Abstract
Protein kinase C (PKC) is a family of kinases that are critical in many cellular events. These enzymes are activated by lipid-derived second messengers, are dependent on binding to negatively charged phospholipids and some members also require calcium to attain full activation. The interaction with lipids and calcium activators is mediated by binding to the regulatory domains C1 and C2. In addition, many protein-protein interactions between PKC and other proteins have been described. These include interactions with adaptor proteins, substrates and cytoskeletal elements. Regulation of the interactions between PKC, small molecules and other proteins is essential for signal transduction to occur. Finally, a number of auto-inhibitory intramolecular protein-protein interactions have also been identified in PKC. This chapter focuses on mapping the sites for many of these inter and intramolecular interactions and how this information may be used to generate selective inhibitors and activators of PKC signaling.
1. Introduction
In the thirty years of research into the human kinome, more than 400 human diseases have been linked to aberrations in kinase-mediated signaling pathways (1). Modulation of protein kinase activity has been a promising target for drug discovery, but the off-target effect of many kinase inhibitors due to high similarity between the kinase families has largely prohibited the use of these molecules in clinics. To design specific modulators of kinase function, a recent approach is focused on targeting intra- and inter-molecular interactions of this family of enzymes.
Protein kinase C (PKC), a family of serine/threonine kinases, provides an excellent example for the complexity of kinase-mediated signaling. Since first identified (2), the 10 members of the PKC isozyme family have been the subject of intense investigation in academia and in industry. PKC isozymes are highly homologous in their catalytic domain, and their regulatory domains determine the response of individual members to activators. The family of classical PKC isozymes (α, βI, βII, γ) are activated by the second messengers calcium and diacylglycerol (DAG), whereas novel PKC isozymes (ε, δ, θ, η) respond only to DAG (Fig. 1). The atypical family (η, λ/ι) are not responsive to either of the second messengers (3). Upon activation, PKCs translocate from the soluble fraction to cellular membranes, where they bind to anionic phospholipids (4), and are localized to diverse subcellular sites by binding to receptors for activated C Kinase (RACKs), which anchor them nearby a subset of protein substrates and away from others (5). Many of the isozymes are expressed in the same cells, respond to the same activators but translocate to different intracellular sites, to mediate unique and sometimes even opposing functions (6, 7). The complexity of PKC activation, targeting to unique subcellular sites to trigger diverse downstream signaling is mediated by multiple isozyme-specific protein-protein interactions. Here we review a number of intra- and inter-molecular interactions that have been identified so far and how this knowledge has been capitalized to generate selective inhibitors and activators of the individual PKC isozymes. Though phosphorylation of PKCs and other post-translational modifications of the enzymes play critical roles in maturation, activation and signaling through this family of protein kinases, these will not be discussed here as they have been extensively reviewed (8–10).
Figure 1. PKC family of isozymes.
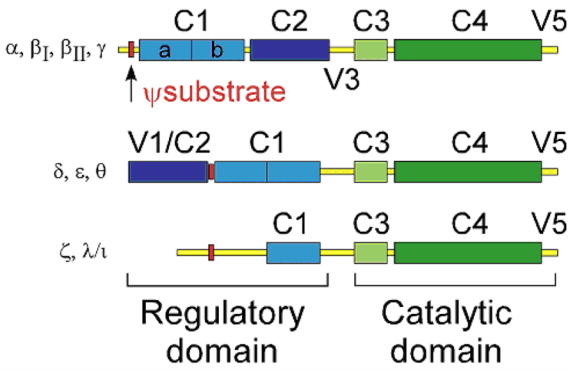
The PKC family of isozymes consists of three classes: the classical (α, βI, βII, γ), novel (ε, δ, θ), and atypical (η,λ/ι) The regulatory domain consists of the C1 and C2 domains, and variable regions (V) 1-3. The V1 region contains the Ψsubstrate sequence (red) that binds the substrate binding site of the catalytic domain; the Ψsubstrate sequence is the most well-known example of inhibitory intramolecular interaction. The classical and novel families contain a duplicate of the C1 domain (light blue) that binds DAG and its analogs, whereas the atypical family contains only one C1 copy. The classical and novel families contain a C2 domain (dark blue), which binds to phosphatidylserine; the classical C2 binds PS in a calcium-dependent manner. The catalytic domain consists of the ATP binding domain C3 (light green) and substrate binding/catalytic domain C4 (dark green). The C-terminus of the protein contains the V5 domain, which contains phosphorylation sites that regulate PKC activity.
Full-length structures of PKC isozymes are still unavailable, likely due to the high degree of flexibility and post-translational modifications within isozymes. However, the structure of each domain has been solved independently and two-dimensional crystals of δPKC present some evidence of the overall orientation of the enzyme (11). Here, we will summarize the known roles for each domain of PKC and discuss the intramolecular interactions that regulate the activation state of the enzyme, as well as intermolecular interactions that determine the specificity of PKC signaling. We will also demonstrate how elucidation of the intramolecular interactions within PKC can lead to the design of effective isozyme-specific activators and inhibitors of PKC function.
2. Mechanism of PKC activation
Cells sense the changes in their environment through signal cascades initiated by receptors on the outer cell membrane. The signal transduction pathway that activates PKC consists of receptor-mediated activation of phopsholipases C, leading to hydrolysis of PtdIns4,5P2 to produce DAG, as well as a rise in intracellular calcium levels (12). Inactive PKCs are found in the soluble fraction of the cells, and translocate upon increases in cellular signaling to various membrane surfaces, where they bind anchoring molecules and phosphorylate neighboring protein substrates (5).
The current model for the activation of the classical PKC isozymes suggests that calcium binding increases the affinity of PKC for phosphatidylserine (PS) at the cell membrane. This, in turn, enables the kinase to laterally ‘search’ for and bind DAG molecules that are found at low abundance in the membrane (13). The binding of the two regulatory domains to the membrane releases the auto-inhibitory pseudosubstrate site (Ψsubstrate) from the active site in the catalytic domain, producing conformational changes that leave the catalytic domain accessible to substrate binding and phosphorylation. The novel family of PKCs do not bind calcium, but have higher affinity for DAG as compared to the classical PKC family (12), producing a fine balance of responsiveness to similar activators for different isozymes. In addition to second-messenger sensing domains, other PKC regions and domains, described below, participate in PKC activation and subcellular localization, resulting in multi-step events leading to PKC activation, localization and function.
Multiple PKC isozymes can be present in the same cell, and can translocate to different subcellular localizations in response to the same stimuli (6). In order to explain this phenomenon, it was hypothesized that each individual PKC isozyme might have an isozyme-selective anchoring protein to which each PKC isozyme binds upon activation. These anchoring proteins, termed receptors for activated C Kinase (RACKs), are hypothesized to anchor specific PKC isozymes at unique subcellular locations (5). Thus, anchoring of a specific PKC isozyme to its respective RACK localizes that PKC isozyme in close proximity to its isozyme-specific protein substrates. Subcellular translocation and binding to isozyme-selective RACKs can therefore bestow functional specificity for each PKC isozyme. Two RACKs have been identified to date: the RACK for βIIPKC, known as RACK1 (14), and the RACK for εPKC, known as RACK2 or β′COP (15). The specificity of PKC-RACK interaction is thought to be mediated by the C2 and the V5 domains, discussed in detail below, though further characterization of these proteins may elucidate more isozyme-selective interaction sites.
