Abstract
Aims
H-Dmt-D-Arg-Phe-Lys-NH2 ([Dmt1]DALDA), a highly selective μ-opioid peptide, is potently analgesic after systemic and intrathecal administration but is less potent given intracerebroventricularly. This study was performed to further characterize the analgesic effects of [Dmt1]DALDA.
Methods
We compared the effects of [Dmt1]DALDA and morphine after systemic administration in two different acute pain tests, the tail flick test and the paw withdrawal test, and examined how antagonizing the spinal opioid actions would affect their analgesic effects.
Results
[Dmt1]DALDA was markedly more potent in the tail flick test than in the hot plate test, while the potencies of morphine were similar in the two tests. Intrathecal naloxone completely blocked the effect of systemic [Dmt1]DALDA in the tail flick test, while it only partially blocked the effect of morphine. At higher doses that produced analgesia in the hot plate test, the effect of [Dmt1]DALDA in this test was only partially blocked by naloxone.
Conclusion
Systemic [Dmt1]DALDA has a unique analgesic property clearly different from that of morphine and it has a propensity to produce spinal analgesia.
Key Words: [Dmt1]DALDA, Morphine, Opioids, Analgesic effect
Introduction
H-Dmt-D-Arg-Phe-Lys-NH2 ([Dmt1]DALDA) is a dermorphin-derived tetrapeptide with extraordinary selectivity for the μ-opioid receptor [1]. It carries a net positive charge of 3+ at physiological pH. Despite its polar nature, [Dmt1]DALDA is readily distributed to the CNS and produces long-lasting analgesic effects after systemic administration [2]. [Dmt1]DALDA also produces very potent analgesic effects after intrathecal administration [3], but is much less potent after intracerebroventricular (i.c.v.) administration [2]. In this regard, it differs from the prototypical μ-agonist, morphine, which is equally potent after intrathecal and i.c.v. administration. Thus, [Dmt1]DALDA and morphine appear to have differential analgesic characteristics even though they are both μ-opioid agonists acting in the CNS. To further characterize the difference between the analgesic effects of [Dmt1]DALDA and morphine, we compared the effects of the two compounds after systemic administration in two different acute pain tests, the tail flick test and the paw withdrawal test. The tail flick test has been widely used in evaluating analgesic effects of opioids. It utilizes a spinal reflex induced by a pain stimulus and, in theory, the CNS actions of analgesics being examined in this test either involve the spinal cord directly or involve descending analgesic pathways to the spinal cord [4]. On the other hand, the hot plate test involves both spinal and supraspinal pathways, and the site of action of analgesic agents in this assay system may be at any level of the pain pathway [4]. A difference in analgesic potency of an opioid agonist in the two tests may reflect its distinctive site or mode of action. To the best of our knowledge, there have been no reports on an opioid agonist that has differential effects in these two tests. In addition, to further investigate the differential effects of [Dmt1]DALDA and morphine, it was examined how antagonizing their spinal opioid actions would affect analgesic activity.
Materials and Methods
Experiments were performed according to the guidelines of the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the United States National Institutes of Health, and were approved by the Institutional Animal Use Committee of the Chiba University Graduate School of Medicine.
Animals and Drugs
Male Sprague-Dawley rats (180–200 g) were used in the experiments. [Dmt1]DALDA was synthesized as described elsewhere [1]. Morphine hydrochloride and naloxone hydrochloride were obtained from Takeda Pharmaceuticals, Osaka, Japan, and Sigma-Aldrich Co., St. Louis, Mo., USA, respectively.
Drug Administration
For subcutaneous (s.c.) administration, [Dmt1]DALDA or morphine were dissolved in saline and were delivered in a volume of 0.1 ml/100 g rat weight. Subcutaneous administration was performed 2 h ([Dmt1]DALDA) or 30 min (morphine) prior to pain testing. Spinal administration of naloxone or saline was carried out under light sevoflurane anesthesia by direct intrathecal injection at the level of L 2/3 via a 30-gauge needle connected to a polyethylene tube using a Hamilton syringe. Spinal injection was performed 10 min prior to pain testing in a volume of 10 μl.
