Metabolism. 2006;3:289–300. doi: 10.1016/j.cmet.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Froystad MK, Volden V, Berg T, Gjoen T. Metabolism of oxidized and chemically modified low density lipoproteins in rainbow trout--clearance via scavenger receptors. Developmental & Comparative Immunology. 2002;26:723–733. doi: 10.1016/s0145-305x(02)00035-6. [DOI] [PubMed] [Google Scholar]
- (16).Kaur H, Jaso-Friedmann L, Evans DL. Identification of a scavenger receptor homologue on nonspecific cytotoxic cells and evidence for binding to oligodeoxyguanosine. Fish & Shellfish Immunology. 2003;15:169–181. doi: 10.1016/s1050-4648(02)00156-0. [DOI] [PubMed] [Google Scholar]
- (17).Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized Phospholipids, Lp(a) Lipoprotein, and Coronary Artery Disease. The New England Journal of Medicine. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- (18).Hartvigsen K, Binder CJ, Hansen LF, Rafia A, Juliano J, Horkko S, Steinberg D, Palinski W, Witztum JL, Li AC. A Diet-Induced Hypercholesterolemic Murine Model to Study Atherogenesis Without Obesity and Metabolic Syndrome. Arterioscler Thromb Vasc Biol. 2007;27:878–885. doi: 10.1161/01.ATV.0000258790.35810.02. [DOI] [PubMed] [Google Scholar]
- (19).Khouw AS, Parthasarathy S, Witztum JL. Radioiodination of low density lipoprotein initiates lipid peroxidation: protection by use of antioxidants. J Lipid Res. 1993;34:1483–1496. [PubMed] [Google Scholar]
- (20).Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- (21).Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Mullick AE, Soldau K, Kiosses WB, Bell TA, III, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Farber SA, Pack M, Ho SY, Johnson ID, Wagner DS, Dosch R, Mullins MC, Hendrickson HS, Hendrickson EK, Halpern ME. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science. 2001;292:1385–1388. doi: 10.1126/science.1060418. [DOI] [PubMed] [Google Scholar]
- (24).Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally Modified LDL Binds to CD14, Induces Macrophage Spreading via TLR4/MD-2, and Inhibits Phagocytosis of Apoptotic Cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- (25).Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- (26).Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- (27).Steinberg D, Glass CK, Witztum JL. Evidence Mandating Earlier and More Aggressive Treatment of Hypercholesterolemia. Circulation. 2008;118:672–677. doi: 10.1161/CIRCULATIONAHA.107.753152. [DOI] [PubMed] [Google Scholar]
- (28).Martinez-Alvarez R, Morales A, Sanz A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Reviews in Fish Biology and Fisheries. 2005;15:75–88. [Google Scholar]
- (29).Kiechl S, Willeit J, Mayr M, Viehweider B, Oberhollenzer M, Kronenberg F, Wiedermann CJ, Oberthaler S, Xu Q, Witztum JL, Tsimikas S. Oxidized Phospholipids, Lipoprotein(a), Lipoprotein-Associated Phospholipase A2 Activity, and 10-Year Cardiovascular Outcomes: Prospective Results From the Bruneck Study. Arterioscler Thromb Vasc Biol. 2007;27:1788–1795. doi: 10.1161/ATVBAHA.107.145805. [DOI] [PubMed] [Google Scholar]
- (30).Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- (31).Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. PNAS. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Miller YI, Worrall DS, Funk CD, Feramisco JR, Witztum JL. Actin polymerization in macrophages in response to oxidized LDL and apoptotic cells: role of 12/15-lipoxygenase and phosphoinositide 3-kinase. Mol Biol Cell. 2003;14:4196–4206. doi: 10.1091/mbc.E03-02-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized LDL. J Biol Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kidd KR, Weinstein BM. Fishing for novel angiogenic therapies. Br J Pharmacol. 2003;140:585–594. doi: 10.1038/sj.bjp.0705496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Jault C, Pichon L, Chluba J. Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Mol Immunol. 2004;40:759–771. doi: 10.1016/j.molimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.