Abstract
Background
Cocaine and methamphetamine (METH) are two commonly abused drugs that have behavioral-stimulant properties. These stimulant effects are partially mediated by the dopaminergic system. Recent evidence has suggested that the histamine H3 receptor (H3R) may modulate the release of dopamine induced by METH. The aim of the present study was to examine the role of H3R in the behavioral-stimulant effects of cocaine and METH in mice and monkeys.
Methods
Nonhabituated, experimentally naïve mice (n = 5–6) were pretreated with the H3R agonist imetit 30 min before METH or cocaine, and activity was measured for 90 min. The behavioral-stimulant effects of METH and cocaine were also studied in squirrel monkeys (n = 3) under a fixed-interval schedule of stimulus termination. Monkeys were pretreated with imetit 30 min before the peak behavioral-stimulant doses of METH or cocaine derived from individual subjects.
Results
Pretreatment with imetit did not affect basal activity in mice. Imetit significantly attenuated the behavioral-stimulant effects of METH, but not cocaine. In monkeys, no dose of imetit tested significantly altered the behavioral-stimulant effects of METH or cocaine.
Conclusion
These results suggest a role of H3R in the behavioral-stimulant effects of METH, but not cocaine, in mice and no role in monkeys.
Copyright © 2009 S. Karger AG, Basel
Key Words: Methamphetamine; Cocaine; Histamine H3 receptor; Locomotor behavior, mice; Fixed-interval schedule, monkeys
Introduction
Methamphetamine (METH) and cocaine are two commonly abused drugs that present significant public health concerns. In behavioral-stimulant assays, such as locomotor activity and responding under a fixed-interval (FI) schedule of stimulus termination, drugs that increase behavior also increase extracellular dopamine levels [1,2,3]. Thus, these two assays might serve as in vivo behavioral screens for examining novel pharmacotherapies in treating METH and cocaine abuse.
The brain histaminergic system may provide novel pharmacotherapies in treating a wide array of central nervous system disorders [4]. Histaminergic cell bodies, originating in the tuberomamillary nucleus of the hypothalamus, project to brain regions believed to be involved in mediating the behavioral-stimulant effects of METH and cocaine [for a review, see [5]]. At present, there are 4 known histamine receptors (H1R, H2R, H3R and H4R). Specifically, the H3R is a Gi/o-protein-coupled receptor with expression in dopaminergic striatal regions of rodents, monkeys and humans [6,7,8]. Thus, H3R may serve as a target for modulating dopamine release and the behavioral-stimulant effects of METH and cocaine; this appears to be the case for METH, since pretreatment with an H3R antagonist potentiates METH-induced dopamine release in rats [9].
However, the literature examining the behavioral interactions between H3R and psychomotor stimulants in rodents has provided conflicting results. H3R antagonists have been shown to attenuate amphetamine- and METH-induced locomotor activity [10,11,12], but potentiate [13, 14] or have no effect [10] on cocaine-induced locomotor activity. Finally, the H3R agonist (R)-α-methylhistamine had no effect on amphetamine-induced locomotor activity [10].
Thus, based on the available literature, there appears to be conflicting evidence for the role of H3R in the behavioral-stimulant effects of psychomotor stimulants, such as METH and cocaine. The aim of the present study was to assess the effects of an H3R agonist, imetit, on the behavioral-stimulant effects of a monoamine releaser (METH) and uptake inhibitor (cocaine) in both mice and squirrel monkeys. To the best of our knowledge, the effects of H3R compounds have not been examined in monkeys. We hypothesized that pretreatment with imetit would attenuate the behavioral-stimulant effects of both METH and cocaine in both species. Furthermore, we hypothesized that cocaine would be more sensitive to imetit pretreatments based on the impulse-dependent release of monoamines compared to METH in both species. These results would provide strong evidence for the involvement of H3R in the behavioral-stimulant effects of METH and cocaine.