Tail Flick and Hot Plate Tests
For the tail flick test, radiant heat was applied to the tail at 5 cm from the tip using a tail flick apparatus (IITC, Woodland Hills, Calif., USA). The time from the onset of the heat to the withdrawal of the tail (tail flick latency) was measured. The intensity of the radiant heat was adjusted so that baseline latencies would fall between 2.5 and 3.5 s. To avoid tissue damage, the heat stimulus was discontinued after 10 s (cut-off latency). For the hot plate test, rats were placed on a 52°C hot plate (Nissin Scientific Corp., Tokyo, Japan) and the latency to paw lick was recorded. A cut-off time was set at 30 s. Baseline latencies for both tests were obtained for each animal prior to the administration of the drug. In both tests, response latencies of each animal were determined at the time of peak effect of the drug administered (2 h for [Dmt1]DALDA and 30 min for morphine).
Data Analysis
Response latencies in the tail flick and hot plate tests were expressed as percentage of baseline values. A one-way ANOVA was used for statistical analysis. A p value <0.05 was considered significant.
Results
Analgesic Effects in Acute Pain Tests
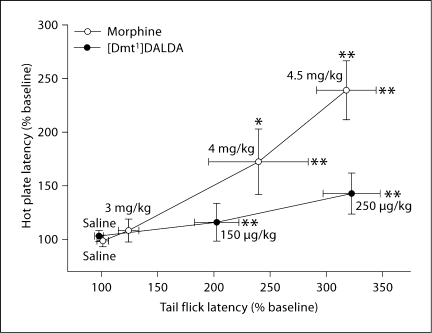
Morphine showed potent dose-dependent analgesic effects in both the tail flick test and the hot plate test (fig. 1). On the other hand, [Dmt1]DALDA at doses of 0.15 and 0.25 mg/kg showed potent dose-dependent analgesic effects in the tail flick test, while no significant effect was seen in the hot plate test at these dose levels. The 0.25 mg/kg dose of [Dmt1]DALDA and the 4.5 mg/kg dose of morphine produced equivalent analgesic effects in the tail flick test, but only the latter dose of morphine elicited analgesia in the hot plate test (fig. 1). [Dmt1]DALDA at a higher dose of 0.35 mg/kg resulted in cut-off latency in the tail flick test in all animals tested, and at the same dose produced an analgesic effect (162.2 ± 12.2%, significantly different from saline control (p < 0.05)) in the hot plate test. Increasing the dose of this compound to 0.5 mg/kg resulted in a further increase in hot plate latency (250.8 ± 20.9%, significantly different from saline control (p < 0.01)). This was equivalent to the effect of morphine at a dose of 4.5 mg/kg in the hot plate test.
Fig. 1.
Comparison of the analgesic effects of [Dmt1]DALDA and morphine in the tail flick test versus the hot plate test. Tail flick and hot plate latencies were measured at 30 min after the administration of morphine or saline and 2 h after the administration of [Dmt1]DALDA or saline. The number of animals was 6 or 7 for each group. Data are expressed as percent of baseline latency values. Tail flick latencies are plotted against hot plate latencies of each dose of morphine and [Dmt1]DALDA. * Significantly different from saline control (p < 0.05). ** Significantly different from saline control (p < 0.01). [Dmt1]DALDA at higher doses of 0.35 and 0.5 mg/kg produced a significant effect in the hot plate test (see text) but resulted in cut-off latency in all tested animals in the tail flick test (results not included in the graph).
