Abstract
We investigated the role of Na+-K+-Cl− cotransporter (NKCC1) in conjunction with Na+/Ca2+ exchanger (NCX) in disruption of endoplasmic reticulum (ER) Ca2+ homeostasis and ER stress development in primary cortical neurons following in vitro ischemia. Oxygen-glucose deprivation (OGD) and reoxygenation (REOX) caused a rise in [Na+]cyt which was accompanied by an elevation in [Ca2+]cyt. Inhibition of NKCC1 with its potent inhibitor bumetanide abolished the OGD/REOX-induced rise in [Na+]cyt and [Ca2+]cyt. Moreover, OGD significantly increased Ca2+ER accumulation. Following REOX, a biphasic change in Ca2+ER occurred with an initial release of Ca2+ER which was sensitive to inositol 1,4,5-trisphosphate receptor (IP3R) inhibition and a subsequent refilling of Ca2+ER stores. Inhibition of NKCC1 activity with its inhibitor or genetic ablation prevented the release of Ca2+ER. A similar result was obtained with inhibition of reversed mode operation of NCX (NCXrev). OGD/REOX also triggered a transient increase of glucose regulated protein 78 (GRP78), phospho-form of the alpha subunit of eukaryotic initiation factor 2 (p-eIF2α), and cleaved caspase 12 proteins. Pretreatment of neurons with NKCC1 inhibitor bumetanide inhibited upregulation of GRP78 and attenuated the level of cleaved caspase 12 and p-eIF2α. Inhibition of NKCC1 reduced cytochrome C release and neuronal death. Taken together, these results suggest that NKCC1 and NCXrev may be involved in ischemic cell damage in part via disrupting ER Ca2+ homeostasis and ER function.
Keywords: oxygen and glucose deprivation, ischemia-reperfusion, unfolded protein response, IP3 receptor, caspase 12, eIF2α
INTRODUCTION
Endoplasmic reticulum (ER) stress has received increased attention in ischemic neurodegeneration because it triggers the unfolded protein response (UPR). The UPR involves protein synthesis inhibition, activation of stress gene expression, and ER-associated protein degradation (DeGracia and Montie 2004; DeGracia and Hu 2007). There is direct evidence of ER stress following brain ischemia/reperfusion (DeGracia and Hu 2007; Hayashi et al. 2003; Hayashi et al. 2005). However, it is not well understood how the UPR is triggered following ischemia. Disruption of ER Ca2+ (Ca2+ER) homeostasis plays an important role in induction of ER stress (Corbett and Michalak 2000; Groenendyk and Michalak 2005). Depletion of Ca2+ER and ATP can disrupt proper peptide folding and trigger the UPR and ER stress. Numerous reports demonstrate that depletion of Ca2+ER with the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor thapsigargin triggers ER stress and the UPR in many cell types (Groenendyk and Michalak 2005). Moreover, augmenting Ca2+ER levels by overexpression of the ER Ca2+ binding protein calreticulin or SERCA increases the amount of ER Ca2+ available for release and amplifies mitochondrial cytochrome C (Cyt. C) release and apoptosis (Groenendyk and Michalak 2005). However, the cellular mechanisms underlying Ca2+ER dysregulation and UPR effector activation following ischemia and reperfusion are not defined.
We hypothesize that dysregulation of Ca2+ER homeostasis following ischemia involves two phases: accumulation of Ca2+ in ER stores and subsequent release of Ca2+ER following ischemia/reoxygenation (REOX). Both accumulation of Ca2+ in ER stores and subsequent release of Ca2+ER may contribute to ER stress and ischemic cell death. Na+-K+-Cl− cotransporter isoform 1 (NKCC1) transports Na+, K+, and Cl − in cells under physiological conditions (Russell 2000). Our previous studies demonstrated that NKCC1 in conjunction with NCXrev are important in disruption of Na+ and Ca2+ homeostasis following ischemia (Kintner et al. 2007; Lenart et al. 2004). However, it is unknown whether these transporter proteins indirectly contribute to changes of Ca2+ER homeostasis and ER dysfunction following ischemia.
In the current study, we present first line evidence that NKCC1 and NCXrev are involved in Ca2+ER dysregulation, which includes overload of Ca2+ER during oxygen and glucose deprivation (OGD) and release of Ca2+ER during REOX. Ca2+ER dysregulation was accompanied with development of ER stress.
MATERIALS AND METHODS
Materials
Eagle's minimal essential medium (EMEM) and Hanks balanced salt solution (HBSS) were from Mediatech Cellgro (Herndon, VA). Fetal bovine serum (FBS) was from Valley Biomedical Inc. (Winchester, VA) and horse serum (HS) was from Hyclone Laboratories (Logan, UT). 1-[6-Amino-2-(5-carboxy-2-oxazolyl)-5-benzofuranyloxy]-2-(2-amino-5-methylphenoxy) ethane-N,N,N′,N′-tetraacetic acid (fura-2 AM), sodium-binding benzofuran isophthalate (SBFI-AM), furaptra-AM (mag-fura-2-AM), calcein-AM, 4-bromo A-23187 and Alexa Fluor 488 anti-mouse IgG were from Invitrogen (Carlsbad, CA). Pluronic acid was purchased from BASF (Ludwigshafen, Germany). Bumetanide, gramicidin, monensin, propidium iodide (PI), thapsigargin, xestospongin C, and (−)-MK-801 were purchased from Sigma (St. Louis, MO). 2-aminoethoxydiphenyl borate (2-APB) and 1-aminoindan-1, 5-dicarboxylic acid (AIDA) were from Tocris (Ellisville, MO). SEA0400 was a kind gift from Taisho Pharmaceutical CO. Ltd. (Omiya, Saitama, Japan). 4′, 6-diamidino-2-phenylindole (DAPI) was from Vector Laboratories (Burlingame, CA). Antibody for β tubulin type-III was from Promega (Madison, WI). Anti-GRP78 antibody was from Assay Design (Ann Arbor, MI). Anti-caspase 12 antibody, anti-phospho-eIF2alpha (p-eIF2α) antibody, and anti-eIF2alpha (eIF2α) antibody were from Cell Signaling Technology (Danvers, MA). Anti-Cyt. C antibody was from Pharmingen (clone 6H2.B4, San Jose, CA).
Enriched cortical neuron cultures
Wild-type (NKCC1+/+) E14-16 pregnant mice (SV129/Black swiss) were anesthetized with 5% halothane and euthanized as described before (Beck et al. 2003). Fetuses were removed and the cortices were dissected in ice-cold HBSS (Beck et al. 2003). The tissues were treated with 0.5 mg/ml trypsin at 37°C for 25 min. The cells were centrifuged at 350 g for 4 min. The cell suspension was diluted in complete EMEM containing 5% FBS and 5% HS. The cells were seeded in 24-well plates or on glass coverslips coated with poly-D-lysine (800 cells/mm2) and incubated at 37°C in an incubator with 5% CO2 and atmospheric air. After 96 h, cultures were treated with 4 μM cytosine-β-D arabinofuranoside for 3 days. Cultures were re-fed with fresh medium every 3 days. Cultures at 10-15 days in vitro (DIV) were used in the study. This cortical neuron culture preparation contained 5–10% astrocytes.
In the experiments using cortical neurons cultured from NKCC1 homozygous mutant (NKCC1−/−) mice, male and female gene-targeted NKCC1 heterozygous mutant (NKCC1+/−) mice were bred as described before (Flagella et al. 1999). NKCC1+/+ and NKCC1−/− cultures were established from the E14–16 fetuses (Chen et al. 2005). The genotype of each mouse was determined by a polymerase chain reaction of DNA from fetus tail biopsies (Su et al. 2002). Experiments were performed in parallel with the wild-type and knockout cells from the littermates.
Oxygen and glucose deprivation (OGD) treatment
DIV 10-15 neuronal cultures were rinsed with an isotonic OGD solution (pH 7.4) containing (in mM): 0 glucose, 20 NaHCO3, 120 NaCl, 5.36 KCl, 0.33 Na2HPO4, 0.44 KH2PO4, 1.27 CaCl2, 0.81 MgSO4, as described before (Lenart et al. 2004). This solution has a K+ concentration (~ 5.8 mM) which is similar to the EMEM (5.4 mM) used for cell cultures. The cells were incubated in 1 ml of OGD solution for 2 h in a hypoxic incubator (model 3130, Thermo Forma, Marietta, OH) containing 94% N2, 1% O2, and 5% CO2. The oxygen level in the OGD solution decreased to ~2-3% after 60 min in the hypoxic incubator (Beck et al. 2003). Normoxic control cells were incubated for 2 h in 5% CO2 and atmospheric air in a buffer identical to the OGD solution except for the addition of 5.5 mM glucose. Reoxygenation (REOX) was achieved by addition of equal volume of EMEM with the final glucose concentration of 5.5 mM, and incubation at 37°C in 5% CO2 and atmospheric air. The oxygen level was ~ 18% during reoxygenation on the stage.
Intracellular Na+ measurement
Intracellular Na+ concentration ([Na+]cyt) was measured with the fluorescent dye SBFI-AM as described previously (Kintner et al. 2004). Cultured neurons grown on coverslips were loaded with 10 μM SBFI-AM plus 0.02% pluronic acid during 2 h OGD. In our previous study (Luo et al. 2005), 2 h OGD did not significantly decrease retention of calcein-AM fluorescence signal and fluorescent intensity of either pH indicator BCECF-AM, Na+ dye SBFI-AM, or Ca2+ dye fura-2-AM. Therefore, SBFI-AM or fura-2-AM was loaded during OGD throughout this study.