Materials and Methods
Subjects
Male Swiss Webster mice (Charles River Laboratories Inc., Wilmington, Mass., USA) weighing approximately 20–30 g were housed 5 animals per 44.5 × 22.3 × 12.7 cm Plexiglas cage. The rodent vivarium was maintained at an ambient temperature of 22 ± 2°C at 45–50% humidity, and lights were set to a 12-hour light/dark cycle. Animals were fed lab diet rodent chow (Laboratory Rodent Diet No. 5001; PMI Feeds Inc., St. Louis, Mo., USA) and water ad libitum immediately before testing. Mice were not used in the experiments until at least 3 days after arrival in the laboratory, and there was no specific handling regimen employed in these studies. Three adult male squirrel monkeys (Saimiri sciureus) with a previous history of responding under the FI schedule [15] weighing 900–1,200 g served as subjects. The monkeys lived in individual home cages and had daily access to food (5045 high-protein monkey diet; Purina Mills International Inc., Brentwood, Mo., USA; fresh fruit and vegetables) and unlimited access to water in the home cage. All monkeys had had prior exposure to cocaine and other drugs with dopaminergic or serotonergic activity [15]. The facilities for housing and care of the animals are accredited by the American Association for the Assessment and Accreditation of Laboratory Animal Care. Animal use procedures were in strict accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Institutional Animal Care and Use Committee of Emory University.
Drug-Induced Locomotor Behavior
Mice (n = 5–6/group) were handled and weighed prior to initiation of activity experiments as previously described [16]. Mice were not habituated to the activity chambers prior to injections. Experiments were conducted during the light phase. Vehicle (water) or imetit (30–100 mg/kg, i.p.) was administered 30 min before METH (3 mg/kg, i.p.), cocaine (30 mg/kg, i.p.) or saline. Doses of METH and cocaine were chosen based on pilot experiments to induce comparable behavioral-stimulant effects across the 90-min session. Following the second injection, animals were directly placed into the locomotor chambers (40 × 40 × 40 cm), and activity was monitored and quantified for 90 min using a modified open-field activity system under low-light conditions (San Diego Instruments, San Diego, Calif., USA). Horizontal, but not vertical, beam breaks were recorded as an index of activity.
FI Stimulus Termination Procedure
Responding under the FI 300-second schedule was as previously described [15]. Daily sessions consisted of 15 FI components. Briefly, the test chamber was illuminated with a red light during the FI. When the interval elapsed, the subject had 3 s to press the lever to terminate the red light and avoid an impending electrical stimulus to the tail. A single dose of drug or saline was administered intramuscularly before the beginning of the session. Vehicle or imetit (3–10 mg/kg, i.m.) was administered 30 min before a peak behavioral-stimulant dose of METH or cocaine derived from individual subjects or saline in a quasi-random order and counterbalanced across subjects.
Statistical Analysis
Mean session activity counts for the locomotor experiments in mice were analyzed with a one-way analysis of variance with imetit dose as the main factor. Due to individual differences in rates of responding in the monkey FI experiment, data were individually normalized to responding after saline administration and then averaged as a group mean. Percent control response rates for the monkey FI experiments were analyzed with one-way repeated-measures analysis of variance with imetit dose as the main factor. In the presence of a significant main effect (F test), a Dunnett post hoc test was conducted comparing each imetit dose to either cocaine or METH after the vehicle pretreatment. Significance was set at the 95% confidence level.
Drugs
METH HCl and cocaine HCl were provided by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, N.C., USA). Imetit HBr was purchased from Sigma (St. Louis, Mo., USA). Cocaine and METH were dissolved in sterile physiological saline, and imetit was dissolved in sterile water. All drug doses are expressed as the salt form.
