Abstract
Alcohol and nicotine (in the form of tobacco) are 2 commonly used recreational drugs and studies show a high correlation between tobacco use and alcohol consumption. In the present study, using C57BL/6J mice, we investigated the ability of mecamylamine (a nicotinic antagonist) to reduce alcohol consumption and alcohol preference with free 24-hour access using a 2-bottle choice test drinking procedure. Male C57BL/6J mice were individually housed and acclimatized to 10% alcohol. Immediately following the last day of alcohol acclimatization, the mice (n = 5/group) received subcutaneous injections of mecamylamine (0.5, 1 and 2 mg/kg) or saline consisting of either intermittent (3 injections given every other day) or daily (injections on all 5 days) exposures. Fluid consumption (alcohol and water) was recorded daily. The results showed that mecamylamine significantly reduced alcohol consumption and alcohol preference in both phases of intermittent and daily drug exposures, while the total fluid consumption was unchanged. These results provide further support that mecamylamine is effective in reducing alcohol consumption and preference, and nicotinic-receptor-based drugs could further be explored as potential treatments for alcoholism.
Copyright © 2009 S. Karger AG, Basel
Key Words: Alcohol consumption, Preference, Mecamylamine, C57BL/6J mice
Introduction
A high correlation exists between cigarette smoking and alcohol consumption [1]. The prevalence of smoking among alcoholics is as high as 90% compared to 30% among the general population, and people who drink alcohol are 2-fold more likely to smoke than the general population [2]. Nicotine is the primary constituent found in tobacco that reinforces the pleasure of smoking. It is widely understood that the rewarding properties of nicotine are mediated through nicotinic acetylcholine receptors (nAChRs), which are primarily located in the central nervous system [3, 4]. The codependence on nicotine and alcohol is common, suggesting that there may be some shared mechanism.
It is known that alcohol alters the function of neuronal nAChRs [5, 6]. For example, alcohol may increase (α4β2) or inhibit (α7) nAChR receptor function [7], or blocks the discriminative stimulus properties of nicotine in rats [8]. Acute intraventricular or intra-accumbens infusion of nicotine has been shown to decrease alcohol consumption [9, 10], and this suppression of alcohol intake by nicotine involved only intermittent doses [11]. Conversely, daily administration of nicotine is known to increase alcohol consumption [12]. Administration of mecamylamine (a nicotinic antagonist) has been shown to reduce alcohol consumption [13,14,15]. Therefore, the objective of the present study was to evaluate if alcohol consumption could differ on intermittent versus daily administration of mecamylamine in C57BL/6J mice.
‘Preference drinking’ is one of the mouse models widely used in alcohol research [16]. In this model, known as the 2-bottle choice test procedure, animals are given access to 2 bottles, one with a diluted solution of alcohol and the other with plain water. Consumption is then monitored every 24 h over several days so as to make an assessment of a subject's preference for alcohol over water. The genotype of animal/strain exerts a high influence in its tendency to prefer alcohol to water. Using this 2-bottle choice test procedure, several mouse lines have been classified as ‘highly alcohol preferring’, or ‘rarely alcohol preferring’. The C57BL/6J (B6) strain of mice is widely known to voluntarily consume significant quantities of alcohol and therefore is classified as an ‘alcohol-preferring’ mouse strain [17]. The B6 mouse could be of great value in investigating the pharmacological profiles of compounds thought to mediate alcohol consumption, considering the fact that a great detail of established pharmacological, behavioral and genetic resources using this inbred mouse line is already known [17]. A 2-bottle choice test procedure is currently being used in our laboratory as the model to evaluate the therapeutic potential of novel drugs in treating alcoholism [18]. The overall objective of this study was to test the effects of intermittent and daily administration of mecamylamine for their ability to attenuate alcohol consumption and alcohol preference in C57BL/6J mice.
Materials and Methods
Animals
Male C57BL/6J mice (n = 5/group) weighing 27–33 g (Jackson Laboratory, Bar Harbor, Me., USA) were housed 1/cage in a room maintained on a 15/9 light/dark cycle (lights on at 06:00 AM and off at 09:00 PM) and kept at a constant temperature with free access to food and water for 7 days upon their arrival. All research was conducted in accordance with the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH publications No. 80-23 revised in 1996) and approved by the University of Kentucky Institutional Animal Use and Care Committee.