The coverslips were quickly (< 2 min) placed in an open-bath imaging chamber (Model RC24, Warner Instruments, Hamden, CT) and superfused (1 ml/min) with HCO3−-MEM at 37°C. HCO3−-MEM contained (in mM): 5.5 glucose, 20 NaHCO3, 120 NaCl, 5.4 KCl, 0.33 Na2HPO4, 0.44 KH2PO4, 1.27 CaCl2, 0.81 MgSO4 and equilibrated with 5% CO2, 21 % O2, and 74 % N2. Using a Nikon TE 300 inverted epifluorescence microscope and a 40X lens, neurons were excited every 10 min at 345 nm and 385 nm and the emission fluorescence at 510 nm recorded. Images were collected and the 345/385 ratios analyzed with the MetaFluor image-processing software as described previously (Su et al. 2002). At the end of each experiment, absolute [Na+]cyt was determined for each cell by performing an in-situ calibration as described before (Su et al. 2002).
Intracellular Ca2+ measurement
Neurons grown on coverslips were incubated with 5 μM fura-2 AM during 2 h OGD. This extended period of dye loading did not lead to compartmentalization of the dye into ER and mitochondria because permeablization of neurons with saponin (3 μg/ml for 30 sec) led to an approximately 94% loss of the fura-2 fluorescence from the cell, indicating little fura-2 was loaded in cellular organelles. Following OGD, the cells were quickly (< 2 min) placed in the open-bath imaging chamber and superfused with HCO3−-MEM at 37°C as described above. Using the Nikon TE 300 inverted epifluorescence microscope and a 40X objective lens, neurons were excited at either every 10 sec or every 5 min at 345 and 385 nm and the emission fluorescence at 510 nm recorded. Images were collected and analyzed with the MetaFluor image-processing software. At the end of each experiment, the cells were exposed to 1 mM MnCl2 in Ca2+-free HCO3−-MEM and 5 μM 4-bromo A-23187. The Ca2+-insensitive fluorescence was subtracted (Luo et al. 2005) and the MnCl2-corrected 345/385 emission ratios were converted to concentration using the Grynkiewicz equation (Grynkiewicz et al. 1985) as described previously (Lenart et al. 2004). A Kd of 370 nM for fura-2 was used (Petr and Wurster 1997). Rmin was obtained in the presence of Ca2+-free HEPES-MEM containing 10 mM EGTA and 5 μM 4-bromo A-23187. Rmax was from the ratio in HEPES-MEM containing 10 mM Ca2+ and 5 μM 4-bromo A-23187 as well as 4 μM rotenone and 2 μM FCCP (to inhibit active mitochondrial Ca2+ transport). The ionic composition of HEPES-MEM was similar to HCO3−-MEM except that 20 mM NaHCO3 was replaced with 20 mM HEPES and NaCl adjusted to maintain constant Na+ (Su et al. 2002).
Determination of NCXrex
NCXrex was determined in cultured neurons using the method of Urbanczyk et al. (Urbanczyk et al. 2006), which minimizes the confounding effects of Ca2+ buffering by intracellular organelles. Briefly, cells on coverslips were loaded with 5 μM fura2-AM during either 2 h normoxia or OGD. Cells were then placed in HEPES-MEM and [Ca2+]cyt was monitored as described above. After a 30 sec baseline, ER Ca2+ stores were emptied by applying ATP (100 μM) and thapsigargin (1 μM) in a Ca2+-free HEPES-MEM buffer. When a new baseline was established, a Na+-free/low Ca2+ (100 μM) HEPES buffer was applied to initiate NCXrev. In the Na+ free buffer, NaCl was replaced with equimolar levels of N-methyl-D-glutamine. NCXrev was quantified by fitting a slope to the increase in [Ca2+]cyt over the first 30 sec.
Ca2+ER measurement
Neurons on coverslips were incubated with 4 μM mag-fura-2 AM and 0.02 % pluronic acid during 2 h OGD. Upon to REOX, the coverslip was quickly (< 2 min) placed on an open-bath imaging chamber in HEPES-MEM at 37°C. Cells were excited every 10 sec at 345 and 385 nm and the emission fluorescence images collected at 510 nm using Nikon TE 300 inverted epifluorescence microscope and a 40X objective lens. To determine Ca2+ER, first, the plasma membrane was permeablized with 30 sec exposure to saponin (3.0 μg/ml) in an intracellular solution to eliminate the cytosolic mag-fura-2 signal. Composition of the intracellular solution was (mM): 25 NaCl, 125 KCl, 10 HEPES, 3 Na2ATP, 0.55 CaCl2, and 1.0 EGTA with free Ca2+ of ~150 nmol/ml using the Max chelator program (Tsien and Pozzan 1989). This treatment caused a decrease in cytosolic mag-fura-2 fluorescence (~ 60%, Figure 2C, a) but an increase in the ratio of 345nm/385nm (F345/385, Rbase), which reflects mag-fura-2 in ER. Next, Rmin was obtained with a minimum F345/385 ratio in a Ca2+-free solution (in mM, 25 NaCl, 125 KCl, 10 HEPES, 4 EGTA, 0.005 4-bromo A-23187, Figure 2C, c). Rmax was the maximum F345/385 ratio in a high Ca2+ solution (containing 10 mM CaCl2, Figure 2C, d). The Ca2+ER values were then calculated using the equation . K was determined as 56 μM in neurons using solutions of known Ca2+ concentrations (Calcium Calibration Buffer Kit, Invitrogen, Carlsbad, CA).
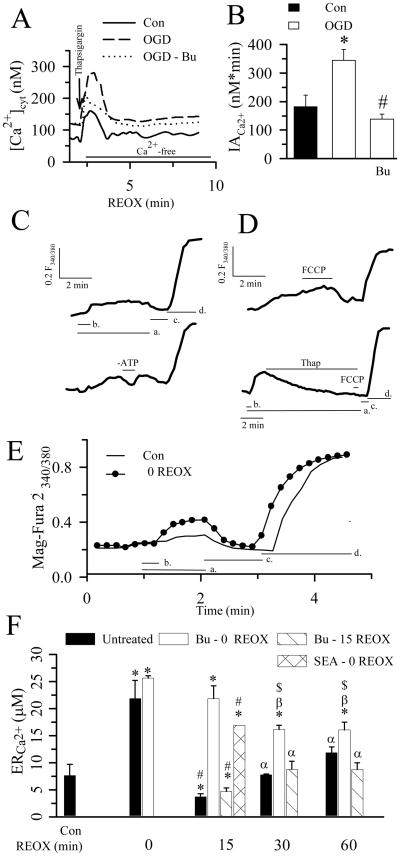
Figure 2. Changes of Ca2+ER in neurons during REOX.
A. Thapsigargin-mediated release of Ca2+ER in single neurons was determined with fura-2 during 2-5 min REOX following 2 h OGD. Thapsigargin (1 μM) was applied under [Ca2+]o-free conditions. Bumetanide (Bu, 5 μM) was present during both OGD and REOX. B. Summary data. Data are means ± SEM. n = 3-4. Means were compared using a student's t-test. * p < 0.05 vs. Con; # p < 0.05 vs. OGD. C. Representative traces for Ca2+ER determination by measuring 340 nm/380 nm fluorescence of mag-fura 2. In the top trace, cells were in a solution mimicking intracellular ion concentrations (a) and permeablized by a brief exposure to saponin (b). The post-saponin 340/380 fluorescence (Ca2+ER) was calibrated by exposing cells to zero Ca2+ (Rmin, c) and 10 mM Ca2+ containing solutions (Rmax, d). In the bottom trace, ATP was removed (-ATP) from the solution to confirm the ATP dependency of the mag-fura 2 fluorescence. Tracings are identically scaled, but off-set to provide clarity. D. Response of mag-fura 2 signals to 1 μM FCCP (top trace) or to 1 μM thapsigargin followed by 1 μM FCCP (bottom trace). Note that the time scale in the bottom trace is somewhat longer than the upper trace. This is due to the slower response of Ca2+ER to thapsigargin compared to FCCP. E. Changes of mag-fura 2 signals in normoxic control or 2 h OGD-treated neurons. F. Summary data of Ca2+ER at 2 h OGD and 0, 15, 30, or 60 min REOX. In bumetanide experiments, the drug was applied at 0 or 15 REOX. The NCXrev antagonist SEA0400 (1 μM) was applied at 0 REOX. Data are means ± SEM. n = 4-6. Means were compared using a One-Way ANOVA with a Bonferroni post-hoc test. * p < 0.05 vs. Con; # p < 0.05 vs. 0 min REOX; α p < 0.05 vs. 15 min REOX; β p < 0.05 vs. 0 min Bu-REOX; $ p < 0.05 vs. untreated group.
Gel electrophoresis and Western blotting
Cells were washed with ice-cold PBS and lysed with 30 sec sonication at 4°C in anti-phosphatase buffer (pH 7.4) containing (mM): 145 NaCl, 1.8 NaH2PO4, 8.6 Na2HPO4, 100 NaF, 10 Na4P2O7, 2 Na3VO4, 2 EDTA and 0.2 μM microcystin and protease inhibitors as described previously (Luo et al. 2005). Protein content was determined by the bicinchoninic acid method. Protein samples (60-80 μg/lane) and pre-stained molecular mass markers (Bio-Rad, Hercules, CA) were denatured in SDS 2X sample buffer and then electrophoretically separated on 8 or 10% SDS gels. The resolved proteins were electrophoretically transferred to a PVDF membrane (Kintner et al. 2004). The blots were incubated in 7.5% nonfat dry milk in tris-buffered saline (TBS) overnight at 4°C and then incubated for 1 h with a primary antibody. The blots were rinsed with TBS and incubated with horseradish peroxidase-conjugated secondary IgG for 1 h. Bound antibody was visualized using an enhanced chemiluminescence assay (Amersham Corp, Piscataway, NJ). Relative changes in protein expression were estimated from the mean pixel density of each protein band using the Scion Image program (Orem, Utah). Rabbit anti-GRP78 polyclonal antibody (1:1000), anti-caspase 12 polyclonal antibody (1:1000), anti-phosphorylated eIF2α (p-eIF2α) polyclonal antibody (1:500), or anti-eIF2α antibody (1:1000), and anti-β tubulin III monoclonal antibody (1:4000) were used.