Results
Effects of Imetit on the Behavioral-Stimulant Effects of METH and Cocaine in Mice
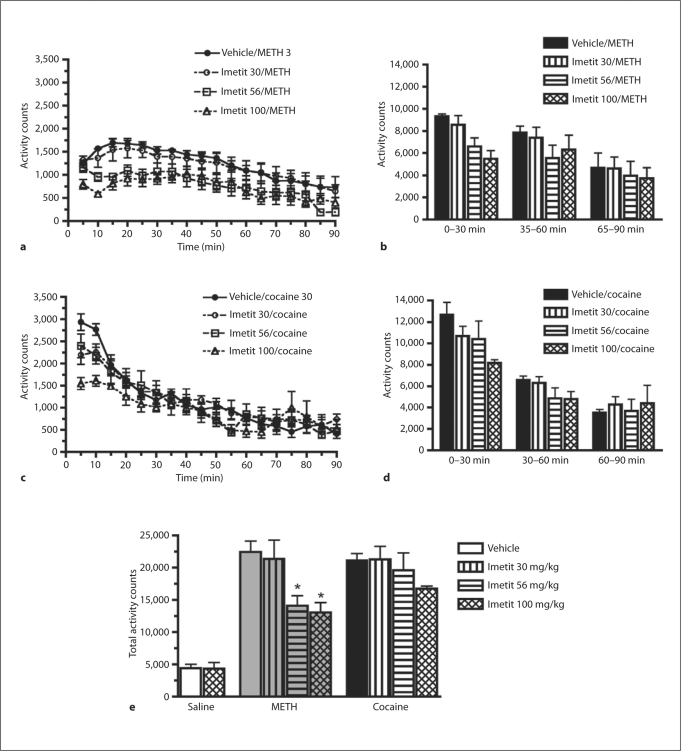
Imetit pretreatment (100 mg/kg, i.p.) 30 min before a saline injection did not significantly alter basal locomotor activity (fig. 1). Pretreatment with imetit significantly decreased (F3, 17 = 4.94, p < 0.05) activity counts after METH, but not cocaine administration (fig. 1). Post hoc analysis demonstrated a significant (p < 0.05) attenuation of locomotor behavior after METH at the 56 and 100 mg/kg imetit pretreatment doses.
Fig. 1.
Effects of imetit (0–100 mg/kg, i.p.) on METH- (a, b) and cocaine-induced (c, d) activity in mice (n = 5–6). Data are shown as means ± SEM. Different symbols represent different pretreatment doses of imetit. * p < 0.05: significantly different from vehicle pretreatment condition.
Effects of Imetit on the Behavioral-Stimulant Effects of METH and Cocaine in Monkeys
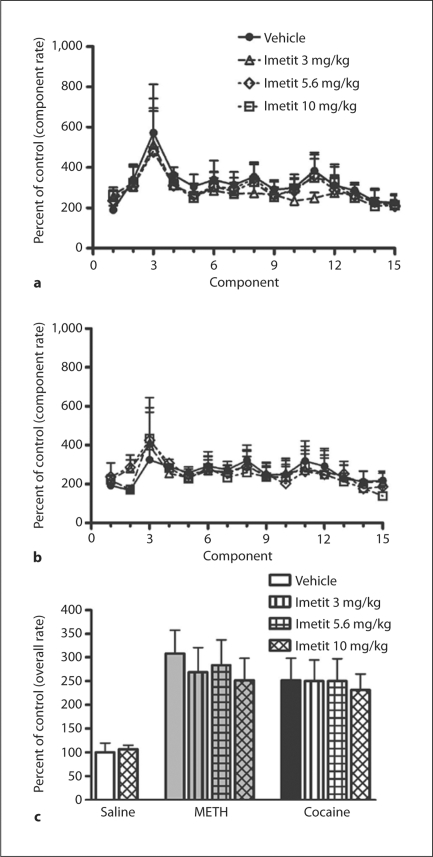
Under the FI schedule, both METH and cocaine dose-dependently increased, responding with the peak dose being 0.3 mg/kg (n = 3) for METH and 0.3 mg/kg (n = 1) or 1.0 mg/kg (n = 2) for cocaine (data not shown). The peak behavioral-stimulant doses for both METH and cocaine determined from individual subjects were used for the imetit interaction studies. Imetit pretreatment (10 mg/kg, i.m.) 30 min before saline administration did not significantly alter baseline responding (fig. 2). Furthermore, imetit pretreatments (3–10 mg/kg, i.m.) had no significant effect on the behavioral-stimulant effects of METH or cocaine (fig. 2).