Drugs
Mecamylamine (2-(methylamino)isocamphane hydrochloride N,2,3,3-tetramethylbicyclo[2.2.1]heptan-2-amine hydrochloride; C11H21N · HCl) was obtained from Sigma-Aldrich, Saint Louis, Mo., USA. The drug was freshly prepared by dissolving in sterile water and injected subcutaneously at doses of 0.5, 1 and 2 mg/kg (injection volume = 10 ml/kg). These doses were chosen based on previous literature [19]. The control animals received subcutaneous saline injections in a similar injection volume.
Behavioral Procedure
Two-Bottle Choice Test Procedure: Alcohol Priming. Prior to the start of the mecamylamine administration, the mice were given 24-hour access to water and alcohol for 7 days using 2 feeding tubes (Dyets Inc., Bethlehem, Pa., USA) inserted directly into the animal cage. Over these 7 days, the concentration of alcohol was gradually increased using the following schedule: days 1–2: 3%; days 3–5: 6%; days 6–7: 10%. The position of the water and alcohol tubes was changed on alternate days in order to avoid potential position bias. Intermittent and daily administration phases were considered separate experiments and involved a separate batch of animals. Intermittent drug administration involved 3 drug injections (on days 1, 3 and 5), whereas daily administration involved drug injections on all 5 days. Every day between 14:00 and 16:00 h, the amount of alcohol and water consumed was measured for 5 days (every 24 h). Following the drug administration, the animals were put into clean cages with fresh alcohol and water. For the intermittent drug exposure group, during the days when the animals did not receive drug injections, they were put into clean cages with fresh alcohol and water immediately after measuring the amount of alcohol and water consumed. Alcohol preference was calculated by using the following equation: preference(percent) = alcohol intake × 100/total fluid intake.
Results
Effects of Intermittent Administration of Mecamylamine
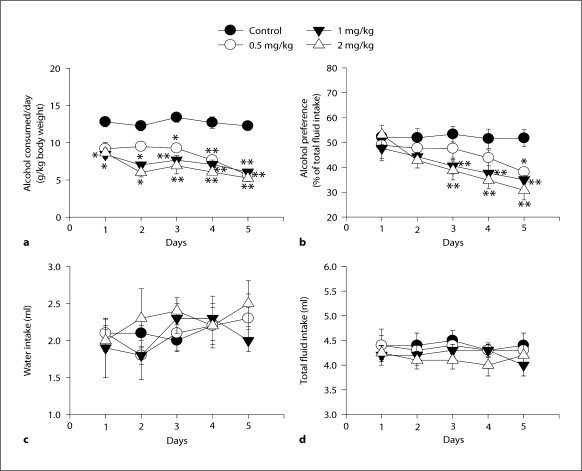
Alcohol Consumption. Mecamylamine at doses 1 and 2 mg/kg reduced alcohol consumption on days 1–2 (p <0.05), at doses 0.5 (p < 0.05), 1 and 2 mg/kg (p < 0.001) reduced alcohol consumption on day 3, and at doses 0.5, 1 and 2 mg/kg reduced alcohol consumption on days 4–5 (p < 0.001). Two-way ANOVA for alcohol consumed: dose, F(3, 97) = 67.927, p < 0.001; day, F(4, 97) = 6.482, p < 0.001, and interaction, F(12, 97) = 1.038, p = 0.424 (fig. 1a).
Fig. 1.
Effects of subcutaneous intermittent administration of mecamylamine (doses of 0.5, 1 and 2 mg/kg) in the 2-bottle choice test procedure. a Alcohol consumption. b Alcohol preference. c Water intake. d Total fluid intake. * p < 0.05, or ** p < 0.001 by post hoc Tukey honestly significant difference against the appropriate control group across days.
Alcohol Preference. The results showed that mecamylamine at dose 0.5 mg/kg reduced alcohol consumption on day 5 (p < 0.05), and at doses 1 and 2 mg/kg on days 3–5 (p <0.001). Two-way ANOVA for alcohol preference: dose, F(3, 95) = 10.658, p < 0.001; day, F(4, 95) = 7.016, p < 0.001, and interaction, F(12, 95) = 1.229, p = 0.279 (fig. 1b).