Cyt. C immunofluorescence
Cyt. C release was determined with a specific antibody staining against Cyt. C. Briefly, cells on coverslips were fixed in 4% paraformaldehyde in PBS for 10 min. After rinsing, cells were incubated with the blocking solution for 20 min followed by incubation at room temperature with the anti-Cyt. C antibody (1:100) for 1 h. After rinsing in PBS, coverslips were incubated with green-fluorescent Alexa Fluor 488 anti-mouse IgG (1:100) for 1 h at 37°C. The coverslips were then covered with Vectashield mounting medium with DAPI. Fluorescence images were captured by the Nikon TE 300 inverted epifluorescence microscope (60X) using a Princeton Instruments MicroMax CCD camera and MetaMorph image-processing software (Universal Imaging Corp., Downingtown, PA).
Quantification of Cyt. C release from mitochondria
Cyt. C release into the cytosol was assessed in subcellular fraction preparations as described before. After removing the culture medium from the plates, cells were incubated in 2 ml of trypsin (0.2 mg/ml)-EDTA (1 mM) solution at 37°C for 10 min. The cells were detached and a cell suspension was prepared in 4 ml of EMEM. The cytosolic and mitochondrial fractions were isolated as described before and protein content in each fraction was determined (Luo et al. 2005). Levels of Cyt. C in cytosol and mitochondrial fractions were measured using the Quantikine M Rat/Mouse Cytochrome C Immunoassay kit (R&D Systems, Minneapolis, MN). Data were expressed as ng/mg protein.
Measurement of cell death
Cell viability was assessed by PI uptake and retention of calcein using a Nikon TE 300 inverted epifluorescence microscope. Cultured neurons were rinsed with HEPES-MEM and incubated with 1 μM calcein-AM and 10 μg/ml PI in the same buffer at 37°C for 30 min. For cell counting, cells were rinsed with the isotonic control buffer and visualized using a Nikon 20X objective lens. Calcein and PI fluorescences were visualized using FITC filters and Texas Red filters as described before (Beck et al. 2003). Images were collected using a Princeton Instruments MicroMax CCD camera. In a blind manner, a total of 1000 cells/condition were counted using MetaMorph image-processing software. Cell mortality was expressed as the ratio of PI-positive cells to the sum of calcein-positive and PI-positive cells.
Measurement of IP3 content
Following OGD/REOX, pure neuron cultures grown on coverslips were deproteinized in ice-cold OGD buffer containing 4% perchloric acid. The cell lystates were centrifuged (2000 g) and the resulting supernatant was neutralized with 1.5 M KOH in 60 mM HEPES. After centrifugation (2000 g), IP3 content in an 100 μL aliquot of the supernatant was analyzed with the D-myo-inositol 1,4,5-triphosphate biotrak assay kit from Amersham (Piscataway, NJ). The radioactivity in each sample was determined in a liquid scintillation counter. Protein content was measured in sister coverslips subjected to identical treatments. Data were expressed as pmol/mg protein.
Statistics
Statistical significance was determined by student's t-test or an ANOVA (Bonferroni post-hoc test) in the case of multiple comparisons. A P-value smaller than 0.05 was considered statistically significant. N values represent the number of cultures used in each experiment.
RESULTS
Changes in [Na+]cyt and [Ca2+]cyt are partially dependent on NKCC1 activity following OGD/REOX
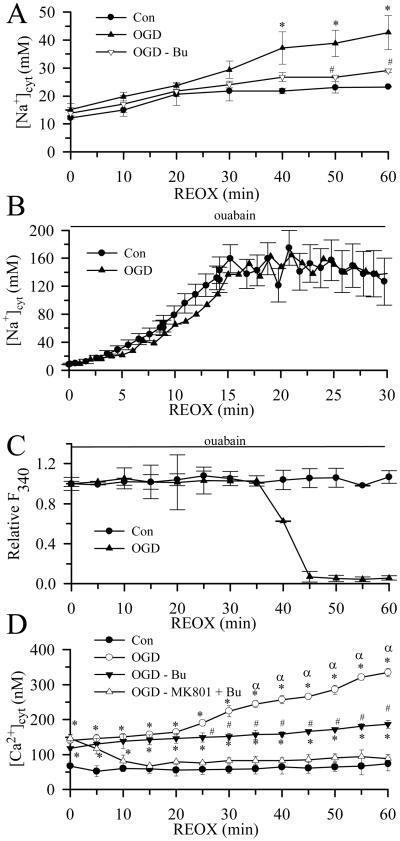
A small increase in [Na+]cyt occurred during 60 min of normoxic perfusion on the microscope stage (Figure 1 A). Two hours of OGD did not cause a significant increase in [Na+]cyt as compared to normoxic neurons (15.5 ± 1.1 vs. 11.5 ± 0.9 mM Figure 1 A). In contrast, REOX triggered a progressive rise in [Na+]cyt which reached 42.7 ± 6.1 mM after 60 min (p < 0.05). When NKCC1 was inhibited with 5 μM bumetanide, the increase in [Na+]cyt at 60 min REOX was significantly reduced (29.1 ± 0.6 mM, p < 0.05).
Figure 1. Changes of [Na+]cyt and [Ca2+]cyt in neurons during REOX.
[Na+]cyt (A, B) and [Ca2+]cyt (D) were monitored during 30-60 min REOX in single neurons following 2 h OGD. In studies with bumetanide, bumetanide (5 μM) was present during both OGD and REOX. In the double treatment study, (−)-MK-801 (10 μM) and bumetanide (5 μM) were added at 0 min REOX. Data are means ± SEM, n=3-4. Means were compared using a One-Way ANOVA with a Bonferroni post-hoc test. * p < 0.05 vs. Con; # p < 0.05 vs. non-treated OGD; α < 0.05 vs. 0 min REOX. In B, in ouabain (1 mM)-treated neurons, the data are expressed as means ± SD of 15-20 cells for each condition. (C) Ouabain-induced changes of relative SBFI fluorescence at 340 nm are shown. The sudden loss of fluorescence represents loss of plasma membrane integrity and cell death. Data are means ± SD of 2-3 cells for each condition.
[Na+]i levels measured in this study are the net result of a balance between Na+ efflux mediated by Na+-K+-ATPase and Na+ influx mediated by various influx pathways, including NKCC1. To illustrate the real Na+ rise, an experiment with 1 mM ouabain was performed. As shown in Figure 1 B, the rise of [Na+]i was 13.8 Na+ mM/min under normoxic conditions when Na+-K+-ATPase function was inhibited. The rate was not significantly changed (13.9 Na+ mM/min ) during the first 30 min REOX after 2 h OGD. However, over 40 min these cells died and the SBFI dye leaked out of cells due to the Na+ overload. This did not occur in normoxic control cells (Figure 1 C).
Sustained increases in [Na+]cyt activate NCXrev and lead to an increase in [Ca2+]cyt in astrocytes (Kintner et al. 2007). Thus, we monitored changes in [Ca2+]cyt at 2 h OGD and during 0-60 min REOX (Figure 1 D). 2 h OGD led to a ~ 2-fold increase in [Ca2+]cyt as compared to normoxic controls (140 ± 17 nM vs. 68 ± 5 nM). REOX triggered a significant secondary rise in [Ca2+]cyt at 30 min and reached 334 ± 12 nM at 60 min REOX (Figure 1 D, p < 0.05). Inhibition of NKCC1 with 5 μM bumetanide during OGD did not affect the OGD-induced increase in [Ca2+]cyt, but abolished the secondary rise in [Ca2+]cyt during 30-60 min REOX (187 ± 11 nM, p < 0.05). [Ca2+]cyt returned to basal level when NMDA receptors and NKCC1 were blocked during 0-60 min REOX with NMDA receptor antagonist (−)-MK-801 and bumetanide, respectively (Figure 1 D). These data suggest that the initial rise in [Ca2+]cyt is largely due to activation of NMDA receptors.
Changes in Ca2+ER following OGD/REOX in neurons
We then investigated whether Ca2+ER was affected by OGD/REOX. First, we examined whether the OGD-induced rise in [Ca2+]cyt would result in an accumulation of Ca2+ in Ca2+ER stores. 1 μM thapsigargin, an inhibitor of SERCA, was applied to neurons in the absence of extracellular Ca2+ to measure releasable Ca2+ from the Ca2+ER stores (Figure 2 A, B). Thapsigargin triggered a transient increase in [Ca2+]cyt that was resolved within 1-2 min (Figure 2 A). The integrated area (IACa2+) under the Ca2+ rise curve was 181 ± 41 nM×min. Following 2 h OGD, thapsigargin-induced changes in IACa2+ were significantly increased (344 ± 39 nM×min, p < 0.05, Figure 2 A, B). When NKCC1 was inhibited with 5 μM bumetanide, the thapsigargin-induced release of Ca2+ was markedly attenuated and not significantly different from normoxic controls. This suggests that OGD causes a significant Ca2+ loading in Ca2+ER stores and that involves NKCC1.
To further study kinetic changes in Ca2+ER, we directly measured Ca2+ER in neurons using the low-affinity Ca2+ dye mag-fura 2. The restriction of the mag-fura-2 signal to ER was confirmed by several experiments. First, as seen in Figure 2C, F345/385 for mag-fura 2 increased when the plasma membrane was permeablized with 30 sec exposure to saponin (top trace, a, b) thereby eliminating the cytosolic mag-fura-2 signal. Secondly, removal of ATP from the intracellular solution caused an abrupt decrease in F345/385, demonstrating that retention of Ca2+ER via SERCA depends on ATP (Figure 2C, lower trace). Moreover, application of FCCP to the permeablized cells caused a small increase in F345/385, presumably reflecting Ca2+ taken up by ER after the FCCP-triggered mitochondrial Ca2+ release (Figure 2D, top trace). In contrast, application of 1 μM thapsigargin resulted in an 85% loss in F345/385 (Figure 2D, lower trace). Subsequent application of FCCP did not cause an increase in the F345/385 because thapsigargin irreversibly inhibits SERCA. Taken together, these data strongly suggest that the F345/385 signal is primarily from ER.