Fig. 2.
Effects of imetit (0–10 mg/kg, i.m.) on the behavioral-stimulant effects of METH (a) and cocaine (b) in squirrel monkeys (n = 3) trained to respond under an FI schedule of stimulus termination. Data are expressed as mean ± SEM percent control (saline) rate per component (a, b) or session (c). Different symbols represent different pretreatment doses of imetit.
Discussion
The purpose of the present study was to examine the effects of the H3R agonist imetit on the behavioral-stimulant effects of METH and cocaine in both mice and monkeys. Imetit significantly attenuated METH-induced but not cocaine-induced locomotor activity in mice. In contrast, imetit had no significant effect on FI responding after METH or cocaine in monkeys. These results suggest a role of H3R in the behavioral-stimulant effects of METH, but not cocaine, in mice and no role in monkeys.
Previous studies investigating the behavioral interactions between H3R compounds and psychostimulants have provided conflicting results. H3R antagonists have been shown to attenuate METH- or amphetamine-induced locomotor activity [10,11,12]. However, in the present study, an H3R agonist also attenuated METH-induced locomotor activity. Recently, imetit was shown to attenuate locomotor activity induced by either a D1 or D2 receptor agonist [17]. Pretreatment with an H3R antagonist has been shown to either potentiate [13, 14] or have no effect [10] on cocaine-induced increases in locomotor activity. Although there appeared to be a trend towards a dose-dependent decrease in cocaine-induced activity in the early phase (0–30 min) of the monitoring period, overall, the present study found no significant effect of an H3R agonist on cocaine-induced locomotor activity. Whether the lack of a significant effect on cocaine-induced activity was a result of the study being underpowered or a true effect remains unknown. Moreover, the H3R agonist (R)-α-methylhistamine had no significant effect on amphetamine-induced locomotor activity [10]. In contrast, we found that pretreatment with imetit attenuated the METH-induced locomotor response. Differences between the results of the present study and those of Clapham and Kilpatrick [10] most likely reflect potency differences in the H3R agonists administered [18].
A possible explanation for the differential response to imetit between METH and cocaine in mice, independently of dopamine, could be related to histamine release. METH acutely increases extracellular levels of histamine in hypothalamic and striatal regions of rodents [19, 20]. In contrast, methylphenidate, a monoamine uptake inhibitor, does not increase extracellular levels of histamine in the hypothalamus [21]. H3R can function as autoreceptor, regulating the release of histamine [22]. Activation of these H3 autoreceptors should attenuate the release of histamine. Thus, if the behavioral-stimulant effects of METH are mediated in part via histamine release, this could explain why imetit attenuated the locomotor response to METH, but not cocaine.
In contrast to the effects of imetit in mice, the present study found no effect of imetit on the behavioral-stimulant effects of METH and cocaine in monkeys. One possible explanation is that H3R may not be involved in modulating METH- or cocaine-induced dopamine release and subsequent behavioral-stimulant effects. In vivo microdialysis studies examining the effects of H3R agonists on METH- and cocaine-induced dopamine release in monkeys would answer this question. Another explanation, although there is currently no data to support this, might be that imetit is less efficacious in the monkey than the rodent. Unfortunately, higher doses of imetit could not be tested due to solubility issues. A third possibility could be due to methodological differences in the procedures used for measuring the behavioral-stimulant effects of METH and cocaine in monkeys and mice. An operant behavioral procedure was used to assess the behavioral-stimulant effects in monkeys, while an activity procedure was used in mice. To the best of our knowledge, there are no studies directly comparing the results of operant and activity behavioral procedures in assessing the behavioral-stimulant effects of drugs in any species, and thus we do not know whether one procedure is more or less sensitive.