In order to better understand isozyme-specific roles and activation mechanisms, whole enzyme and individual PKC domains are used to study protein-protein interactions and translocation of PKC isozymes. The complexity of PKC signaling becomes increasingly apparent as our understanding of the mechanism of PKC function is elucidated. For example, the mechanism underlying PKC movement from the cytosol to the membrane is still debated. There is evidence that PKC translocation is dependent on cytoskeletal elements (16, 17), yet studies calculating the accumulation of PKC at the membrane suggest that the translocation process is diffusion-limited (e.g., (18)). However, studies concluding that PKC translocation depends only on the speed of diffusion utilized over-expressed tagged PKC, whereas those favoring active transport were conducted with endogenous proteins. It is therefore possible that over-expression saturates the putative machinery required for active PKC translocation. If PKC translocation involves active transport along the cytoskeletal elements, a new set of protein-protein interactions may be involved in the translocation mechanism.
3. Chimera studies
The high degree of similarity between PKC isozymes has led to the adoption of various approaches aimed at elucidating specific roles of PKC domains in regulating PKC isozyme-specific functions. A number of these approaches have been recently reviewed (19, 20) and will not be discussed extensively here. One approach to elucidate the role of different domains of homologous proteins with minimal protein perturbation is through construction of chimera enzymes. This approach has helped to identify domains in PKC that provide unique properties for each isozyme, as well as the inter-domain interaction relationships. For example, chimera studies of regulatory domains of ε and δPKC have helped to understand the roles of these isozymes in cellular morphological changes, differentiation, proliferation, and tumorgenicity. While the εPKC regulatory domain was shown to have a growth-promoting effect and its catalytic domain confers tumorgenicity, the δPKC regulatory domain inhibits cell growth and the catalytic domain drives macrophage differentiation (21–23). The catalytic domains of the chimeras sometimes affected the responsiveness of the regulatory domains to activators, suggesting complex contributions of the different domains to the overall PKC activation mechanism and a role for protein-protein interactions within the enzyme (24). Chimera studies also showed that substrate specificity can be determined by the regulatory domain, suggesting that domains other than the catalytic core bind substrates or affect selectivity indirectly (25). Therefore, a complex network of intermolecular interactions with activators, substrates and regulatory proteins, as well as intramolecular interactions between the domains, contributes to the unique activity of each PKC isozyme.
4. Role of PKC domains in intra- and inter-molecular interactions
4.1 The pseudosubstrate site
A pseudosubstrate site (Ψ–substrate), located in the N-terminus of the C1 domain, was the first partner for intramolecular inhibitory interactions described for PKC (26) (see Fig. 2 for a schema). This site, identified by Kemp and colleagues, mimics the substrate consensus sequence, except that instead of a phosphorylatable serine/threonine residue, there is an alanine within the PKC substrate consensus site. This feature enables the Ψ-substrate sequence to bind to the substrate-binding site in the catalytic domain without serving as a substrate for the enzyme. Mutation of this sequence (alanine for serine or for glutamate, to mimic the charge of a phosphorylated residue) abrogates the inhibitory intramolecular interaction, resulting in activated PKC (27). Interestingly, deletion of the Ψ–substrate site did not abrogate the inhibitory effect of the regulatory domain of αPKC in the full-length enzyme (28). It was therefore apparent that the Ψ–substrate sequence is likely to be one of several sites of inhibitory intramolecular interactions.
Figure 2. A schematic showing how some inter- and intra- molecular interactions are disrupted and others established when PKC is activated.
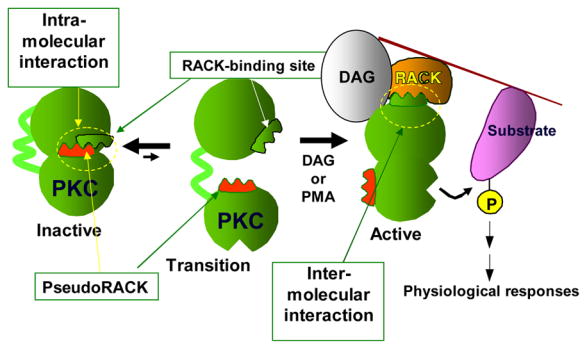
PKC contains several intra-molecular interactions that keep the enzyme in the inactive state. For simplicity, we focus on one such interaction between the RACK binding site and a site in PKC that is homologous to the RACK sequence and is therefore termed pseudoRACK. These intra-molecular interactions can be disrupted spontaneously to create an open transition state. However, most of the time, the inter-molecular interactions are maintained. When there is a rise in diacylglycerol (DAG) (and calcium for the calcium-sensitive PKCs) or when cells are treated with the tumor promoter phorbol ester (PMA), the open state is stabilized by binding of the enzyme to membranes and the corresponding RACK, resulting in its anchoring nearby a particular substrate and away from others. The numerous intra- and inter-molecular interactions can be disrupted to yield selective regulators of PKC functions.
The Ψ-substrate site is proposed to participate also in several intermolecular interactions. It is rich in basic residues and was found to bind directly to acidic lipids in the membrane (29) to drive subcellular localization of recombinant proteins to the plasma membrane and cytoskeletal components (30). In addition, the Ψ–substrate site binds to PKC substrates, such as ZIP (31). Therefore, once released from intramolecular inhibitory interaction with the catalytic domain, the Ψ–substrate may mediate a variety of intermolecular interactions, bringing the kinase to its cellular substrates. However, since the Ψ–substrate in all the classical isozymes is identical and is quite well conserved among other isozymes (Arg19-Phe-Ala-Arg-Lys-Gly-Ala25-Leu-Arg-Gln-Lys-Asn-Val-His-Glu-Val-Lys-Asn36), it is unlikely that Ψ–substrate alone can provide sufficient selectivity to explain the diverse location and functions of individual isozymes.
4.2. The C1 domain
The C1 domains in the regulatory domain of the classical and novel PKC isozymes bind the second messenger, DAG, as well as the tumor promoters, phorbol esters. The C1 domain in both classical and novel families of PKC consists of two tandem repeats of cysteine-rich zinc fingers, A and B, that depend on zinc ions for both proper folding and function (32); the atypical PKC family has only one such subdomain. While both C1 repeats are thought to be oriented for potential membrane interaction and can bind DAG and phorbol esters, only one of the C1 repeats usually binds this second messenger (33). Interestingly, these sub-domains differ between the isozymes in their binding affinity for DAG and phorbol ester. For example, α and δ PKC bind DAG with greater affinity in the C1A and phorbol ester in the C1B sub-domain (34), whereas γPKC binds DAG and phorbol esters equally well with both repeats. In addition, the γC1 domain is not conformationally restricted by other domains, as discussed below, allowing higher sensitivity of γPKC to DAG increases than observed for αPKC (35). Similarly, in the novel PKC family, the C1 domain repeats of εPKC are much less conformationally restricted as compared to the C1 domain of δPKC, thus allowing higher sensitivity to DAG for εPKC (36). In this manner, PKC isozymes of the same family can be preferentially activated based on the amplitude of the signal generated upon cellular activation. However, not all ligands of the C1 domain are activating: resveratrol, a polyphenolic phytoaxelin found in red wine and an anti-tumor compound AD 198 compete with phorbol esters for the C1b binding site, but, although they cause association of PKC with membranes, they do not activate the enzyme (37).