Basal level of Ca2+ER in normoxic neurons was 8.6 ± 1.0 μM (Figure 2 E, F). Following 2 h OGD, Ca2+ER increased by ~ 2.5-fold (21.9 ± 2.1 μM, p < 0.05, Figure 2 E, F), which is consistent with the findings of the thapsigargin experiments described above. Interestingly, there were transient changes in Ca2+ER during 0-60 min REOX. 85% of the Ca2+ER in ischemic neurons was released at 15 REOX (3.1 ± 0.9 μM, Figure 2 F). Ca2+ER levels recovered at 30 min REOX and reached 9.5 ± 3.0 μM at 60 min REOX. In contrast, when 5 μM bumetanide was applied at the beginning of REOX, REOX failed to trigger the release of Ca2+ER (Figure 2 F). Ca2+ER remained overloaded at 15 min REOX (21.8 ± 2.4 μM). Only a small amount of Ca2+ER was slowly released during 30-60 min REOX (~ 16.2 μM). However, delayed application of 5 μM bumetanide (at 15 min REOX) failed to block the release of Ca2+ER and did not affect the subsequent refilling of Ca2+ER stores (Figure 2 F). In addition, inhibition of NCXrev with the NCX reverse-mode inhibitor SEA0400 (1 μM) during 15 min REOX also blocked the OGD/REOX-induced Ca2+ER release (Figure 2 F). Incubation of 5 μM bumetanide in normoxic neurons for 30 min did not significantly changed basal levels of Ca2+ER (11.3 ± 2.1 μM, n = 3, p > 0.05).
Bumetanide inhibits NKCC1, K+- Cl− cotransporters, and other anion transport processes (e.g., Cl−/HCO3− exchange, Cl− channels etc.) at higher concentrations. Although the concentration of bumetanide (5 or 10 μM) used in this study is relatively specific for NKCC1 (Russell 2000), the role of NKCC1 in changes of Ca2+ER was further confirmed in NKCC1−/− neurons. In contrast to an increase in Ca2+ER in NKCC1+/+ neurons at 2 h OGD (21.9 ± 2.1 μM), NKCC1−/− neurons exhibited significantly less Ca2+ER accumulation (16.4 ± 0.8 μM, n = 4, p < 0.05). At 15 min REOX, no significant release of Ca2+ER was detected in NKCC1−/− neurons (13.0 ± 2.2 μM, n = 4, p > 0.05). Taken together, these data suggest that OGD/REOX transiently perturbs Ca2+ER homeostasis. NKCC1 activity not only contributes to Ca2+ER overload during OGD, but also to the subsequent Ca2+ER release. This further supports the view that activation of NKCC1/NCXrev is involved in the Ca2+ER accumulation and subsequent release of Ca2+ER.
Inhibition of IP3 receptors prevents OGD/REOX-mediated Ca2+ER release
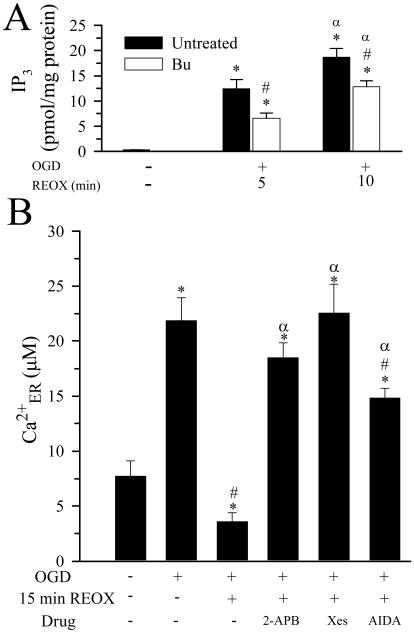
Release of Ca2+ER following REOX could be mediated through several pathways including activation of IP3 receptors (IP3R). To investigate this possibility, we first determined if IP3 levels in neurons were elevated during early REOX. Basal level of IP3 in pure neuronal cultures averaged 0.32 ± 0.01 pmol/mg protein, but increased dramatically at 5 min REOX (12.8 ± 1.4 pmol/mg protein (Figure 3 A). IP3 levels were further increased to 18.7 ± 1.7 pmol/mg protein at 10 min REOX. In the presence of 5 μM bumetanide, the OGD/REOX-mediated increase in IP3 was decreased by ~ 50% at 5 min REOX (6.6 ± 1.04 pmol/mg protein). IP3 remained significantly reduced at 10 min REOX when NKCC1 was inhibited by bumetanide (Figure 3 A).
Figure 3. IP3 formation and its role in OGD/REOX-induced Ca2+ER release.
A. IP3 levels in pure neuronal cultures were determined under basal conditions and at 5 or 10 min REOX. In bumetanide experiments, bumetanide was present during OGD and REOX. Data are means ± SEM. n = 3. Means were compared with a student's t-test. * p < 0.05 vs. Con; # p < 0.05 vs. untreated; α p < 0.05 vs. 10 min REOX. B. IP3 receptor antagonists 2-APB (100 μM) or xestospongin C (20 μM) or the mGluR I antagonist AIDA (100 μM) were applied at the end of OGD. Ca2+ER was determined at 15 min of REOX. Data are means ± SEM. n = 3-4. Means were compared using a One-Way ANOVA with a Bonferroni post-hoc test. * p < 0.05 vs. Con; # p < 0.05 vs. 0 min REOX; α p < 0.05 vs. 15 min REOX.
We then investigated whether inhibition of the IP3R could affect Ca2+ER release after OGD/REOX. When IP3R were inhibited during 0-15 min REOX with 2-APB, REOX-induced release of Ca2+ER was blocked (~ 18.5 μM, Figure 3 B). To further establish the role of IP3R in REOX-mediated Ca2+ER release, xestospongin C, a more potent inhibitor of IP3R, was used. Xestospongin C (20 μM) applied during 0-15 min REOX completely prevented the REOX-mediated release of Ca2+ER (~ 22.0 μM, Figure 3 B).
IP3 production can be stimulated via activation of group I metabotropic glutamate receptors (mGluR I) and subsequent stimulation of phospholipase C (PLC). We further investigated the role of mGluR I in REOX-mediated Ca2+ER release. Inhibition of mGluR I with the specific mGluR I antagonist AIDA (100 μM) during 0-15 min of REOX blocked ~ 66% of the REOX-induced release of Ca2+ER (Figure 3 B). Taken together, the data suggest that IP3 production and activation of IP3R lead to Ca2+ER release following OGD/REOX.
Inhibition of NKCC1 activity reduces OGD/REOX-induced ER stress in neurons
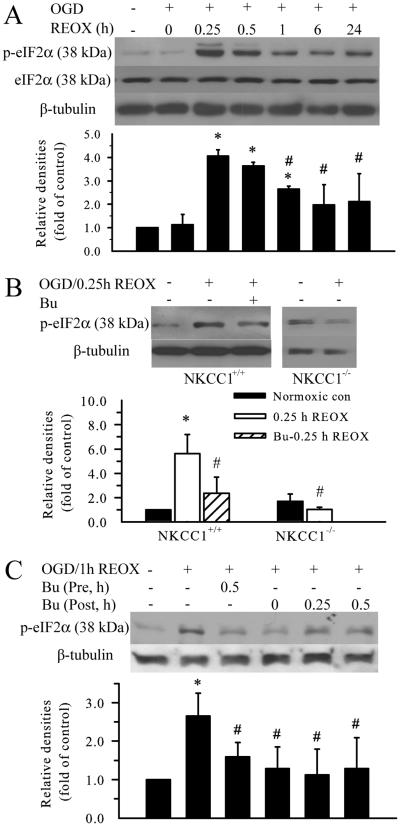
To elucidate the role of Ca2+ER dysregulation in development of ER stress following OGD/REOX, expression of several ER stress markers was examined. Phosphorylation of eIF2α can be triggered by RNA-dependent protein kinase-like ER eIF2α kinase (PERK) activation following cerebral ischemia and reperfusion (Kumar et al. 2001; Paschen 2003). In the current study, the level of p-eIF2α did not change at 0 h REOX, but was dramatically increased at 0.25, 0.5, and 1 h REOX (p < 0.05, Figure 4 A). The peak level of p-eIF2α signal was ~ 4 fold of control at 0.25 h REOX. It remained elevated by ~ 2.5 fold at 1 h REOX and above the resting level at 24 h REOX (Figure 4 A). Interestingly, inhibition of NKCC1 with bumetanide during OGD and REOX (pretreatment) significantly attenuated OGD/REOX-mediated up-regulation of p-eIF2α (p < 0.05, Figure 4 B). Moreover, addition of bumetanide at 0 min REOX was effective in reducing p-eIF2α levels at 1 h REOX (Figure 4 C). Inhibition of NKCC1 starting at 15 min or 30 min REOX still had some protective effects. Consistent with the bumetanide result, NKCC1−/− neurons did not show up-regulation of p-eIF2α following OGD/REOX (Figure 4 B). Taken together, these results indicate that early REOX triggers ER stress and inhibition of NKCC1 activity reduces the level of p-eIF2α.
Figure 4. Inhibition of NKCC1 activity attenuates OGD/REOX-mediated upregulation of p-eIF2α.