Effects of Spinal Naloxone
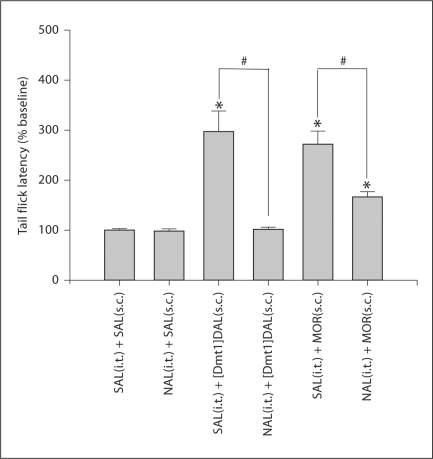
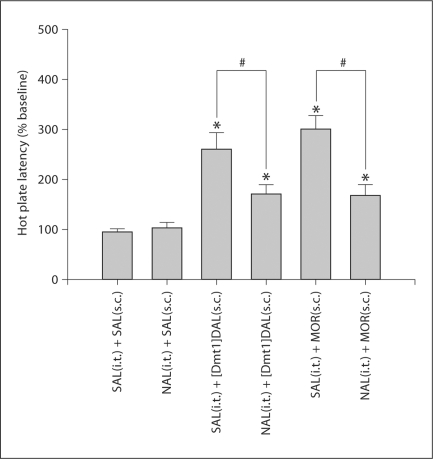
Twenty μg of naloxone given spinally 10 min prior to the tail flick test completely blocked the analgesic effect of s.c. [Dmt1]DALDA (0.25 mg/kg) (fig. 2). In contrast, the same dose of naloxone only partially blocked the analgesic effect of s.c. morphine at the dose of 4.5 mg/kg, a dose which is equipotent to a dose of 0.25 mg/kg [Dmt1]DALDA in the tail flick test. In the hot plate test, 20 μg of spinal naloxone only partially blocked the effects of both morphine at 4.5 mg/kg and of [Dmt1]DALDA at 0.5 mg/kg (fig. 3). These doses of the two compounds produced equivalent effects in the hot plate test. No effect on [Dmt1]DALDA or morphine analgesia was observed in both pain tests by spinally injecting the same volume of saline (fig. 2, 3). Naloxone pretreatment itself did not affect tail flick or hot plate latency (fig. 2, 3).
Fig. 2.
Spinal naloxone (NAL) abolished the analgesic effects of s.c. [Dmt1]DALDA ([Dmt1]DAL) in the tail flick test but only partially blocked the effects of s.c. morphine (MOR). Tail flick testing was performed 2 h ([Dmt1]DALDA) or 30 min (morphine and saline) after s.c. administration of [Dmt1]DALDA (0.25 mg/kg), morphine (4.5 mg/kg) or saline (SAL). Intrathecal (i.t.) naloxone (20 μg) or saline were given 10 min prior to testing. The number of animals was 5 for each group. # Significantly different between groups (p < 0.05). * Significantly different from saline control (p < 0.05).
Fig. 3.
Spinal naloxone (NAL) partially blocked the effects of both s.c. [Dmt1]DALDA ([Dmt1]DAL) and morphine (MOR) in the hot plate test. The hot plate test was performed 2 h ([Dmt1]DALDA) or 30 min (morphine and saline) after s.c. administration of [Dmt1]DALDA (0.5 mg/kg), morphine (4.5 mg/kg) or saline (SAL). Intrathecal (i.t.) naloxone (20 μg) or saline were given 10 min prior to testing. The number of animals was 5 or 6 for each group. # Significantly different between groups (p < 0.05). * Significantly different from saline control (p < 0.05).
Discussion
The tail flick test utilizes a spinal reflex induced by a pain stimulus, thus theoretically, the CNS actions produced by analgesic agents in this test directly involve the spinal cord or activate descending analgesic pathways to the spinal cord [4]. On the other hand, the hot plate test involves both spinal and supraspinal pathways, and the site of action of analgesic agents that affect this test may be at any level of the pain pathway [4]. Both the tail flick and hot plate tests are highly sensitive to opioids and have been widely utilized to demonstrate opioid analgesia in animal studies. The systemic administration of the prototypical μ-opioid agonist morphine has been shown to produce analgesia in both pain tests [5, 6]. In agreement with these earlier works, in the present study, we observed potent dose-dependent analgesic effects of morphine in the two tests at the same dose level. On the other hand, we found that systemic administration of [Dmt1]DALDA was markedly more effective in the tail flick test than in the hot plate test. Doses that produced potent analgesic effects in the tail flick test elicited no analgesic effect in the hot plate test. Furthermore, the opioid receptor antagonist naloxone given spinally completely abolished the analgesic effect of systemic [Dmt1]DALDA in the tail flick test, while the same dose of naloxone only partially blocked the analgesic effect of an equipotent dose of morphine. These results demonstrate that the characteristics of [Dmt1]DALDA's analgesic action are clearly different from those of morphine, despite the fact that both compounds are μ-opioids with CNS activity after systemic administration.