In addition to DAG binding, the C1 domain has been implicated in PKC targeting to subcellular sites through both lipid and protein interactions. αPKC can be activated by alcohols and anesthetics, which bind in spatially distinct regions from the DAG on the C1 domain (38). The C1B domain has been reported to be important for specific subcellular targeting of PKC to Golgi (39), possibly through arachidonic acid binding (40). In addition, the C1 domain of εPKC contains a unique motif between the two cysteine-rich repeats, which specifically binds to actin at cell adhesion sites upon activation of εPKC (41). This motif is not available for protein-protein interaction when εPKC is inactive, indicating that conformational changes occur within the C1 domain after PKC activation. The same region was also observed to be crucial for neurite induction (42). Atypical PKCs shuttle from the cytoplasm to the nucleus and contain both a nuclear localization (NLS) and a nuclear export (NES) sequence in the N-terminal and C-terminal part of the C1 region (43). Together, these data indicate the importance of the C1 domain not only in binding and responding to second messenger generation, but also in PKC isozyme-specific sub-cellular targeting and activation responses.
4.3. The C2 domain – a site for lipid and calcium binding
The C2 domain of the classical PKC family binds two to three calcium ions, which induce both electrostatic and conformational changes, as well as enable phosphatidylserine binding and membrane penetration of the domain (44). The classical PKC isozymes differ in their calcium binding affinities, cooperativity and stoichiometry of binding. Therefore, similar to the difference in the response to DAG through the C1 domains of each PKC isozyme, the C2 domains of the classical PKC isozymes are differentially regulated by the amount of cellular calcium released in response to cell stimulation (45). The C2 domains of the novel PKC isozymes share very low sequence identity with the classical C2 domains (~15%) and do not have a calcium-binding site (46), rendering the novel C2 domain insensitive to calcium. Nevertheless, both classical and novel isozymes have the same protein fold consisting of an eight-stranded, anti-parallel, beta-sandwich (47). The C2 domain of the novel PKC isozymes is regulated further by phosphorylation, which increases the affinity of the domain towards membranes, possibly to substitute for the requirement for calcium (48).
The C2 domain binds to the membrane with its beta-sheet parallel to the membrane surface (49), and in the case of αPKC, this position places the lysine-rich cluster, located on beta-strands 3 and 4, to bind PtdIns4,5P2 (50). Retinoic acid binds both lipid-binding sites of the αC2: the site of calcium-mediated binding, as well as the lysine-rich cluster (51), Therefore, the C2 domain is regulated by at least two classes of cellular signaling molecules, calcium and lipids. Differential production of these signaling molecules is likely to regulate the activation of different PKC isozymes (52).
Membrane binding is only a part of the function of the C2 domain, and the importance of this domain in intramolecular interaction is well documented (53). The C2 domain affects the affinity of the C1 domain to activators (54), and removal of this domain increases the sensitivity of PKC to DAG/phorbol esters (55). The C2 domain activation through calcium binding can mediate association of the C1 domain with lipids such as arachidonic acid (56), and molecular modeling suggests that the C1 domain may provide carboxylate or carbonyl groups of specific amino acids for calcium binding of the C2 domain (57). Interaction of the C1 and C2 domains also provides means for PKC dimerization, an interaction that may also lead to cross-regulating each other (58). Together, these data suggest that the C2 domain participates in inhibitory intramolecular interaction with the C1 domain which is relieved when intracellular calcium increases, leading first to a conformation change within the C2 domain and subsequent binding of the C1 domain to DAG, which causes further activation of the enzyme.
4.4. The C2 domain – a critical domain for subcellular location
A critical role for the C2 domain in anchoring of individual PKC isozymes to diverse subcellular sites has been demonstrated by a number of studies from our own laboratory. The first study to suggest a role for the C2 domain in protein-protein-mediated anchoring stemmed from an observation in 1992 that the C2 domain is present in synaptotagmin (aka p65). p65 contains mainly two C2 domain repeats and the location of the protein is in synaptic vesicles, a site where PKC is not present. We therefore reasoned that in addition to calcium sensing and PS binding activities, the C2 domain must mediate also unique protein-protein interactions. We subsequently showed that the C2 domain binds to the βPKC-specific RACK with an affinity that is about 100 times lower than that of βPKC (59) and that peptides derived from the sequence that are most homologous between the C2 domains in p65 and in PKC are those containing the protein-interaction sequences; peptides derived from these regions in the C2 domain of βPKC selectively inhibit βPKC translocation and function (60).
Subsequent studies using the C2 domains of δ and εPKC demonstrated that the C2 domain acts as a selective inhibitor of translocation and function of the corresponding isozymes. Using this fragment (amino acids 2-142) derived from δPKC and εPKC, we demonstrated that the two isozymes have opposing roles in regulating contraction rate of heart muscle cells in culture (61). Subsequent studies (described in part in another chapter in this volume) identified short peptides that correspond to the RACK-binding sites on the C2 domains; peptides corresponding to these sequences acted as selective inhibitors of the respective isozymes. Table 1 summarizes some of the cell functions and potential disease association of individual PKC isozymes identified by using such peptide inhibitors of individual PKC isozymes.
Table 1.
Examples of application of PKC regulating peptide in models of human diseases
| PKC ISOZYME | MECHANISM | INDICATIONS |
|---|---|---|
| ↓αPKC | Tumor proliferation | Oncology |
| ↓βI/βIIPKC | Cell growth
Insulin release |
Heart failure
Diabetes |
| ↓γPKC | Dorsal root signaling | Pain, addiction |
| ↓δPKC | Reperfusion injury
Fibroblast proliferation |
Myocardial infarction
Stroke Heart failure |
| ↑δPKC | Apoptosis, free radicals | Oncology |
| ↓εPKC | nociception | Pain |
| ↑εPKC | Cytoprotection, preconditioning | Organ transplant |
| ↓θPKC | Immune modulation | Immune suppression |
| ↑θPKC | Immune modulation | Immune enhancement |
Each C2 domain of the PKCs also contains a short sequence that is homologous to a sequence in their corresponding RACKs. This sequence, measuring 6-10 amino acids in length, was termed pseudo-RACK (ΨRACK) site, for its homology with RACK. The first ΨRACK sequence was originally identified through a sequence comparison of βPKC with its RACK, RACK1 (62). The sequence SVEIWD in βPKC (amino acids 241-246) is homologous to the sequence SIKIWD in RACK1 (amino acids 234-241), the βIIPKC-selective RACK (14). It contains a relatively rare amino acid sequence WD, but also one apparent charge difference (K to E). We proposed and subsequently demonstrated that this charge difference confers a lower intramolecular affinity of the ΨRACK for the RACK-binding site within PKC as compared with the intermolecular affinity of the RACK-binding site for RACK itself (63); the lower affinity of the intramolecular auto-inhibitory interaction allows its disruption and consequent binding of PKC to RACK. The involvement of ΨRACK sequences in auto-inhibitory intramolecular interactions was demonstrated using three approaches. First, using εPKC as an example, we showed that a single amino-acid mutation in the ΨRACK site alters the kinetics of enzyme activation in cells (64). Second, a peptide designed from this region activates the PKC isozyme from which it was derived, thus allowing isozyme-specific activity modulation (62). Finally, a single amino acid substitution at the charged position to alanine rendered ΨεRACK inactive and a substitution to the charge found in the εRACK conferred higher affinity of the peptide for εPKC, and thus that peptide acted as a competitive inhibitor of εPKC with its RACK (Liron et al., submitted). Therefore, the ΨRACK site participates in an auto-inhibitory interaction, similar to the Ψ–substrate site, and activation of the enzyme depends on the disruption of this intramolecular interaction (Fig. 2)
Importantly, peptides corresponding to the ΨRACK sequence served as isozyme-selective activators and, together with the C2-derived inhibitors, helped identify isozyme-selective functions in vitro and in vivo (Table 1). Note that although these 6-10 amino acid long peptides are not cell permeable, they can be readily delivered into cells by conjugating them to cell permeable arginine-rich polypeptide, such as antennapedia or TAT47–57 (63). Conjugation of the PKC regulating peptide (cargo) to the carrier (Arg-rich peptide) was mediated by adding a cystine residue to each peptide and conjugation through an S-S bond. Because of the redox potential, this S-S bond likely breaks inside cells, releasing the cargo, trapping it inside the cells and allowing it to interact with the target protein without steric hindrance from the carrier (63). We showed that this conjugate can be effectively delivered in vitro and in vivo, following intraperitoneal, intra-arterial or subcutaneous sustained delivery, using an Alzet pump, without desensitization or adverse side effects.