A. Expression of p-eIF2α protein was determined at 2 h OGD, or 0.25, 0.5, 1, 6, and 24 h REOX. Levels of total eIF2α and β tubulin were shown for loading controls. The bar graph represents the relative expression of p-eIF2α protein estimated from the mean pixel density normalized to β tubulin. B. Effect of NKCC1 inhibitor bumetanide or gene ablation of NKCC1 on p-eIF2α protein expression. NKCC1+/+ neurons were treated with 10 μM bumetanide 30 min prior to OGD and the drug was present in all subsequent incubations. In gene ablation studies, NKCC1+/+ or NKCC1−/− neuronal cultures were prepared from NKCC1+/+ and NKCC1−/− littermates. The mean data of the NKCC1+/+ cultures were then pooled together with the original data sets, so systematic differences were reduced to the minimum. Data in A-B are means ± SD. n = 3. * p < 0.05 vs. NKCC1+/+ con; # p < 0.05 vs. NKCC1+/+ 0.25 h REOX. C. Bumetanide post-ischemia treatment. 10 μM bumetanide was applied at 0, 0.25 or 0.5 h REOX. Expression of p-eIF2α protein was determined at 1 h REOX. Data are means ± SD. n = 3. Means were compared using a One-Way ANOVA with a Bonferroni post-hoc test. * p < 0.05 vs. con; # p < 0.05 vs. 1 h REOX.
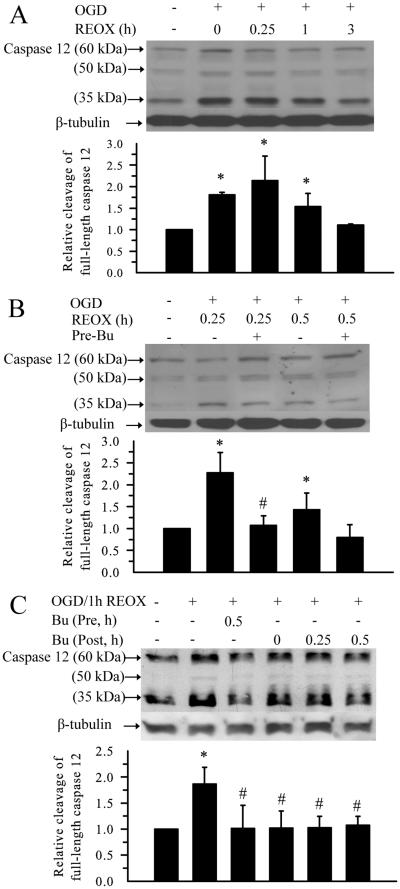
We also studied activation of caspase 12, a marker for ER stress-dependent apoptotic pathway (Glazner and Fernyhough 2002). Both procaspase 12 (60 kDa band) and the cleaved forms of caspase 12 (35 kDa and 50 kDa) were recognized by the anti-caspase12 antibody (Figure 5 A, B). The relative ratio of 35 plus 50 kDa /60 kDa intensity was calculated to reflect caspase 12 activation. 2 h OGD triggered caspase 12 activation by 80%. The level of cleaved caspase 12 remained elevated during 15-60 min REOX. It returned to control levels by 3 h REOX (Figure 5 A, B). Treatment of neurons with bumetanide during OGD and REOX significantly reduced the activation of caspase 12 during 15-30 min REOX. Moreover, addition of bumetanide at 0, 15 or 30 min REOX was effective in reducing the activation of caspase 12 (Figure 5 C). Taken together, our results demonstrate that inhibition of NKCC1 not only attenuates the dysregulation of Ca2+ER homeostasis during early REOX but also concurrently reduces caspase 12 cleavage following OGD/REOX.
Figure 5.
Inhibition of NKCC1 activity reduces OGD/REOX-mediated caspase 12 activation
A. Changes of caspase 12 protein at 2 h OGD and 0.25, 1, or 3 h REOX. Relative activation of caspase 12 was determined by the ratio of the cleavage bands (35 kDa + 50 kDa) vs. 60 kDa full length band. Sister cultures were incubated in normoxic buffers as control. B. Effect of bumetanide pre-ischemia treatment on the caspase 12 protein expression. Cells were pretreated with 10 μM bumetanide for 30 min and the drug was present in all subsequent incubations. Cells were then collected at either 0.25 h or 0.5 h REOX. Data are means ± SD. n = 3-4. Means were compared using a One-Way ANOVA with a Bonferroni post-hoc test. * p < 0.05 vs. Con; # p < 0.05 vs. 0.25 h REOX. C. Effect of bumetanide post-ischemia treatment on caspase 12 protein expression. 10 μM bumetanide was applied to cultures at 0, 0.25 or 0.5 h REOX. Cells were collected at 1 h REOX. Data are means ± SD. n = 3. * p < 0.05 vs. Con; # p < 0.05 vs. 1 h REOX.
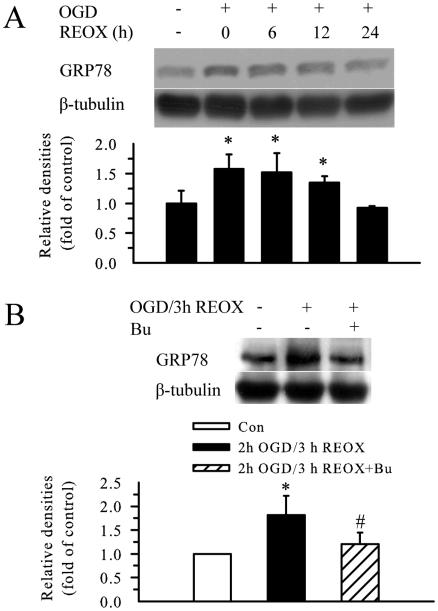
GRP78 protein, a commonly used marker for ER stress, was up-regulated by ~ 50 % at 2 h OGD. This elevation continued at 3, 6, and 12 h REOX (p < 0.05, Figure 6 A, B). GRP78 protein level returned to baseline by 24 h REOX. Inhibition of NKCC1 activity during OGD and REOX blocked the up-regulation of GRP78 protein (p < 0.05, Figure 6 B).
Figure 6. Inhibition of NKCC1 activity reduces OGD/REOX-mediated GRP78 upregulation.
A. Expression of GRP78 protein at 2 h OGD or at 6, 12, and 24 h REOX. The bar graph represents the relative expression in GRP78 protein normalized to β tubulin. B. Effect of bumetanide pre-ischemia treatment on GRP78 protein expression. Data are means ± SD. n = 3. Means were compared using a One-Way ANOVA with a Bonferroni post-hoc test. * p < 0.05 vs. Con.; # p < 0.05 vs. REOX.
Prevention of Ca2+ER dysregulation is neuroprotective
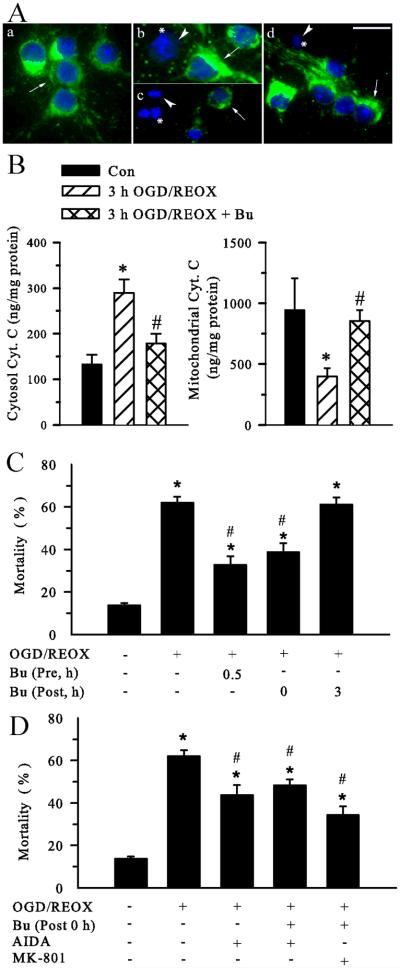
We further investigated whether prevention of Ca2+ dysregulation reduces neuronal damage. First, we determined Cyt. C release. Normoxic control neurons express an abundant level of Cyt. C with a punctuate and perinuclear expression pattern (Figure 7 A a, green, arrow). Some punctate Cyt. C staining was also observed in neurites. DAPI staining shows normal nuclear morphology (Figure 7 A a, blue). In contrast, after 3 h OGD/1 h REOX, Cyt. C immunoreactive signals became diffuse in the cytosol in some neurons (Figure 7 A b, arrowhead). Moreover, some neurons lost Cyt. C immunoreactivity (* in Figure 7 A b). After 3 h OGD/21 h REOX, Cyt. C staining could not be detected in most neurons (Figure 7 A c). These neurons concurrently exhibited condensed or fragmented chromatin in the nuclei (* in Figure 7 A b & c). The loss of Cyt. C immunoreactive signals suggests that Cyt. C may be released from mitochondria and subsequently degraded following OGD/REOX. However, when NKCC1 activity was inhibited with 10 μM bumetanide, OGD/REOX-triggered Cyt. C release was significantly attenuated. Figure 7 A (d) illustrates strong Cyt. C immunoreactive signals in the bumetanide treated neurons with near normal nuclear morphology (Figure 7 A d, arrow).
Figure 7. Inhibition of NKCC1 activity reduces OGD/REOX-mediated Cyt. C release and neuronal death.
A. Cyt. C release in neurons was visualized by immunofluorescence staining. a - d: double staining with the Cyt. C antibody and DAPI. Images shown are representative of 4 experiments. For bumetanide treatment, neurons were incubated in EMEM in the presence of 10 μM Bu at 37°C during 3 h OGD and 21 h REOX. Sister cultures were incubated for 24 h in normoxic control buffers as controls (Con). a. Con. b. 3 h OGD/1 h REOX. c. 3 h OGD/21 h REOX. d. 3 h OGD/21 h REOX plus 10 μM Bu. Scale bar, 20 μm. Arrow: cells with retained Cyt. C; Arrowhead: loss of Cyt. C; *: nuclei displayed with condensed or fragmented chromatin. B. Cyt. C content in cytosolic fraction (left) and mitochondrial fraction (right) was determined in Con, 3 h OGD/21h REOX, or 3 h OGD/21h REOX plus Bu. Data are means ± SD. n = 3. Means were compared using a One-Way ANOVA with a Bonferroni post-hoc test. * p < 0.05 vs. Con; # p < 0.05 vs. OGD/REOX. C. Neuronal protection with bumetanide (10 μM) during OGD or REOX. Cell mortality was assessed after 2 h OGD and 22 h REOX. D. No additive effects with AIDA or (−)-MK-801. For drug treatment, neurons were incubated in EMEM containing 100 μM AIDA or 10 μM (−)-MK-801 during 0-22 h REOX. Data are means ± SEM. n = 4-8. * p < 0.05 vs. Con. # p < 0.05 vs. OGD/REOX.