Water Intake. There were no statistically significant differences in water intake across treatment conditions (fig. 1c). Two-way ANOVA for water intake: dose, F(3, 99) = 0.229, p = 0.570; day, F(4, 99) = 0.908, p = 0.463, and interaction, F(12, 99) = 0.374, p = 0.969.
Total Fluid Intake. There were also no statistically significant differences in total fluid intake across days (fig. 1d). Two-way ANOVA for total fluid intake: dose, F(3, 97) = 1.651, p = 0.185; day, F(4, 97) = 0.278, p = 0.891, and interaction, F(12, 97) = 0.286, p = 0.990.
Effects of Daily Administration of Mecamylamine
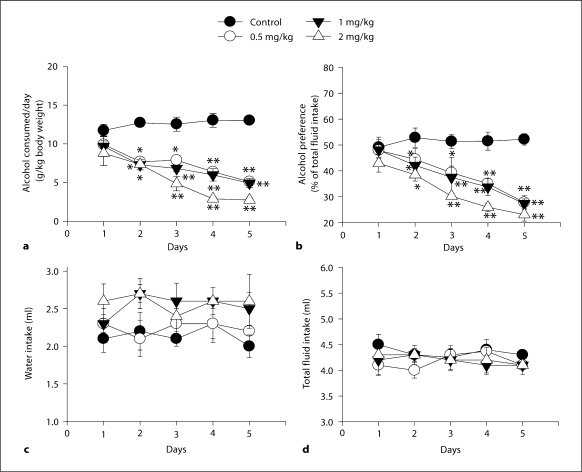
Alcohol Consumption. The results showed that mecamylamine at doses 0.5, 1 and 2 mg/kg reduced alcohol consumption on day 2 (p <0.05), at doses 0.5 (p < 0.05), 1 and 2 mg/kg (p < 0.001) reduced alcohol consumption on day 3, and at doses 0.5, 1 and 2 mg/kg reduced alcohol consumption on days 4–5 (p <0.001) (fig. 2a). Two-way ANOVA for alcohol consumed: dose, F(3, 97) = 73.715, p < 0.001; day, F(4, 97) = 12.325, p < 0.001, and interaction, F(12, 97) = 3.133, p < 0.001.
Fig. 2.
Effects of subcutaneous daily administration of mecamylamine (doses of 0.5, 1 and 2 mg/kg) in the 2-bottle test choice procedure. a Alcohol consumption. b Alcohol preference. c Water intake. d Total fluid intake. * p < 0.05, or ** p < 0.001 by post hoc Tukey honestly significant difference against the appropriate control group across days.
Alcohol Preference. The results showed that mecamylamine at doses 0.5, 1 and 3 mg/kg reduced alcohol consumption on day 2 (p < 0.05), and thereafter at doses 0.5, 1 and 3 mg/kg on days 3–5 (p <0.001) (fig. 2b). Two-way ANOVA for alcohol preference: dose, F(3, 95) = 25.762, p < 0.001; day, F(4, 95) = 10.709, p < 0.001, and interaction, F(12, 95) = 1.669, p = 0.091.
Water Intake. There was no statistically significant difference in water intake across days (fig. 2c). Two-way ANOVA for water intake: dose, F(3, 98) = 5.080, p < 0.05; day, F(4, 98) = 0.279, p = 0.891, and interaction, F(12, 98) = 0.305, p = 0.987.
Total Fluid Intake. There was no statistically significant difference in total fluid intake across days (fig. 2d). Two-way ANOVA for total fluid intake: dose, F(3, 97) = 0.950, p = 0.421; day, F(4, 97) = 0.274, p = 0.881, and interaction, F(12, 97) = 0.359, p = 0.974.