The results of the naloxone study utilizing the tail flick test suggest that [Dmt1]DALDA after systemic administration has a propensity to produce analgesia mainly in the spinal cord, while morphine produces both supraspinal and spinal analgesia. This finding is compatible with previous reports which demonstrated that [Dmt1]DALDA is highly potent in the tail flick test when given spinally [3], while much less potent when given i.c.v. [2]. The results suggest that [Dmt1]DALDA acts differently in the spinal cord and in the brain. This difference may be due to [Dmt1]DALDA's activation of other systems in the spinal cord that would potentiate the analgesic μ-opioid effect. In a previous study we showed that [Dmt1]DALDA inhibited norepinephrine reuptake, thereby increasing the effects of norepinephrine [3]. Norepinephrine has been shown to act synergistically with opioids to produce spinal analgesia [7, 8]. Furthermore, GTPγS-binding studies showed that [Dmt1]DALDA was a full agonist when the assay was performed with spinal cord membranes, whereas it showed partial agonism in the assay using brain membranes [9]. These findings indicate that [Dmt1]DALDA has differential actions on μ-receptors in the spinal cord versus the brain. This might be explained by differential distribution of μ-receptor variants in the spinal cord and in the brain. It has been shown that [Dmt1]DALDA differentially activated different μ-receptor variants as compared to the selective μ-agonist DAMGO [9].
We found that [Dmt1]DALDA at high doses, as compared to the low doses required in the tail flick test, produced an analgesic effect in the hot plate test which was only partially blocked by spinal naloxone. Thus, at these high doses, [Dmt1]DALDA can act similarly to morphine by blocking nociceptive pathways involved in the hot plate test and exerting analgesic action in the brain as well as in the spinal cord. However, lower doses of this compound that produced a potent effect in the tail flick test had no effect in the hot plate test. This greater potency in the tail flick test as compared to the hot plate test cannot be explained simply by the propensity of [Dmt1]DALDA to produce analgesic actions in the spinal cord as discussed above. Since the nociceptive impulse is transmitted through the spinal cord in both pain tests, if spinal μ-receptor activation uniformly suppresses nociceptive transmission, it would be anticipated that the potent spinal analgesia produced by [Dmt1]DALDA would result in analgesic effects in both tests. The differential effects of [Dmt1]DALDA in the two tests seems to suggest that the nociceptive transmissions are not uniform and that they may be differentially affected by drugs. Although both pain tests are based on thermal nociception, there is a difference in the pain stimuli between the two tests. In general, the hot plate test utilizes a lower intensity thermal stimulation that results in a longer latency for the animal to react as compared to the tail flick test. Such difference in stimulus intensity may activate different pathways in the spinal cord. We have previously shown that neuronal endothelin-1 has an effect in the tail flick test only when the stimulus intensity is markedly decreased [10]. Why [Dmt1]DALDA but not morphine appears to produce differential effects on nociceptive transmissions is unclear. It might involve non-uniformity within the spinal cord of [Dmt1]DALDA's potentiating effects on μ-opioid inhibition by the non-opioid mechanisms mentioned above, or possibly the activation of different μ-receptor variants distributed inhomogeneously among different nociceptive pathways within the spinal cord.