To further quantify Cyt. C release, we measured Cyt. C content from cytosolic and mitochondrial fractions with an immunoassay. As shown in Figure 7 B, under control conditions, Cyt. C content in the cytosol was low (133 ± 21 ng/mg protein). OGD/REOX treatment led to a nearly ~ 2 fold increase in the cytosolic Cyt. C (289 ± 30 ng/mg protein). In contrast, mitochondrial Cyt.C was significantly decreased (399 ± 68 ng/mg protein, p < 0.05, Figure 7 B), compared to normoxic controls (944 ± 262 ng/mg protein). In the presence of bumetanide, Cyt. C release into cytosol was almost blocked (178 ± 22 ng/mg protein, p < 0.05). Consist with this, inhibition of NKCC1 activity with bumetanide prevented the decrease in the mitochondrial Cyt.C (854 ± 89 ng/mg protein, p < 0.05, Figure 7 B). Taken together, the data of immunostaining and immunoassay for Cyt. C consistently demonstrate that inhibition of NKCC1 activity preserves mitochondrial integrity in neurons following OGD/REOX.
We then examined the effects of NKCC1 or mGluR I inhibition on neuroprotection following OGD/REOX. A low level of cell death occurred in normoxic control neurons (13.8 ± 1.0 %). 2 h OGD and 22 h REOX triggered a significant increase in cell mortality (65.1 ± 2.8 %, p < 0.05, Figure 7 C). Inhibition of NKCC1 activity with 10 μM bumetanide either prior to OGD (32.7 ± 4.0 %, p < 0.05) or only during 0-24 h REOX (38.7 ± 4.2 %, p < 0.05) significantly attenuated OGD/REOX-mediated neuronal death. However, addition of bumetanide at 3 h REOX failed to reduce ischemic cell death.
Inhibition of mGluR I with 100 μM AIDA during 0-24 h REOX caused similar protection as bumetanide (43.3 ± 3.4 %, p < 0.05, Figure 7 D). Compared to bumetanide treatment alone, no additive effects were found in neuroprotection when either AIDA or 10 μM (-)-MK-801 was added together with bumetanide during 0-24 h REOX (Figure 7 D). These findings suggest that NKCC1 activity is important for neuronal damage during early REOX.
Thermodynamic analysis of NCXrev
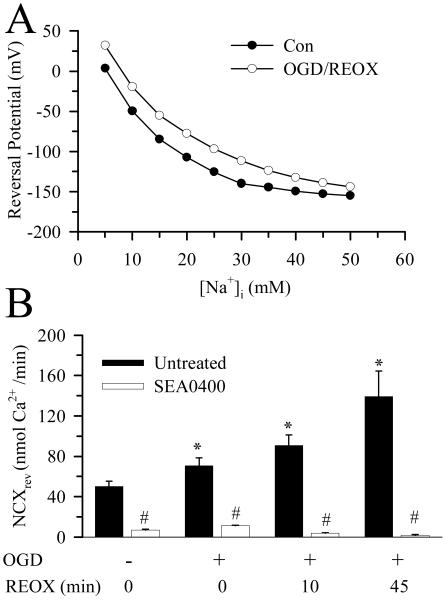
To further support the role of NCXrev in Ca2+ homeostasis, we performed theoretical thermodynamic analysis of NCXrev in neurons with LabHEART [version 4.9.5 simulation software; (Puglisi and Bers 2001)]. The key variables, [Na+]o, [Ca2+]o, [Na+]cyt, and [Ca2+]cyt, were modified with our experimental data under normoxic control and 60 min REOX. The membrane voltage at which each simulation generated zero current was then plotted against [Na+]cyt (Figure 8 A). Under control conditions, with a [Ca2+]cyt of 68 nM, and [Na+]cyt of 10 mM in neurons, the model predicts a reversal potential of −50 mV. At ~ 60 mV resting plasma membrane potential in cultured cortical neuron (Zhu et al. 2005), the forward-mode of NCX would be favored. At 60 min REOX, [Ca2+]cyt rises to 334 nM. Although this increase in [Ca2+]cyt would further favor the forward-mode operation of NCX, the significant concurrent increase in [Na+]cyt strongly favors an inwardly directed Ca2+ current via NCXrev (Figure 8 A). The simulation predicts that even if plasma membrane potential remains at −60 mV, NCX will function in the reverse mode when [Na+]cyt increases to ~ 19 mM with [Ca2+]cyt of 334 nM.
Figure 8. NCXrev and its dependence on intracellular Na+.
A. The NCX module of the LabHEART version 4.9.5 software was used to model changes in NCX reversal potential with intracellular Na+ under control and OGD/REOX conditions. It is assumed that NCX is an electrogenic mechanism that transports Na+ and Ca2+ with a stoichiometry of 3:1. Values of Na+ and Ca2+ affinities, voltage saturation, and the position of the energy barrier were set at the default values for the simulator. I-V curves for NCX were generated with known values of extracellular Na+ and Ca2+ (Methods) and intracellular Ca2+ and Na+ values (Figure 1). For each intracellular Na+ value, the reversal potential was taken as the voltage when NCX current equaled zero. B. NCXrev was determined under normoxic conditions and at 0, 10, and 60 min REOX following 2 h OGD. In SEA0400 experiments, SEA0400 (1 μM) was present throughout the NCXrev determination period. Data are the means ± SD of 15-20 cells from one coverslip for each condition. Means were compared using a One-Way ANOVA with a Bonferroni post-hoc test. * p < 0.05 vs. con, # p < 0.05 vs. non-treated.
We also directly determined NCXrev in neurons at 0, 10 and 45 min REOX following 2 h OGD (Figure 8 B). When NCXrev was initiated in a Na+-free/low Ca2+ buffer, Ca2+ influx rate was ~ 50 nM/min. In the presence of SEA0400 (1 μM), NCXrev was reduced by 90 % (Figure 8 B). Following 2 h OGD, NCXrev activity was increased by ~ 40%. 10 min REOX led to ~ 80% increase in NCXrev activity, which was doubled at 45 min REOX. This activation of NCXrev during post-OGD was nearly abolished with the NCXrev inhibitor SEA0400 (Figure 8 B). These data are consistent with the temporal changes of [Na+]cyt as described above. Taken together, the role of NCXrev in Ca2+ homeostasis following in vitro ischemia is supported by these studies.
DISCUSSION
Dysregulation of Ca2+ER in cortical neurons following OGD/REOX
The disruption of Ca2+ER homeostasis has been linked to numerous pathological conditions including neuronal death (Glazner and Fernyhough 2002). Depletion of Ca2+ER has been suggested as an initial signal for ER dysfunction in ischemic neurons, based on high IP3R density and low SERCA in the vulnerable regions of the hippocampus, and expression of ER-resident stress proteins similar to that seen following thapsigargin-induced Ca2+ER depletion (Paschen and Doutheil 1999). However, kinetic changes of Ca2+ER during ischemia and post-ischemia are not well understood.
In the current study, we observed that 2 h OGD led to ~2 fold increase in thapsigargin-induced Ca2+ release, which implies that OGD causes an increase in Ca2+ER loading in neurons. Direct measurement of Ca2+ER by the ER Ca2+ fluorescence dye mag-fura 2 revealed an ~ 3 fold increase in Ca2+ER at the end of 2 h OGD. The results from the two different methods are consistent and in agreement with the well defined function of ER in buffering of Ca2+cyt. Simulations from a quantitative model predict that mild depolarization in bullfrog sympathetic neurons would result in Ca2+cyt elevation to 300-400 nM and an increase in Ca2+ER (Albrecht et al. 2002). Our finding directly demonstrates that accumulation of Ca2+ER occurs when the Ca2+cyt level is moderately elevated during OGD.
Many studies with indirect measurement of Ca2+ER reveal that there is a release of Ca2+ER in ischemic neurons. When Ca2+ER is depleted with cyclopiazonic acid or Ca2+ER release is blocked by ryanodine receptor inhibition prior to OGD, aspiny interneurons show less increase in Ca2+cyt (Pisani et al. 2000). These data suggest that a portion of the increase in Ca2+cyt originates from ER under severe metabolic stress conditions.
In the current study, significant elevation of IP3 content was detected in neurons during 5-10 min REOX. 85% loss of Ca2+ER occurred within 15 min REOX. Inhibition of the IP3R with either 2-APB or xestospongin C blocked the Ca2+ER release. This implies that Ca2+ER release is largely mediated by IP3R activation during early REOX. Because activation of group I mGluR coupled with PLC can stimulate IP3 production and release of Ca2+ from ER (Bruno et al. 2001), we investigated whether inhibition of group I mGluR affected Ca2+ER release. Indeed, group I mGluR antagonist AIDA nearly blocked OGD/REOX-mediated Ca2+ER release. Moreover, we found that treatment of neurons with AIDA during 0-24 h REOX caused significant neuroprotection. Neuroprotective effects of inhibition of group I mGluRs have been shown in numerous reports (Bruno et al. 2001; Allen et al. 2000). Our study further suggests that group I mGluRs may be involved in ischemic damage in part via triggering IP3R-mediated Ca2+ER release and ER dysfunction (Figure 9).