The C2 domain also participates in other types of intermolecular interactions. Binding of PKC substrates, for example GAP-43 and lamin (65), has been shown to be stabilized by the C2 domain, and new modes of interaction, such as binding of δC2 to phosphotyrosine peptides (66), have been described. In addition, a number of PKC-regulating interactions through binding to the C2 domain have been reported, for example the interaction of PKC with calponin (67), calseqestrin (68), actin (69) and annexin V (70).
In the case of annexin V, we demonstrated a transient interaction between annexin V and δPKC that occurs in cells after δPKC stimulation, but before δPKC translocates to the particulate fraction (70). Evidence of δPKC/annexin V binding is provided by both FRET studies on cells and by in vitro binding studies, where both the C2 domain and the V5 domain (see in the following) of δPKC and not εPKC bind to recombinant annexin V. We also found that depletion of endogenous annexin V but not annexin IV, using siRNA, inhibited δPKC translocation following PKC stimulation and that dissociation of the δPKC/annexin V complex in cells requires ATP and microtubule integrity. Further demonstration of the physiological importance of this interaction was provided by the use of a rationally designed eight amino acid peptide, corresponding to the interaction site for δPKC on annexin V. This peptide inhibited δPKC translocation and δPKC-mediated function (70). A role for annexins in PKC activation may be a common theme in PKC signaling. Previous reports suggest that individual PKC isozymes interact with unique members of the annexin family (e.g., βPKC/annexin I, εPKC/annexin II and αPKC/annexin VI) (71). It is not known, however, whether as for δPKC and annexin V, the biological activity of other PKC isozymes requires a step of binding to annexin and whether this step precedes translocation of the corresponding PKC isozyme.
Together, the C2 domain inter- and intra-molecular interactions have been demonstrated to be critical for PKC activation, translocation, binding to protein substrates and anchoring. Importantly, inhibition of both intra and intermolecular protein-protein interactions via the C2 domain has proved to be a successful tactic for regulation of individual isozymes (63).
4.5. The V3 region
The V3 region, sometimes referred to as the hinge region, is situated between the regulatory domain and the catalytic domain of PKC, and is highly accessible to proteolytic cleavage upon activation and conformational change of PKC (72). Cleavage at the V3 region results in release of a constitutively active catalytic domain, suggesting that the majority or possibly all the inhibitory intramolecular interactions occur between the regulatory and catalytic domain. In δPKC, proteolytic cleavage of this region is highly regulated by phosphorylation (10). Though the V3 region is mostly known for its proteolytic sites, its importance in protein-protein interaction has been suggested. The V3 region is involved in targeting of α and εPKC to cell-cell contacts (73), binding of αPKC to β1-integrin (74) and potentially in targeting εPKC for ubiquitin-dependent destruction (75).
4.6. The catalytic domain and the V5 region
The C3 domain, containing the ATP binding site, and the C4 domain, responsible for substrate binding, make up the catalytic core of PKC (76). These domains are highly homologous between many kinases (77), and therefore will not be discussed in detail here.
The carboxy-terminus of all PKCs contains phosphorylation sites, such as the turn, and hydrophobic motifs that are vital for kinase processing, localization, and activity (9). Regulation of the V5 region autophosphorylation involves a balance between HSP70 and PDK1 binding, which protects the region from de-phosphorylation and degradation (78). Crystal structure of the catalytic domain illustrates that the phosphorylation motifs participate in an intramolecular clamp with the N-terminal lobe of the kinase and order both the structure of the domain and the activation loop (79), similar to that seen with AKT (80). Interestingly, phosphorylation at the hydrophobic motif affects Ca2+ affinity of PKC (81), demonstrating that the V5 domain interacts with the C2 domain. This interaction is terminated upon PKC activation (9), which releases both the V5 and the C2 regions to participate in intermolecular protein-protein, as well as lipid interactions (82, 83). In fact, there are multiple examples of participation of the V5 domain in important interactions. For example, the V5 region of βIIPKC drives translocation of PKC to nuclear membranes through binding to phosphatidylglycerol (84), and the V5 of αPKC contains a PDZ binding domain, which interacts with PICK for proper localization (85). Similarly, βIIV5 contains a RACK-binding site, and inhibition of this interaction blocks βIIPKC specific signaling (82). The RACK-binding site on V5 likely participates in inhibitory intramolecular interaction with the ΨRACK site, located in the C2 region, while PKC is in an inactive conformation (83). Studies comparing the rate of translocation of the regulatory domain, isolated C2, and holo-enzyme also suggested that the αV5 domain interacts directly with the αC2 domain (86).
We recently demonstrated that the V5 and the C2 domain of εPKC bind to each other in vitro. A pulldown assay of MBP-εC2 was conducted with immobilized GST-εV5. εC2, but not δC2, bound to εV5 (Kheifets and Mochly-Rosen, unpublished results), demonstrating that intramolecular interaction occurs in vitro between the V5 and the C2 domains of εPKC. Further, the data demonstrate that the affinity of the εV5 to the C2 domain is isozyme specific: the interaction between domains of the same isozyme is greater than between two different isozymes of the same family.
Finally, in addition to participating in numerous intramolecular and intermolecular interactions, the V5 region contains a nuclear localization sequence (NLS), present most clearly in δPKC, but to some degree in all V5 regions of PKC isozymes (87). It is likely that abrogation of inhibitory intramolecular interaction with both the catalytic core and the C2 domain upon PKC activation liberates the V5 region to participate in PKC targeting through protein-protein interactions. Together, these data indicate that the V5 region is a good target for design of isozyme specific modulators of PKC activity.