One limitation in interpreting the results of the present study is the selectivity of imetit. For example, the cardiovascular effects of imetit were blocked by cholinergic and serotonergic (5-HT3) antagonists, but not histaminergic (H3) antagonists in anesthetized rats [23]. Furthermore, the recent discovery of the histamine H4 receptor has demonstrated that imetit may only be approximately 10-fold selective for the H3 versus the H4 receptor [24]. Thus, while imetit is one of the most potent H3R agonists available [18, 25], further research with more selective compounds investigating the role of H3R in the behavioral-stimulant effects of METH and cocaine is warranted.
In conclusion, the results of the present study suggest a role of H3R in the behavioral-stimulant effects of METH, but not cocaine, in mice. In contrast to mice, there was no significant effect of the H3R agonist on the behavioral-stimulant effects of METH or cocaine in squirrel monkeys. At present, there is not enough information on H3R and nonhuman primates to propose definitive mechanisms.
Acknowledgements
We appreciate the technical assistance of Mi Zhou and Peggy Plant. This study was funded by National Institutes of Health grants DA15040, DA00517, DA12514, DA21476 and Yerkes base grant RR00165.
References
- 1.Itoh K, Fukumori R, Suzuki Y. Effect of methamphetamine on the locomotor activity in the 6-OHDA dorsal hippocampus lesioned rat. Life Sci. 1984;34:827–833. doi: 10.1016/0024-3205(84)90199-1. [DOI] [PubMed] [Google Scholar]
- 2.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- 4.Passani MB, Lin JS, Hancock A, Crochet S, Blandina P. The histamine H3 receptor as a novel therapeutic target for cognitive and sleep disorders. Trends Pharmacol Sci. 2004;25:618–625. doi: 10.1016/j.tips.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 6.Arrang JM, Roy J, Morgat JL, Schunack W, Schwartz JC. Histamine H3 receptor binding sites in rat brain membranes: modulations by guanine nucleotides and divalent cations. Eur J Pharmacol. 1990;188:219–227. doi: 10.1016/0922-4106(90)90005-i. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Mir MI, Pollard H, Moreau J, Arrang JM, Ruat M, Traiffort E, Schwartz JC, Palacios JM. Three histamine receptors (H1, H2 and H3) visualized in the brain of human and non-human primates. Brain Res. 1990;526:322–327. doi: 10.1016/0006-8993(90)91240-h. [DOI] [PubMed] [Google Scholar]
- 8.Jansen FP, Mochizuki T, Maeyama K, Leurs R, Timmerman H. Characterization of histamine H3 receptors in the mouse brain using the H3 antagonist [125I]iodophenpropit. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:60–67. doi: 10.1007/s002100000227. [DOI] [PubMed] [Google Scholar]
- 9.Munzar P, Tanda G, Justinova Z, Goldberg SR. Histamine H3 receptor antagonists potentiate methamphetamine self-administration and methamphetamine-induced accumbal dopamine release. Neuropsycho- pharmcology. 2004;29:705–717. doi: 10.1038/sj.npp.1300380. [DOI] [PubMed] [Google Scholar]
- 10.Clapham J, Kilpatrick GJ. Thioperamide, the selective histamine H3 receptor antagonist, attenuates stimulant-induced locomotor activity in the mouse. Eur J Pharmacol. 1994;259:107–114. doi: 10.1016/0014-2999(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 11.Morisset S, Pilon C, Tardivel-lacombe J, Weinstein D, Rostene W, Betancur C, Sokoloff P, Schwartz JC, Arrang JM. Acute and chronic effects of methamphetamine on tele-methylhistamine levels in the mouse brain: selective involvement of the D2 and not D3 receptor. J Pharmacol Exp Ther. 2002;300:621–628. doi: 10.1124/jpet.300.2.621. [DOI] [PubMed] [Google Scholar]