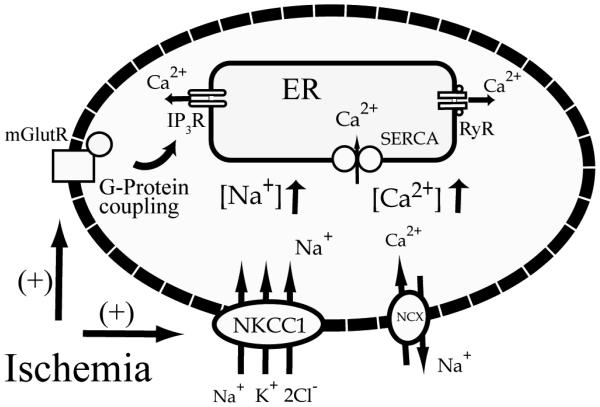
Figure 9. A proposed mechanism for a role of NKCC1 and NCXrev in Ca2+ER dysregulation following in vitro ischemia.
Following ischemia, activation of NKCC1 causes an increase in [Na+]cyt which triggers NCXrev and leads to increases in [Ca2+]cyt. The [Ca2+]cyt overload is initially buffered by SERCA and results in Ca2+ER overload during ischemia. Ca2+ER release subsequently occurs via activation of IP3R and mGluR-mediated pathways.
Role of NKCC1/NCXrev in disruption of Ca2+ER following OGD/REOX
NCXrev-mediated intracellular Ca2+ overload following [Na+]cyt elevation has been suggested as a contributing mechanism leading to neuronal death (Hoyt et al. 1998), axonal damage (Li et al. 2000), and astrocyte dysfunction (Kintner et al. 2006). On the other hand, it has been proposed that NCX operates in the forward mode to clear Ca2+ in astrocytes from neonatal rat optic nerve during OGD/REOX (Fern 1998). In this study, we found that inhibition of NKCC1 during OGD prevented Ca2+ER accumulation. This is because NKCC1-mediated Na+ flux may trigger Ca2+ influx through NCXrev and contribute to Ca2+ER loading (Figure 9). This possibility has been confirmed with thermodynamic modeling analysis and NCXrev measurement (Figure 8). Our current findings suggest that coupling of NKCC1/NCXrev may play a role in Ca2+ER release during REOX (Figure 9). We found that either application of bumetanide at 0 min REOX or genetic ablation of NKCC1 abolished the release of Ca2+ER. The role of NCXrev is further supported by our data with the NCXrev inhibitor SEA0400.
It has been reported that a significant portion of ischemic axoplasmic Ca2+ increase originates from the ER and the Ca2+ release is strongly dependent on axonal Na+ influx (Nikolaeva et al. 2005). The authors speculate that ischemic Na+ influx promotes glutamate release, which in turn stimulates mGluRs, activates PLC, and generates IP3 (Nikolaeva et al. 2005). Although our data are consistent with the report on the dependency of Ca2+ER release on accumulation of Na+cyt and activation of IP3R, the exact underlying cellular mechanisms which involve NKCC1/NCXrev remain to be determined. We speculate that inhibition of NKCC1/NCXrev reduces Na+cyt, Ca2+cyt, and swelling (Figure 9). Presumably, these changes will attenuate glutamate release from both vesicle-mediated (Ca2+-dependent) and swelling-induced (volume-sensitive organic anion channels) pathways. Therefore, when NKCC1 activity is inhibited by bumetanide, a reduced release of glutamate would lessen activation of mGluRs and subsequent IP3 formation following ischemia. This view is supported by our IP3 data. Moreover, inhibition of NKCC1 activity either pharmacologically or by genetic ablation significantly attenuates swelling of astrocytes and neurons as well as glutamate release from astrocytes (Su et al. 2002; Beck et al. 2003).
It has been suggested that IP3R-mediated Ca2+ER release can enter the adjacent mitochondria and trigger Cyt. C release (Rizzuto and Pozzan 2006). Cyt. C binds to IP3R and further augment Ca2+ER release by inhibiting Ca2+-mediated inhibition of IP3R (Hara and Snyder 2007). This feed-forward cycle of Ca2+ transmission can cause synchronized Ca2+ ER release and apoptosis (Hara and Snyder 2007). We detected Cyt. C release at 1 h REOX following OGD and inhibition of NKCC1 with bumetanide not only blocked the Ca2+ER release but also attenuated Cyt. C release and neuronal death. However, we did not monitor the time-dependent release of Cyt. C during the entire REOX period. It remains to be determined whether the feed-forward cycle plays a role in Ca2+ER release following OGD/REOX.
ER stress following OGD/REOX
Depletion of Ca2+ER stores with thapsigargin can lead to ER stress (Paschen and Doutheil 1999), which induces suppression of protein synthesis, polyribosomal disaggregation, and activation of ER stress genes in neurons (Doutheil et al. 1997; Mengesdorf et al. 2001). This ER stress response is almost identical to the responses found in transient cerebral ischemia (Paschen et al. 1996). GRP78 is detached from PERK by 1-4 hour reperfusion following 30-min focal ischemia, which is accompanied by a significant increase in p-eIF2α (Hayashi et al. 2004). After 6 h reperfusion, the level of p-eIF2α is three fold higher than basal levels (Althausen et al. 2001). PERK is also activated by 10-min reperfusion following 10-min global ischemia (Kumar et al. 2003). PERK is the only kinase to phosphorylate eIF2α in ischemic brain at least during the early stage of reperfusion (DeGracia et al. 1999; Kumar et al. 2001). However, it is unknown whether this is the case in primary neurons after OGD/REOX.
In the current study, p-eIF2α signals were not changed during 2 h OGD but dramatically increased upon REOX. The p-eIF2α signals reached a peak level at 0.25 h REOX and remained elevated until 24 h REOX, which is different from in vivo ischemia (Althausen et al. 2001). This sustained elevation in p-eIF2α in neurons suggests that some type of stress, which is independent of intracellular ATP level, may be involved. We have reported that ATP is reduced by ~ 85% at 2 h OGD (Luo et al. 2005) and returned to ~ 50 % of control by 1 h REOX (data not shown). These changes in ATP levels are similar in differentiated PC12 cells, however, 4 h OGD in PC12 cells leads to a transient increase in p-eIF2α that then returns to the basal level by 30 min REOX (Munoz et al. 2000). This suggests that primary cortical neurons have less tolerance to ischemic insult and exhibit sustained UPR following OGD.
We observed that 2 h OGD led to 80% activation of caspase 12, which remained elevated during 15-60 min REOX. GRP78 protein elevation and caspase 12 activation occurred at 2 h OGD when Ca2+ER was significantly loaded. The increase of p-eIF2a level did not occur until Ca2+ER was depleted during REOX. These data imply that altered Ca2+ER homeostasis (either overload or depletion) may trigger ER dysfunction and ER stress.
Inhibition of NKCC1 activity with its inhibitor bumetanide during OGD and REOX significantly reduced the up-regulation of GRP78, expression of p-eIF2α, and activation of caspase 12 during REOX. Similar results of p-eIF2α were found in NKCC1−/− neurons. These data suggest that NKCC1 activation is involved in ER stress development. Bumetanide pretreatment-mediated effects on ER stress may result from less Ca2+ER loading during OGD and subsequent less release of Ca2+ER during REOX. Moreover, inhibition of NKCC1 during REOX also shows some protective effects. This may result from less glutamate release and retention of Ca2+ER as discussed above.
In the case of GRP78, 2 h OGD induced 50 % up-regulation of GRP78 protein, which remained elevated during 1-12 h REOX. Multiple mechanisms, including glucose deprivation, govern GRP78 expression (Shiu et al. 1977; Shintani-Ishida et al. 2006). The OGD-mediated upregulation of GRP78 may in part result from both glucose deprivation and changes of Ca2+ER. Moreover, the high p-eIF2α levels during REOX may inhibit protein synthesis and could preclude further increases in GRP78 expression.
In summary, we report here that biphasic changes in Ca2+ER were triggered in neurons following OGD and REOX. NKCC1 and NCXrev are involved in the overload of Ca2+ER during OGD and IP3R-mediated release of Ca2+ER during REOX. The changes of Ca2+ER were concurrently accompanied with elevation of several ER stress markers such as p-eIF2α, caspase 12 cleavage, and GRP78. Reduction of Ca2+ER disruption by blocking of NKCC1/NCXrev significantly attenuated ER stress proteins and cell death.
The abbreviations used
- Cyt. C
cytochrome C
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- GRP78
glucose regulated protein 78
- ICS
intracellular solution
- IP3
inositol 1,4,5-trisphosphate
- IP3R
inositol 1,4,5-trisphosphate receptor
- mGluR I
group I metabotropic glutamate receptor
- NCX
Na+/Ca2+ exchanger
- NCXrev
reversed mode operation of NCX
- NKCC1
Na+-K+-Cl− cotransporter isoform 1
- OGD
Oxygen and glucose deprivation
- PERK
RNA-dependent protein kinase-like ER kinase
- PLC
phospholipase C
- REOX
reoxygenation
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- UPR
unfolded protein response
Footnotes
We would like to thank Dr. Donald J. DeGracia for many helpful discussions and reading the manuscript. This work was supported in part by NIH grants R01NS38118 and R01NS048216 (D. Sun), and an AHA Established-Investigator award grant N0540154 (D. Sun).