The propensity of [Dmt1]DALDA to produce spinal analgesia may have significance in the clinical setting. Firstly, [Dmt1]DALDA at analgesic doses may produce less supraspinal opioid side effects. Secondly, the potent spinal analgesia produced by [Dmt1]DALDA may be effective in pathological pain involving spinal mechanisms, in particular neuropathic pain. On the other hand, the mechanism that produces its relatively low effect in the hot plate test may result in relative ineffectiveness in clinical pain. Although morphine and other opioids are effective in neuropathic pain, high doses are frequently required that may be intolerable clinically. It remains to be shown whether [Dmt1]DALDA may be more effective than morphine in neuropathic pain due to its propensity to act in the spinal cord, and also due to its non-opioid actions such as norepinephrine reuptake inhibition and ROS scavenging actions [11].
Acknowledgements
This study was supported in part by a Grant-in-Aid for Cancer Research (Study Group on Palliative Care and Psycho-oncology in Cancer Treatment) from the Ministry of Health and Welfare, Japan (N.S.), and a Multi-Center Program Project Grant (DA08924) from the National Institute on Drug Abuse, NIH, USA (H.H.S., P.W.S.)
Footnotes
Conflict of Interest Disclosure
Patent applications have been filed by Cornell Research Foundation (CRF) for the technology (SS peptides) described in this article. Hazel H. Szeto and Peter W. Schiller are the inventors. CFR, on behalf of Cornell University, has licensed the technology for future research and development to a commercial enterprise in which CRF and Dr. Szeto have financial interest.
References
- 1.Schiller PW, Nguyen TM, Berzowska I, Dupuis S, Weltrowska G, Chung NN, Lemieux C. Synthesis and in vitro activity profiles of DALDA analogues. Eur J Med Chem. 2000;35:895–901. doi: 10.1016/s0223-5234(00)01171-5. [DOI] [PubMed] [Google Scholar]
- 2.Zhao GM, Wu D, Soong Y, Shimoyama M, Berezowska I, Schiller PW, Szeto HH. Profound spinal tolerance after repeated exposure to a highly selective μ-opioid peptide agonist: role of δ-opioid receptors. J Pharmacol Exp Ther. 2002;302:188–196. doi: 10.1124/jpet.302.1.188. [DOI] [PubMed] [Google Scholar]
- 3.Shimoyama M, Shimoyama N, Zhao GM, Schiller PW, Szeto HH. Antinociceptive and respiratory effects of intrathecal H-Tyr- D-Arg-Phe-Lys-NH2 (DALDA) and [Dmt1] DALDA. J Pharmacol Exp Ther. 2001;297:364–371. [PubMed] [Google Scholar]
- 4.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 5.Yeung JC, Rudy TA. Sites of antinociceptive action of systemically injected morphine: involvement of supraspinal loci as revealed by intracerebroventricular injection of naloxone. J Pharmacol Exp Ther. 1980;215:626–632. [PubMed] [Google Scholar]
- 6.Sora I, Takahash N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define μ-receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–858. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan AF, Dashwood MR, Dickenson AH. Alpha-2-adrenoceptor modulation of nociception in rat spinal cord: location, effects and interaction with morphine. Eur J Pharmacol. 1987;138:169–177. doi: 10.1016/0014-2999(87)90430-4. [DOI] [PubMed] [Google Scholar]
- 9.Neilan CL, Janvey AJ, Bolan E, Berezowska I, Nguyen TM, Schiller PW, Pasternak GW. Characterization of the binding of [3H][Dmt1]H-Dmt-D-Arg-Phe-Lys-NH2, a highly potent opioid peptide. J Pharmacol Exp Ther. 2003;306:430–436. doi: 10.1124/jpet.103.049742. [DOI] [PubMed] [Google Scholar]
- 10.Hasue F, Kuwaki T, Kisanuki YY, Yanagisawa M, Moriya H, Fukuda Y, Shimoyama M. Increased sensitivity to acute and persistent pain in neuron-specific endothelin-1 knockout mice. Neuroscience. 2005;130:349–358. doi: 10.1016/j.neuroscience.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]