5. Lessons from intra- and inter-molecular interactions of PKC
This review demonstrates that over 20 molecules (proteins, lipids, and second messengers) can interact with PKC through different domains in the enzyme and that there are several regulatable intramolecular protein-protein interactions within PKC (Fig. 3). We also show that modulators of these interactions provide effective isozyme-specific activators and inhibitors. A disruption of, or competition with, inhibitory intramolecular interactions such as those between the C2 and C1 domains, and the C2 and V5 domains, appear to cause or stabilze a conformational change leading to activation of the enzyme. Proof of concept already exists for the inhibitory intramolecular interaction mediated by the ΨRACK sites in all the classical and novel PKC isozymes. Incubation of PKC with a peptide corresponding to the ΨRACK sequence located in the C2 domain increases the enzyme sensitivity to proteolysis in vitro and leads to selective PKC activation in vivo (62, 64, 88). These data suggest that the ΨRACK site interacts with the RACK-binding site, possibly on the V5 domain, and that incubation of the enzyme with the ΨRACK peptide stabilizes the open enzyme, shifting the equilibrium towards the active state (89) (Fig. 2]. Since each PKC isozyme contains specific sequences that modulate its activation state, peptides derived from such isozyme-specific interaction sites activate only the PKC isozyme from which they were designed. In this manner, the first truly selective modulators of PKC activity were made.
Figure 3. PKC inter- and intra- molecular interaction sites mapped on individual PKC domains.
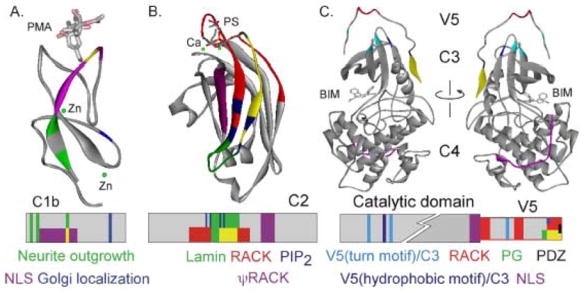
A. Interactions are mapped on the δC1b domain (adapted from 1PTQ (93)) crystallized with PMA (stick figure) and zinc (green). Regions responsible for neurite outgrowth are indicated in green (42), nuclear localization sequence in purple (43), and golgi localization signal (94) in blue. Intersection of green and purple region is indicated in yellow. Lower panel shows the schematic of the domain with color-coded interaction regions. B. Interactions are mapped on the αC2 domain (adapted from 1DSY (95)) crystallized with phosphatidylserine (stick figure) and calcium (green). Regions responsible for RACK binding (red (60)), PIP2 binding (dark blue (50)), lamin binding (green (92)) and the ΨRACK site (purple (62)) are indicated. Intersection of green and red region is indicated in yellow. Lower panel shows the schematic of the domain with color-coded interaction regions. C. Interactions are mapped on the ιPKC catalytic domain (adapted from 1ZRZ (79)) crystallized with bis(indolyl)maleimide inhibitor (BIM1). Regions responsible for RACK binding (red (82)), nuclear localization sequence (purple (87)), phosphatidylglycerol binding (green (84)), PDZ interaction domain (black (85)), and V5/catalytic core interactions (light and dark blue (79)) are indicated. The front and back of the structure is shown for ease of visualization. Intersection of green and red region is indicated in yellow. Lower panel shows the schematic of the domain with color-coded interaction regions.
As the isozyme-specific PKC activator peptides were shown to be selective, the same concept was used to design inhibitors of one of the last steps of the PKC activation mechanism—binding of PKC to its RACK. Since each isozyme translocates to a unique subcellular site and phosphorylates a specific set of substrates at that location, it is highly likely that individual PKC isozymes bind to isozyme-specific RACKs. Disruption of both inter- and intra-molecular interaction using inhibitor and activator peptides designed by these methods have been shown to modulate PKC translocation and function in cells, organs, and animal [56, 57, 79, 85, 87–90] (Table 1).
Importantly, peptides corresponding to the interaction site of PKCs with their respective RACKs are not the only isozyme-specific modulators of interaction. Interaction of βIIPKC with actin (90) and with phosphatidylglycerol (84) were successfully disrupted using a V5 domain-derived peptide. Similarly, the interaction of εPKC with actin (91) was inhibited using a peptide corresponding to the unique actin binding-site on εPKC. Interaction of αPKC with lamin was similarly disrupted with an αPKC-derived peptide designed from their interaction sequence (92), as was the binding with beta 1 integrin (74) from the respective interaction site. In addition, it was recently shown that interference with the mechanism of translocation, as seen for δPKC and annexin V, is also an effective and isozyme-specific method to modulate PKC activity and function (70).
6. Conclusions
In this review, we describe a fine-tuned mechanism for the regulation of PKC involving a series of intra- and inter-molecular interactions. We demonstrate that in-depth knowledge of such interactions can lead to the identification of specific activators and inhibitors of PKC activity. Together, these data indicate that we can utilize the uniqueness and selectivity of intra- and intermolecular interaction of the PKC family of kinases toward the design of isozyme-specific modulators of PKC activity and function. As we progress in our understanding of interactions that occur within PKC and with other proteins, inhibitors or activators of these interactions will allow control of the activity of each isozyme in the system and stimulus of interest. As other kinase families share many similarities with PKC, knowledge gained from the study of the PKC family will greatly advance the goal of controlling the 400 human diseases linked to the aberrations of kinase-mediated signaling pathways.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 2.Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977;252(21):7610–6. [PubMed] [Google Scholar]
- 3.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332 ( Pt 2):281–92. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JE, Giorgione J, Newton AC. The C1 and C2 domains of protein kinase C are independent membrane targeting modules, with specificity for phosphatidylserine conferred by the C1 domain. Biochemistry. 2000;39(37):11360–9. doi: 10.1021/bi000902c. [DOI] [PubMed] [Google Scholar]
- 5.Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995;268(5208):247–51. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 6.Disatnik MH, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res. 1994;210(2):287–97. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 7.Goodnight JA, Mischak H, Kolch W, Mushinski JF. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. Isoform-specific association with microfilaments, Golgi, endoplasmic reticulum, and nuclear and cell membranes. J Biol Chem. 1995;270(17):9991–10001. doi: 10.1074/jbc.270.17.9991. [DOI] [PubMed] [Google Scholar]