REFERENCES
- Albrecht MA, Colegrove SL, Friel DD. Differential regulation of ER Ca2+ uptake and release rates accounts for multiple modes of Ca2+-induced Ca2+ release. J Gen Physiol. 2002;119:211–233. doi: 10.1085/jgp.20028484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JW, Knoblach SM, Faden AI. Activation of group I metabotropic glutamate receptors reduces neuronal apoptosis but increases necrotic cell death in vitro. Cell Death Differ. 2000;7:470–476. doi: 10.1038/sj.cdd.4400678. [DOI] [PubMed] [Google Scholar]
- Althausen S, Mengesdorf T, Mies G, Olah L, Nairn AC, Proud CG, Paschen W. Changes in the phosphorylation of initiation factor eIF-2 alpha, elongation factor eEF-2 and p70 S6 kinase after transient focal cerebral ischaemia in mice. J Neurochem. 2001;78:779–787. doi: 10.1046/j.1471-4159.2001.00462.x. [DOI] [PubMed] [Google Scholar]
- Beck J, Lenart B, Kintner DB, Sun D. Na-K-Cl cotransporter contributes to glutamate-mediated excitotoxicity. J Neurosci. 2003;23:5061–5068. doi: 10.1523/JNEUROSCI.23-12-05061.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Copani A, D'Onofrio M, Di Iorio P, De Blasi A, Melchiorri D, Flor PJ, Nicoletti F. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab. 2001;21:1013–1033. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Chen H, Luo J, Kintner DB, Shull GE, Sun D. Na+-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:54–66. doi: 10.1038/sj.jcbfm.9600006. [DOI] [PubMed] [Google Scholar]
- Corbett EF, Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem Sci. 2000;25:307–311. doi: 10.1016/s0968-0004(00)01588-7. [DOI] [PubMed] [Google Scholar]
- DeGracia DJ, Adamczyk S, Folbe AJ, Konkoly LL, Pittman JE, Neumar RW, Sullivan JM, Scheuner D, Kaufman RJ, White BC, Krause GS. Eukaryotic initiation factor 2 alpha kinase and phosphatase activity during postischemic brain reperfusion. Exp Neurol. 1999;155:221–227. doi: 10.1006/exnr.1998.6986. [DOI] [PubMed] [Google Scholar]
- DeGracia DJ, Hu BR. Irreversible translation arrest in the reperfused brain. J Cereb Blood Flow Metab. 2007;27:875–893. doi: 10.1038/sj.jcbfm.9600388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
- Doutheil J, Gissel C, Oschlies U, Hossmann KA, Paschen W. Relation of neuronal endoplasmic reticulum calcium homeostasis to ribosomal aggregation and protein synthesis: implications for stress-induced suppression of protein synthesis. Brain Res. 1997;775:43–51. doi: 10.1016/s0006-8993(97)00899-8. [DOI] [PubMed] [Google Scholar]
- Fern R. Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ. J Neurosci. 1998;18:7232–7243. doi: 10.1523/JNEUROSCI.18-18-07232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Fernyhough P. Neuronal survival in the balance: are endoplasmic reticulum membrane proteins the fulcrum? Cell Calcium. 2002;32:421–433. doi: 10.1016/s014341600200194x. [DOI] [PubMed] [Google Scholar]
- Groenendyk J, Michalak M. Endoplasmic reticulum quality control and apoptosis. Acta Biochim Pol. 2005;52:381–395. [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hara MR, Snyder SH. Cell signaling and neuronal death. Annu Rev Pharmacol Toxicol. 2007;47:117–141. doi: 10.1146/annurev.pharmtox.47.120505.105311. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Chan PH. Oxidative injury to the endoplasmic reticulum in mouse brains after transient focal ischemia. Neurobiol Dis. 2004;15:229–239. doi: 10.1016/j.nbd.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Chan PH. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J Cereb Blood Flow Metab. 2005;25:41–53. doi: 10.1038/sj.jcbfm.9600005. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Nishi T, Maier CM, Kinouchi H, Chan PH. Oxidative damage to the endoplasmic reticulum is implicated in ischemic neuronal cell death. J Cereb Blood Flow Metab. 2003;23:1117–1128. doi: 10.1097/01.WCB.0000089600.87125.AD. [DOI] [PubMed] [Google Scholar]
- Hoyt KR, Arden SR, Aizenman E, Reynolds IJ. Reverse Na+/Ca2+ exchange contributes to glutamate-induced intracellular Ca2+ concentration increases in cultured rat forebrain neurons. Mol Pharmacol. 1998;53:742–749. [PubMed] [Google Scholar]
- Kintner DB, Luo J, Gerdts J, Ballard AJ, Shull GE, Sun D. Na+-K+-Cl− cotransport and Na+/Ca2+ exchange in astrocyte mitochondrial dysfunction in response to in vitro ischemia. J Neurosci. 2006 doi: 10.1152/ajpcell.00412.2006. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Luo J, Gerdts J, Ballard AJ, Shull GE, Sun D. Role of Na+-K+-Cl− cotransport and Na+/Ca2+ exchange in mitochondrial dysfunction in astrocytes following in vitro ischemia. Am J Physiol Cell Physiol. 2007;292:C1113–C1122. doi: 10.1152/ajpcell.00412.2006. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Su G, Lenart B, Ballard AJ, Meyer JW, Ng LL, Shull GE, Sun D. Increased tolerance to oxygen and glucose deprivation in astrocytes from Na+/H+ exchanger isoform 1 null mice. Am J Physiol Cell Physiol. 2004;287:C12–C21. doi: 10.1152/ajpcell.00560.2003. [DOI] [PubMed] [Google Scholar]
- Kumar R, Azam S, Sullivan JM, Owen C, Cavener DR, Zhang P, Ron D, Harding HP, Chen JJ, Han A, White BC, Krause GS, DeGracia DJ. Brain ischemia and reperfusion activates the eukaryotic initiation factor 2 alpha kinase, PERK. J Neurochem. 2001;77:1418–1421. doi: 10.1046/j.1471-4159.2001.00387.x. [DOI] [PubMed] [Google Scholar]
- Kumar R, Krause GS, Yoshida H, Mori K, DeGracia DJ. Dysfunction of the unfolded protein response during global brain ischemia and reperfusion. J Cereb Blood Flow Metab. 2003;23:462–471. doi: 10.1097/01.WCB.0000056064.25434.CA. [DOI] [PubMed] [Google Scholar]
- Lenart B, Kintner DB, Shull GE, Sun D. Na-K-Cl cotransporter-mediated intracellular Na+ accumulation affects Ca2+ signaling in astrocytes in an in vitro ischemic model. J Neurosci. 2004;24:9585–9597. doi: 10.1523/JNEUROSCI.2569-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jiang Q, Stys PK. Important role of reverse Na+-Ca2+ exchange in spinal cord white matter injury at physiological temperature. J Neurophysiol. 2000;84:1116–1119. doi: 10.1152/jn.2000.84.2.1116. [DOI] [PubMed] [Google Scholar]
- Luo J, Chen H, Kintner DB, Shull GE, Sun D. Decreased neuronal death in Na+/H+ exchanger isoform 1-null mice after in vitro and in vivo ischemia. J Neurosci. 2005;25:11256–11268. doi: 10.1523/JNEUROSCI.3271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengesdorf T, Althausen S, Oberndorfer I, Paschen W. Response of neurons to an irreversible inhibition of endoplasmic reticulum Ca2+-ATPase: relationship between global protein synthesis and expression and translation of individual genes. Biochem J. 2001;356:805–812. doi: 10.1042/0264-6021:3560805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz F, Martin ME, Manso-Tomico J, Berlanga J, Salinas M, Fando JL. Ischemia-induced phosphorylation of initiation factor 2 in differentiated PC12 cells: role for initiation factor 2 phosphatase. J Neurochem. 2000;75:2335–2345. doi: 10.1046/j.1471-4159.2000.0752335.x. [DOI] [PubMed] [Google Scholar]
- Nikolaeva MA, Mukherjee B, Stys PK. Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J Neurosci. 2005;25:9960–9967. doi: 10.1523/JNEUROSCI.2003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W. Endoplasmic reticulum: a primary target in various acute disorders and degenerative diseases of the brain. Cell Calcium. 2003;34:365–383. doi: 10.1016/s0143-4160(03)00139-8. [DOI] [PubMed] [Google Scholar]
- Paschen W, Doutheil J. Disturbances of the functioning of endoplasmic reticulum: a key mechanism underlying neuronal cell injury? J Cereb Blood Flow Metab. 1999;19:1–18. doi: 10.1097/00004647-199901000-00001. [DOI] [PubMed] [Google Scholar]
- Paschen W, Doutheil J, Gissel C, Treiman M. Depletion of neuronal endoplasmic reticulum calcium stores by thapsigargin: effect on protein synthesis. J Neurochem. 1996;67:1735–1743. doi: 10.1046/j.1471-4159.1996.67041735.x. [DOI] [PubMed] [Google Scholar]
- Petr MJ, Wurster RD. Determination of in situ dissociation constant for Fura-2 and quantitation of background fluorescence in astrocyte cell line U373-MG. Cell Calcium. 1997;21:233–240. doi: 10.1016/s0143-4160(97)90047-6. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Centonze D, Giacomini P, Calabresi P. Involvement of intracellular calcium stores during oxygen/glucose deprivation in striatal large aspiny interneurons. J Cereb Blood Flow Metab. 2000;20:839–846. doi: 10.1097/00004647-200005000-00011. [DOI] [PubMed] [Google Scholar]
- Puglisi JL, Bers DM. LabHEART: an interactive computer model of rabbit ventricular myocyte ion channels and Ca transport. Am J Physiol Cell Physiol. 2001;281:C2049–C2060. doi: 10.1152/ajpcell.2001.281.6.C2049. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Shintani-Ishida K, Nakajima M, Uemura K, Yoshida K. Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun. 2006;345:1600–1605. doi: 10.1016/j.bbrc.2006.05.077. [DOI] [PubMed] [Google Scholar]
- Shiu RP, Pouyssegur J, Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977;74:3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G, Kintner DB, Flagella M, Shull GE, Sun D. Astrocytes from Na+-K+-Cl− cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am J Physiol Cell Physiol. 2002;282:C1147–C1160. doi: 10.1152/ajpcell.00538.2001. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Pozzan T. Measurement of Cytosolic Free Ca2+ with Quin2. In: Fleischer S, editor. Biomembranes. Part S, Transport : membrane isolation and characterization. Academic Press; San Diego: 1989. pp. 230–262. [DOI] [PubMed] [Google Scholar]
- Urbanczyk J, Chernysh O, Condrescu M, Reeves JP. Sodium-calcium exchange does not require allosteric calcium activation at high cytosolic sodium concentrations. J Physiol. 2006;575:693–705. doi: 10.1113/jphysiol.2006.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Lovinger D, Delpire E. Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J Neurophysiol. 2005;93:1557–1568. doi: 10.1152/jn.00616.2004. [DOI] [PubMed] [Google Scholar]