- 12.Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, Brownman KE, Buckley MJ, Ballard ME, Komater VA, Miner H, Zhang M, Faghih R, Rueter LE, Bitner RS, Drescher KU, Wetter J, Marsh K, Lemaire M, Porsolt RD, Bennani YL, Sullivan JP, Cowart MD, Decker MW, Hancock AA. Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]. II. Neurophysiologi- cal characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther. 2005;313:176–190. doi: 10.1124/jpet.104.078402. [DOI] [PubMed] [Google Scholar]
- 13.Hyytiä P, Bäckström P, Piepponen P, Ahtee L. Histamine H3-receptor antagonist thioperamide potentiates behavioural effects of cocaine. Eur J Pharm Sci. 2003;19:S25. [Google Scholar]
- 14.Brabant C, Quertemont E, Tirelli E. Effects of the H3-receptor inverse agonist thioperamide on the psychomotor effects induced by acutely and repeatedly given cocaine in C57BL/6J mice. Pharmacol Biochem Behav. 2006;83:561–569. doi: 10.1016/j.pbb.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Kimmel HL, O'Connor JA, Carrol FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86:45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarosh HL, Katz EB, Coop A, Fantegrossi WE. MDMA-like behavioral effects of N-substituted piperazines in the mouse. Pharmacol Biochem Behav. 2007;88:18–27. doi: 10.1016/j.pbb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrada C, Ferre S, Casado V, Cortes A, Justinova Z, Barnes C, Canela EI, Goldberg SR, Leurs R, Lluis C, Franco R. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55:190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krueger KM, Witte DG, Ireland-Denny L, Miller TR, Baranowski JL, Buckner S, Milicic I, Esbenshade TA, Hancock AA. G protein-dependent pharmacology of histamine H3 receptor ligands: evidence for heterogeneous active state receptor conformations. J Pharmacol Exp Ther. 2005;314:271–281. doi: 10.1124/jpet.104.078865. [DOI] [PubMed] [Google Scholar]
- 19.Ito C, Onodera K, Sakurai E, Sato M, Watanabe T. Effects of dopamine antagonists on neuronal histamine release in the striatum of rats subjected to acute and chronic treatments with methamphetamine. J Pharmacol Exp Ther. 1996;279:271–276. [PubMed] [Google Scholar]
- 20.Ito C, Onodera K, Yamatodani A, Watanabe T, Sato M. The effect of methamphetamine on histamine release in the rat hypothalamus. Psychiatry Clin Neurosci. 1997;51:79–81. doi: 10.1111/j.1440-1819.1997.tb02911.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishizuka T, Murakami M, Yamatodani A. Involvement of central histaminergic systems in modafinil-induced but not methylphenidate-induced increases in locomotor activity in rats. Eur J Pharmacol. 2008;578:209–215. doi: 10.1016/j.ejphar.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- 23.Coruzzi G, Gambarelli E, Bertaccini G, Timmerman H. Cardiovascular effects of selective agonists and antagonists of histamine H3 receptors in the anaesthetized rat. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:569–575. doi: 10.1007/BF00170155. [DOI] [PubMed] [Google Scholar]
- 24.Hough LB. Genomics meets histamine receptors: new subtypes, new receptors. Mol Pharmacol. 2001;59:415–419. [PubMed] [Google Scholar]
- 25.Garbarg M, Arrang JM, Rouleau A, Ligneau X, Tuong MD, Schwartz JC, Ganellin CR. S-[2-(imidazyolyl)ethyl]isothiourea, a highly specific and potent histamine H3 receptor agonist. J Pharmacol Exp Ther. 1992;263:304–310. [PubMed] [Google Scholar]