- 8.Borner C, Filipuzzi I, Wartmann M, Eppenberger U, Fabbro D. Biosynthesis and posttranslational modifications of protein kinase C in human breast cancer cells. J Biol Chem. 1989;264(23):13902–9. [PubMed] [Google Scholar]
- 9.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370(Pt 2):361–71. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem J. 2004;384(Pt 3):449–59. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solodukhin AS, Caldwell HL, Sando JJ, Kretsinger RH. Two-dimensional crystal structures of protein kinase C-delta, its regulatory domain, and the enzyme complexed with myelin basic protein. Biophys J. 2002;82(5):2700–8. doi: 10.1016/S0006-3495(02)75611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233(4761):305–12. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 13.Nalefski EA, Newton AC. Membrane binding kinetics of protein kinase C betaII mediated by the C2 domain. Biochemistry. 2001;40(44):13216–29. doi: 10.1021/bi010761u. [DOI] [PubMed] [Google Scholar]
- 14.Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci U S A. 1994;91(3):839–43. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Csukai M, Chen CH, De Matteis MA, Mochly-Rosen D. The coatomer protein beta′-COP, a selective binding protein (RACK) for protein kinase Cepsilon. J Biol Chem. 1997;272(46):29200–6. doi: 10.1074/jbc.272.46.29200. [DOI] [PubMed] [Google Scholar]
- 16.Schmalz D, Kalkbrenner F, Hucho F, Buchner K. Transport of protein kinase C alpha into the nucleus requires intact cytoskeleton while the transport of a protein containing a canonical nuclear localization signal does not. J Cell Sci. 1996;109 ( Pt 9):2401–6. doi: 10.1242/jcs.109.9.2401. [DOI] [PubMed] [Google Scholar]
- 17.Dykes AC, Fultz ME, Norton ML, Wright GL. Microtubule-dependent PKC-alpha localization in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2003;285(1):C76–87. doi: 10.1152/ajpcell.00515.2002. [DOI] [PubMed] [Google Scholar]
- 18.Saito K, Ito E, Takakuwa Y, Tamura M, Kinjo M. In situ observation of mobility and anchoring of PKCbetaI in plasma membrane. FEBS Lett. 2003;541(1–3):126–31. doi: 10.1016/s0014-5793(03)00324-7. [DOI] [PubMed] [Google Scholar]
- 19.Schechtman D, Mochly-Rosen D, Ron D. Glutathione S-transferase pull-down assay. Methods Mol Biol. 2003;233:345–50. doi: 10.1385/1-59259-397-6:345. [DOI] [PubMed] [Google Scholar]
- 20.Schechtman D, Murriel C, Bright R, Mochly-Rosen D. Overlay method for detecting protein-protein interactions. Methods Mol Biol. 2003;233:351–7. doi: 10.1385/1-59259-397-6:351. [DOI] [PubMed] [Google Scholar]
- 21.Acs P, Wang QJ, Bogi K, et al. Both the catalytic and regulatory domains of protein kinase C chimeras modulate the proliferative properties of NIH 3T3 cells. J Biol Chem. 1997;272(45):28793–9. doi: 10.1074/jbc.272.45.28793. [DOI] [PubMed] [Google Scholar]
- 22.Wang QJ, Acs P, Goodnight J, et al. The catalytic domain of protein kinase C-delta in reciprocal delta and epsilon chimeras mediates phorbol ester-induced macrophage differentiation of mouse promyelocytes. J Biol Chem. 1997;272(1):76–82. doi: 10.1074/jbc.272.1.76. [DOI] [PubMed] [Google Scholar]
- 23.Wang QJ, Acs P, Goodnight J, Blumberg PM, Mischak H, Mushinski JF. The catalytic domain of PKC-epsilon, in reciprocal PKC-delta and -epsilon chimeras, is responsible for conferring tumorgenicity to NIH3T3 cells, whereas both regulatory and catalytic domains of PKC-epsilon contribute to in vitro transformation. Oncogene. 1998;16(1):53–60. doi: 10.1038/sj.onc.1201507. [DOI] [PubMed] [Google Scholar]
- 24.Acs P, Bogi K, Lorenzo PS, et al. The catalytic domain of protein kinase C chimeras modulates the affinity and targeting of phorbol ester-induced translocation. J Biol Chem. 1997;272(35):22148–53. doi: 10.1074/jbc.272.35.22148. [DOI] [PubMed] [Google Scholar]
- 25.Pears C, Schaap D, Parker PJ. The regulatory domain of protein kinase C-epsilon restricts the catalytic-domain-specificity. Biochem J. 1991;276 ( Pt 1):257–60. doi: 10.1042/bj2760257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238(4834):1726–8. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 27.Pears CJ, Kour G, House C, Kemp BE, Parker PJ. Mutagenesis of the pseudosubstrate site of protein kinase C leads to activation. Eur J Biochem. 1990;194(1):89–94. doi: 10.1111/j.1432-1033.1990.tb19431.x. [DOI] [PubMed] [Google Scholar]
- 28.Parissenti AM, Kirwan AF, Kim SA, Colantonio CM, Schimmer BP. Inhibitory properties of the regulatory domains of human protein kinase Calpha and mouse protein kinase Cepsilon. J Biol Chem. 1998;273(15):8940–5. doi: 10.1074/jbc.273.15.8940. [DOI] [PubMed] [Google Scholar]
- 29.Mosior M, McLaughlin S. Peptides that mimic the pseudosubstrate region of protein kinase C bind to acidic lipids in membranes. Biophys J. 1991;60(1):149–59. doi: 10.1016/S0006-3495(91)82038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehel C, Olah Z, Jakab G, et al. Protein kinase C epsilon subcellular localization domains and proteolytic degradation sites. A model for protein kinase C conformational changes. J Biol Chem. 1995;270(33):19651–8. doi: 10.1074/jbc.270.33.19651. [DOI] [PubMed] [Google Scholar]
- 31.Liao L, Hyatt SL, Chapline C, Jaken S. Protein kinase C domains involved in interactions with other proteins. Biochemistry. 1994;33(5):1229–33. doi: 10.1021/bi00171a024. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda H, Irie K, Nakahara A, Ohigashi H, Wender PA. Solid-phase synthesis, mass spectrometric analysis of the zinc-folding, and phorbol ester-binding studies of the 116-mer peptide containing the tandem cysteine-rich C1 domains of protein kinase C gamma. Bioorg Med Chem. 1999;7(6):1213–21. doi: 10.1016/s0968-0896(99)00037-1. [DOI] [PubMed] [Google Scholar]
- 33.Giorgione J, Hysell M, Harvey DF, Newton AC. Contribution of the C1A and C1B domains to the membrane interaction of protein kinase C. Biochemistry. 2003;42(38):11194–202. doi: 10.1021/bi0350046. [DOI] [PubMed] [Google Scholar]
- 34.Stahelin RV, Digman MA, Medkova M, et al. Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Cdelta. J Biol Chem. 2004;279(28):29501–12. doi: 10.1074/jbc.M403191200. [DOI] [PubMed] [Google Scholar]
- 35.Ananthanarayanan B, Stahelin RV, Digman MA, Cho W. Activation mechanisms of conventional protein kinase C isoforms are determined by the ligand affinity and conformational flexibility of their C1 domains. J Biol Chem. 2003;278(47):46886–94. doi: 10.1074/jbc.M307853200. [DOI] [PubMed] [Google Scholar]
- 36.Stahelin RV, Digman MA, Medkova M, et al. Diacylglycerol-induced membrane targeting and activation of protein kinase Cepsilon: mechanistic differences between protein kinases Cdelta and Cepsilon. J Biol Chem. 2005;280(20):19784–93. doi: 10.1074/jbc.M411285200. [DOI] [PubMed] [Google Scholar]