Discussion
Alcohol and nicotine are frequently coabused, and therefore it is of interest to investigate whether alcohol consumption could be altered after the administration of a nicotinic drug. Specifically, we examined whether mecamylamine (a nicotine antagonist), administered intermittently or daily, could have an effect on alcohol consumption and alcohol preference. The results showed that mecamylamine significantly attenuated alcohol consumption and preference when administered both intermittently and daily. More importantly, a pronounced reduction in alcohol consumption and preference was noticed following daily administration of mecamylamine. Throughout these studies, the total fluid intake following drug administration was not significantly different from baseline consumption. This eliminates any possibility that differences in alcohol preference may have been due to a sedative effect of the drug. In the present study, mecamylamine was given at doses that did not have any adverse effect on locomotor activity, a finding that has been substantiated by others [19].
The effects of mecamylamine outlined in the present study are in agreement with other studies, both in animals and in humans, which suggest that mecamylamine decreases alcohol preference and consumption in animals [13] and in human subjects [20]. In humans, studies by Blomqvist et al. [20] used 20 human subjects that were pretreated with various doses of mecamylamine (7.5–12 mg/kg) or saline followed by exposure to alcohol. The results showed that mecamylamine significantly attenuated alcohol consumption. Previous studies in rodents indicate that mecamylamine when administered systemically or directly into the ventral tegmental area blocks the elevation of alcohol-mediated dopamine release in the nucleus accumbens [21, 22]. From this inference, it is likely that in the present study, mecamylamine is reducing alcohol and preference intake via a similar mechanism. Considering the stimulant and reinforcing properties of alcohol, the findings of the present study could also suggest that mecamylamine attenuates the reinforcing properties of alcohol such as alcohol consumption and alcohol preference. It is of interest to note that less alcohol consumption was observed in mecamylamine-treated (intermittent) mice than in control mice on each of the days, including days 2 and 4 when mecamylamine was not administered. One possibility for this could be due to the sensitization of nAChRs. This hypothesis is supported by reports that suggest the α7 nAChRs display functional properties very different from those of the α4β2 nAChR, such as a very fast desensitization effect, or higher Ca2+ permeability [23, 24].
Mecamylamine is reported to act as a noncompetitive antagonist of the NMDA receptors due to its action at the phencyclidine site [25]. For example, studies by Blomqvist et al. [21] demonstrated that mecamylamine did not have a significant effect on mesoaccumbal dopamine flow, which could suggest that mecamylamine-sensitive receptors may not influence mesoaccumbal dopamine. However, it should be noted that mecamylamine is a nonselective and noncompetetive NMDA receptor antagonist [25]. Therefore, the possibility that mecamylamine could partly have reduced alcohol consumption through nonnicotinergic pathways cannot be completely ruled out. The results of the present study, combined with that of a previous report [26], support the notion that nAChRs could have a multifaceted interaction at the receptor levels, particularly those associated with the psychopathology of addictive behaviors as this is evident by the ability of mecamylamine to attenuate alcohol consumption over a period of days.
Conclusion
The data presented here indicate that the intermittent and daily subcutaneous administration of mecamylamine over a range of doses attenuates the voluntary consumption of alcohol in a 2-bottle choice drinking procedure. This suggests that nAChRs may play a role in alcohol-seeking behaviors in animals having a preference for alcohol consumption.
Acknowledgements
This work was supported in part by a petite research grant awarded to J.M.F. from the Center on Drug and Alcohol Research, University of Kentucky, and supplemented by an NIAAA Grant awarded to J.M.L. (No. 12600). We thank Noel R. Monks, PhD, (Naprogenix, Inc.) for a valuable critique of the manuscript.