- 37.Roaten JB, Kazanietz MG, Caloca MJ, et al. Interaction of the novel anthracycline antitumor agent N-benzyladriamycin-14-valerate with the C1-regulatory domain of protein kinase C: structural requirements, isoform specificity, and correlation with drug cytotoxicity. Mol Cancer Ther. 2002;1(7):483–92. [PubMed] [Google Scholar]
- 38.Slater SJ, Malinowski SA, Stubbs CD. The nature of the hydrophobic n-alkanol binding site within the C1 domains of protein kinase Calpha. Biochemistry. 2004;43(23):7601–9. doi: 10.1021/bi049755z. [DOI] [PubMed] [Google Scholar]
- 39.Schultz A, Jonsson JI, Larsson C. The regulatory domain of protein kinase Ctheta localises to the Golgi complex and induces apoptosis in neuroblastoma and Jurkat cells. Cell Death Differ. 2003;10(6):662–75. doi: 10.1038/sj.cdd.4401235. [DOI] [PubMed] [Google Scholar]
- 40.Kashiwagi K, Shirai Y, Kuriyama M, Sakai N, Saito N. Importance of C1B domain for lipid messenger-induced targeting of protein kinase C. J Biol Chem. 2002;277(20):18037–45. doi: 10.1074/jbc.M111761200. [DOI] [PubMed] [Google Scholar]
- 41.Prekeris R, Mayhew MW, Cooper JB, Terrian DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C and participates in the regulation of synaptic function. J Cell Biol. 1996;132(1–2):77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ling M, Troller U, Zeidman R, Stensman H, Schultz A, Larsson C. Identification of conserved amino acids N-terminal of the PKC epsilon C1b domain crucial for protein kinase C epsilon-mediated induction of neurite outgrowth. J Biol Chem. 2005;280(18):17910–9. doi: 10.1074/jbc.M412036200. [DOI] [PubMed] [Google Scholar]
- 43.Perander M, Bjorkoy G, Johansen T. Nuclear import and export signals enable rapid nucleocytoplasmic shuttling of the atypical protein kinase C lambda. J Biol Chem. 2001;276(16):13015–24. doi: 10.1074/jbc.M010356200. [DOI] [PubMed] [Google Scholar]
- 44.Medkova M, Cho W. Mutagenesis of the C2 domain of protein kinase C-alpha. Differential roles of Ca2+ ligands and membrane binding residues. J Biol Chem. 1998;273(28):17544–52. doi: 10.1074/jbc.273.28.17544. [DOI] [PubMed] [Google Scholar]
- 45.Kohout SC, Corbalan-Garcia S, Torrecillas A, Gomez-Fernandez JC, Falke JJ. C2 domains of protein kinase C isoforms alpha, beta, and gamma: activation parameters and calcium stoichiometries of the membrane-bound state. Biochemistry. 2002;41(38):11411–24. doi: 10.1021/bi026041k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sossin WS, Schwartz JH. Ca(2+)-independent protein kinase Cs contain an amino-terminal domain similar to the C2 consensus sequence. Trends Biochem Sci. 1993;18(6):207–8. doi: 10.1016/0968-0004(93)90189-t. [DOI] [PubMed] [Google Scholar]
- 47.Pappa H, Murray-Rust J, Dekker LV, Parker PJ, McDonald NQ. Crystal structure of the C2 domain from protein kinase C-delta. Structure. 1998;6(7):885–94. doi: 10.1016/s0969-2126(98)00090-2. [DOI] [PubMed] [Google Scholar]
- 48.Pepio AM, Sossin WS. Membrane translocation of novel protein kinase Cs is regulated by phosphorylation of the C2 domain. J Biol Chem. 2001;276(6):3846–55. doi: 10.1074/jbc.M006339200. [DOI] [PubMed] [Google Scholar]
- 49.Kohout SC, Corbalan-Garcia S, Gomez-Fernandez JC, Falke JJ. C2 domain of protein kinase C alpha: elucidation of the membrane docking surface by site-directed fluorescence and spin labeling. Biochemistry. 2003;42(5):1254–65. doi: 10.1021/bi026596f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corbalan-Garcia S, Garcia-Garcia J, Rodriguez-Alfaro JA, Gomez-Fernandez JC. A new phosphatidylinositol 4,5-bisphosphate-binding site located in the C2 domain of protein kinase Calpha. J Biol Chem. 2003;278(7):4972–80. doi: 10.1074/jbc.M209385200. [DOI] [PubMed] [Google Scholar]
- 51.Ochoa WF, Torrecillas A, Fita I, Verdaguer N, Corbalan-Garcia S, Gomez-Fernandez JC. Retinoic acid binds to the C2-domain of protein kinase C(alpha) Biochemistry. 2003;42(29):8774–9. doi: 10.1021/bi034713g. [DOI] [PubMed] [Google Scholar]
- 52.Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95(3):307–18. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- 53.Kirwan AF, Bibby AC, Mvilongo T, et al. Inhibition of protein kinase C catalytic activity by additional regions within the human protein kinase Calpha-regulatory domain lying outside of the pseudosubstrate sequence. Biochem J. 2003;373(Pt 2):571–81. doi: 10.1042/BJ20030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pepio AM, Sossin WS. The C2 domain of the Ca(2+)-independent protein kinase C Apl II inhibits phorbol ester binding to the C1 domain in a phosphatidic acid-sensitive manner. Biochemistry. 1998;37(5):1256–63. doi: 10.1021/bi971841u. [DOI] [PubMed] [Google Scholar]
- 55.Quest AF, Bell RM. The regulatory region of protein kinase C gamma. Studies of phorbol ester binding to individual and combined functional segments expressed as glutathione S-transferase fusion proteins indicate a complex mechanism of regulation by phospholipids, phorbol esters, and divalent cations. J Biol Chem. 1994;269(31):20000–12. [PubMed] [Google Scholar]
- 56.Lopez-Nicolas R, Lopez-Andreo MJ, Marin-Vicente C, Gomez-Fernandez JC, Corbalan-Garcia S. Molecular Mechanisms of PKCalpha localization and Activation by Arachidonic Acid. The C2 Domain also Plays a Role. J Mol Biol. 2006;357(4):1105–20. doi: 10.1016/j.jmb.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Sutton RB, Sprang SR. Structure of the protein kinase Cbeta phospholipid-binding C2 domain complexed with Ca2+ Structure. 1998;6(11):1395–405. doi: 10.1016/s0969-2126(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 58.Slater SJ, Seiz JL, Cook AC, et al. Regulation of PKC alpha activity by C1-C2 domain interactions. J Biol Chem. 2002;277(18):15277–85. doi: 10.1074/jbc.M112207200. [DOI] [PubMed] [Google Scholar]
- 59.Mochly-Rosen D, Miller KG, Scheller RH, Khaner H, Lopez J, Smith BL. p65 fragments, homologous to the C2 region of protein kinase C, bind to the intracellular receptors for protein kinase C. Biochemistry. 1992;31(35):8120–4. doi: 10.1021/bi00150a003. [DOI] [PubMed] [Google Scholar]
- 60.Ron D, Luo J, Mochly-Rosen D. C2 region-derived peptides inhibit translocation and function of beta protein kinase C in vivo. J Biol Chem. 1995;270(41):24180–7. doi: 10.1074/jbc.270.41.24180. [DOI] [PubMed] [Google Scholar]
- 61.Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem. 1996;271(40):24962–6. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- 62.Ron D, Mochly-Rosen D. An autoregulatory region in protein kinase C: the pseudoanchoring site. Proc Natl Acad Sci U S A. 1995;92(2):492–6. doi: 10.1073/pnas.92.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Souroujon MC, Mochly-Rosen D. Peptide modulators of protein-protein interactions in intracellular signaling. Nat Biotechnol. 1998;16(10):919–24. doi: 10.1038/nbt1098-919. [DOI] [PubMed] [Google Scholar]
- 64.Schechtman D, Craske ML, Kheifets V, Meyer T, Schechtman J, Mochly-Rosen D. A critical intramolecular interaction for protein kinase Cepsilon translocation. J Biol Chem. 2004;279(16):15831–40. doi: 10.1074/jbc.M310696200. [DOI] [PubMed] [Google Scholar]
- 65.Dekker LV, Parker PJ. Regulated binding of the protein kinase C substrate GAP-43 to the V0/C2 region of protein kinase C-delta. J Biol Chem. 1997;272(19):12747–53. doi: 10.1074/jbc.272.19.12747. [DOI] [PubMed] [Google Scholar]
- 66.Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121(2):271–80. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 67.Leinweber B, Parissenti AM, Gallant C, et al. Regulation of protein kinase C by the cytoskeletal protein calponin. J Biol Chem. 2000;275(51):40329–36. doi: 10.1074/jbc.M008257200. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez MM, Chen CH, Smith BL, Mochly-Rosen D. Characterization of the binding and phosphorylation of cardiac calsequestrin by epsilon protein kinase C. FEBS Lett. 1999;454(3):240–6. doi: 10.1016/s0014-5793(99)00697-3. [DOI] [PubMed] [Google Scholar]
- 69.Liedtke CM, Hubbard M, Wang X. Stability of actin cytoskeleton and PKC-delta binding to actin regulate NKCC1 function in airway epithelial cells. Am J Physiol Cell Physiol. 2003;284(2):C487–96. doi: 10.1152/ajpcell.00357.2002. [DOI] [PubMed] [Google Scholar]
- 70.Kheifets V, Bright R, Inagaki K, Schechtman D, Mochly-Rosen D. Protein kinase C delta (deltaPKC)-annexin V interaction: a required step in deltaPKC translocation and function. J Biol Chem. 2006;281(32):23218–26. doi: 10.1074/jbc.M602075200. [DOI] [PubMed] [Google Scholar]
- 71.Mochly-Rosen D, Khaner H, Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci U S A. 1991;88(9):3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kishimoto A, Mikawa K, Hashimoto K, et al. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain) J Biol Chem. 1989;264(7):4088–92. [PubMed] [Google Scholar]
- 73.Quittau-Prevostel C, Delaunay N, Collazos A, Vallentin A, Joubert D. Targeting of PKCalpha and epsilon in the pituitary: a highly regulated mechanism involving a GD(E)E motif of the V3 region. J Cell Sci. 2004;117(Pt 1):63–72. doi: 10.1242/jcs.00832. [DOI] [PubMed] [Google Scholar]
- 74.Parsons M, Keppler MD, Kline A, et al. Site-directed perturbation of protein kinase C- integrin interaction blocks carcinoma cell chemotaxis. Mol Cell Biol. 2002;22(16):5897–911. doi: 10.1128/MCB.22.16.5897-5911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21(7):267–71. [PubMed] [Google Scholar]
- 76.Hofmann J. The potential for isoenzyme-selective modulation of protein kinase C. Faseb J. 1997;11(8):649–69. doi: 10.1096/fasebj.11.8.9240967. [DOI] [PubMed] [Google Scholar]
- 77.Parker PJ, Coussens L, Totty N, et al. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986;233(4766):853–9. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- 78.Gao T, Toker A, Newton AC. The carboxyl terminus of protein kinase c provides a switch to regulate its interaction with the phosphoinositide-dependent kinase, PDK-1. J Biol Chem. 2001;276(22):19588–96. doi: 10.1074/jbc.M101357200. [DOI] [PubMed] [Google Scholar]
- 79.Messerschmidt A, Macieira S, Velarde M, et al. Crystal structure of the catalytic domain of human atypical protein kinase C-iota reveals interaction mode of phosphorylation site in turn motif. J Mol Biol. 2005;352(4):918–31. doi: 10.1016/j.jmb.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 80.Yang J, Cron P, Thompson V, et al. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol Cell. 2002;9(6):1227–40. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 81.Edwards AS, Newton AC. Phosphorylation at conserved carboxyl-terminal hydrophobic motif regulates the catalytic and regulatory domains of protein kinase C. J Biol Chem. 1997;272(29):18382–90. doi: 10.1074/jbc.272.29.18382. [DOI] [PubMed] [Google Scholar]
- 82.Stebbins EG, Mochly-Rosen D. Binding specificity for RACK1 resides in the V5 region of beta II protein kinase C. J Biol Chem. 2001;276(32):29644–50. doi: 10.1074/jbc.M101044200. [DOI] [PubMed] [Google Scholar]
- 83.Banci L, Cavallaro G, Kheifets V, Mochly-Rosen D. Molecular dynamics characterization of the C2 domain of protein kinase Cbeta. J Biol Chem. 2002;277(15):12988–97. doi: 10.1074/jbc.M106875200. [DOI] [PubMed] [Google Scholar]
- 84.Gokmen-Polar Y, Fields AP. Mapping of a molecular determinant for protein kinase C betaII isozyme function. J Biol Chem. 1998;273(32):20261–6. doi: 10.1074/jbc.273.32.20261. [DOI] [PubMed] [Google Scholar]
- 85.Staudinger J, Lu J, Olson EN. Specific interaction of the PDZ domain protein PICK1 with the COOH terminus of protein kinase C-alpha. J Biol Chem. 1997;272(51):32019–24. doi: 10.1074/jbc.272.51.32019. [DOI] [PubMed] [Google Scholar]
- 86.Raghunath A, Ling M, Larsson C. The catalytic domain limits the translocation of protein kinase C alpha in response to increases in Ca2+ and diacylglycerol. Biochem J. 2003;370(Pt 3):901–12. doi: 10.1042/BJ20021420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DeVries TA, Neville MC, Reyland ME. Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. Embo J. 2002;21(22):6050–60. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L, Hahn H, Wu G, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001;98(20):11114–9. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mochly-Rosen D, Gordon AS. Anchoring proteins for protein kinase C: a means for isozyme selectivity. Faseb J. 1998;12(1):35–42. [PubMed] [Google Scholar]
- 90.Blobe GC, Stribling DS, Fabbro D, Stabel S, Hannun YA. Protein kinase C beta II specifically binds to and is activated by F-actin. J Biol Chem. 1996;271(26):15823–30. doi: 10.1074/jbc.271.26.15823. [DOI] [PubMed] [Google Scholar]
- 91.Zeidman R, Troller U, Raghunath A, Pahlman S, Larsson C. Protein kinase Cepsilon actin-binding site is important for neurite outgrowth during neuronal differentiation. Mol Biol Cell. 2002;13(1):12–24. doi: 10.1091/mbc.01-04-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martelli AM, Bortul R, Tabellini G, et al. Molecular characterization of protein kinase C-alpha binding to lamin A. J Cell Biochem. 2002;86(2):320–30. doi: 10.1002/jcb.10227. [DOI] [PubMed] [Google Scholar]
- 93.Zhang G, Kazanietz MG, Blumberg PM, Hurley JH. Crystal structure of the cys2 activator-binding domain of protein kinase C delta in complex with phorbol ester. Cell. 1995;81(6):917–24. doi: 10.1016/0092-8674(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 94.Schultz A, Ling M, Larsson C. Identification of an amino acid residue in the protein kinase C C1b domain crucial for its localization to the Golgi network. J Biol Chem. 2004;279(30):31750–60. doi: 10.1074/jbc.M313017200. [DOI] [PubMed] [Google Scholar]
- 95.Verdaguer N, Corbalan-Garcia S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. Embo J. 1999;18(22):6329–38. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]