References
- 1.Littleton J, Barron S, Prendergast M, Nixon SJ. Smoking kills (alcoholics)! Shouldn't we do something about it? Alcohol Alcohol. 2007;42:167–173. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- 2.Carmody TP, Brischetto CS, Matarazzo JD, O'Donnell RP, Connor WE. Co-occurrent use of cigarettes, alcohol, and coffee in healthy, community-living men and women. Health Psychol. 1985;4:323–335. doi: 10.1037//0278-6133.4.4.323. [DOI] [PubMed] [Google Scholar]
- 3.Wonnacott S, Sidhpura N, Balfour DJK. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- 5.Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci Biobehav Rev. 2004;27:713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Korkosz A, Zatorski P, Taracha E. Effects of ethanol on nicotine-induced conditioned place preference in C57BL/6J mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1283–1290. doi: 10.1016/j.pnpbp.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Zuo Y, Nagata K, Yeh JZ, Narahashi T. Single-channel analyses of ethanol modulation of neuronal nicotinic acetylcholine receptors. Alcohol Clin Exp Res. 2004;28:688–696. doi: 10.1097/01.alc.0000125349.99823.8a. [DOI] [PubMed] [Google Scholar]
- 8.Korkosz A, Taracha E, Plaznik A, Wrobel E, Kostowski W, Bienkowski P. Extended blockade of the discriminative stimulus effects of nicotine with low doses of ethanol. Eur J Pharmacol. 2005;512:165–172. doi: 10.1016/j.ejphar.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Katner SN, McBride WJ, Lumeng L, Li TK, Murphy JM. Involvement of CNS cholinergic systems in alcohol drinking of P rats. Addict Biol. 1997;2:215–223. doi: 10.1080/13556219772769. [DOI] [PubMed] [Google Scholar]
- 10.Nadal R, Chappell AM, Samson HH. Effects of nicotine and mecamylamine microinjections into the nucleus accumbens on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 1998;22:1190–1198. [PubMed] [Google Scholar]
- 11.Gauvin DV, Moore KR, Holloway FA. Do rat strain differences in ethanol consumption reflect differences in ethanol sensitivity or the preparedness to learn? Alcohol. 1993;10:37–43. doi: 10.1016/0741-8329(93)90051-o. [DOI] [PubMed] [Google Scholar]
- 12.Lê AD. Effects of nicotine on alcohol consumption. Alcohol Clin Exp Res. 2002;26:1915–1916. doi: 10.1097/01.ALC.0000040963.46878.5D. [DOI] [PubMed] [Google Scholar]
- 13.Lê AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- 14.Ericson M, Blomqvist O, Engel JA, Söderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- 15.Blomqvist O, Ericson M, Johnson DH, Engel JA, Söderpalm B. Voluntary alcohol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- 16.Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- 17.Crabbe JC, Phillips TJ. Pharmacogenetic studies of alcohol self-administration and withdrawal. Psychopharmacology. 2004;174:539–560. doi: 10.1007/s00213-003-1608-6. [DOI] [PubMed] [Google Scholar]
- 18.Farook JM, Lewis B, Littleton JM, Barron S. Topiramate attenuates the stress-induced increase in alcohol consumption and preference in male C57BL/6J mice. Physiol Behav. 2009;96:189–193. doi: 10.1016/j.physbeh.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Miller DK, Segert IL. Mecamylamine attenuates ephedrine-induced hyperactivity in rats. Pharmacol Biochem Behav. 2005;81:165–169. doi: 10.1016/j.pbb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin Exp Res. 2002;26:326–331. [PubMed] [Google Scholar]
- 21.Blomqvist O, Engel JA, Nissbrandt H, Söderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- 22.Blomqvist O, Ericson M, Engel JA, Söderpalm B. Accumbal dopamine overflow after ethanol: localization of the antagonizing effect of mecamylamine. Eur J Pharmacol. 1997;334:149–156. doi: 10.1016/s0014-2999(97)01220-x. [DOI] [PubMed] [Google Scholar]
- 23.Castro NG, Albuquerque EX. α-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkondon M, Pereira EFR, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of a7 nicotinic acetylcholine receptors. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 25.O'Dell TJ, Christensen BN. Mecamylamine is a selective non-competitive antagonist of N-methyl-D-aspartate- and aspartate-induced currents in horizontal cells dissociated from the catfish retina. Neurosci Lett. 1988;94:93–98. doi: 10.1016/0304-3940(88)90276-5. [DOI] [PubMed] [Google Scholar]
- 26.Beani L, Antonelli T, Tomasini MC, Marani L, Bianchi C. The nicotinic modulation of [3H]D-aspartate outflow in primary cultures of rat neocortical neurons: effect of acute and long-term nicotine treatment. Neuropharmacology. 2000;39:2646–2653. doi: 10.1016/s0028-3908(00)00155-6. [DOI] [PubMed] [Google Scholar]