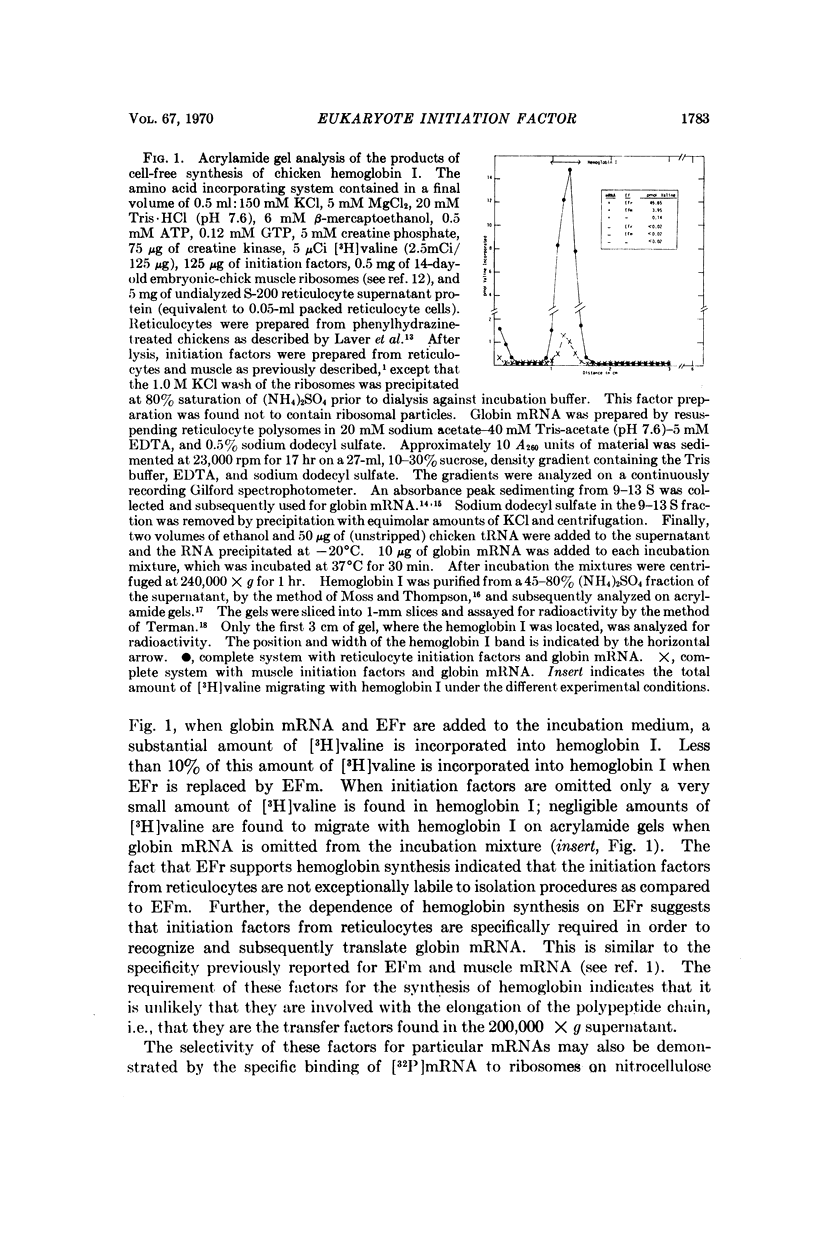
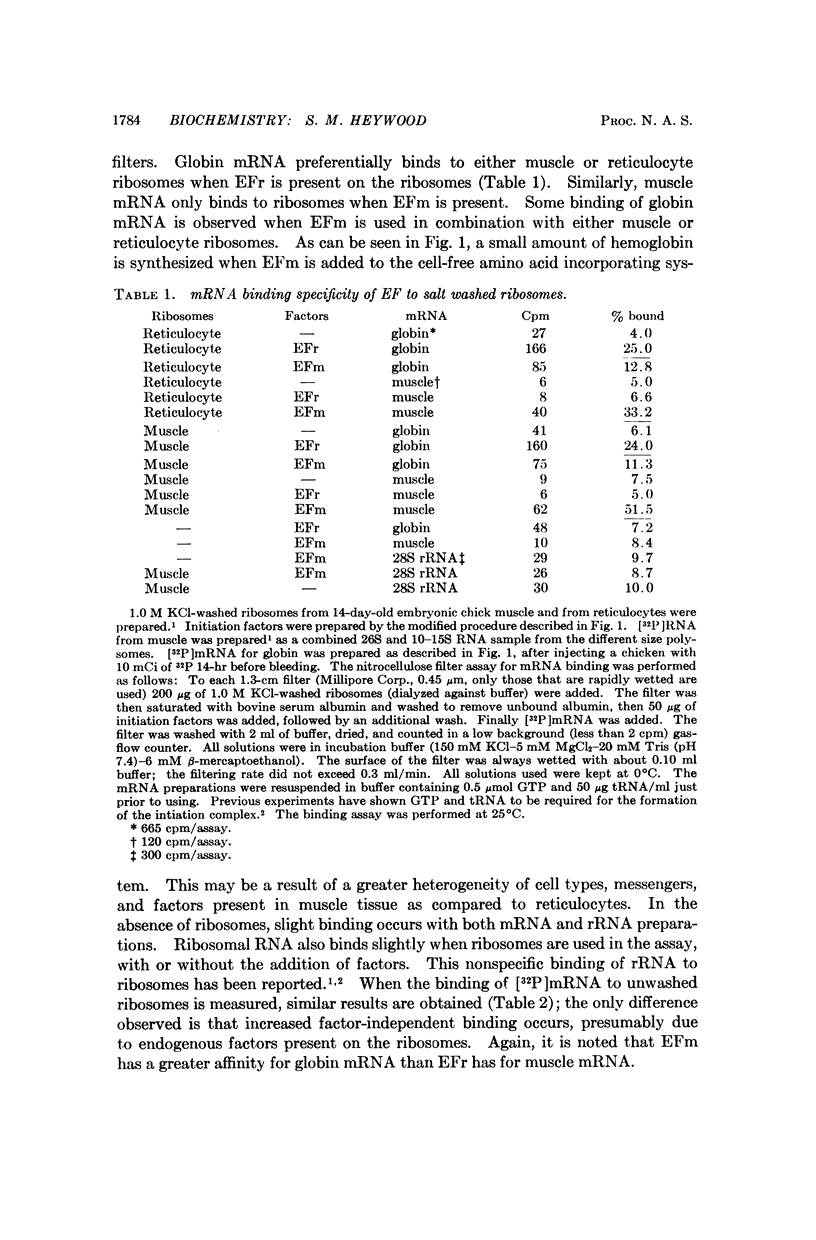
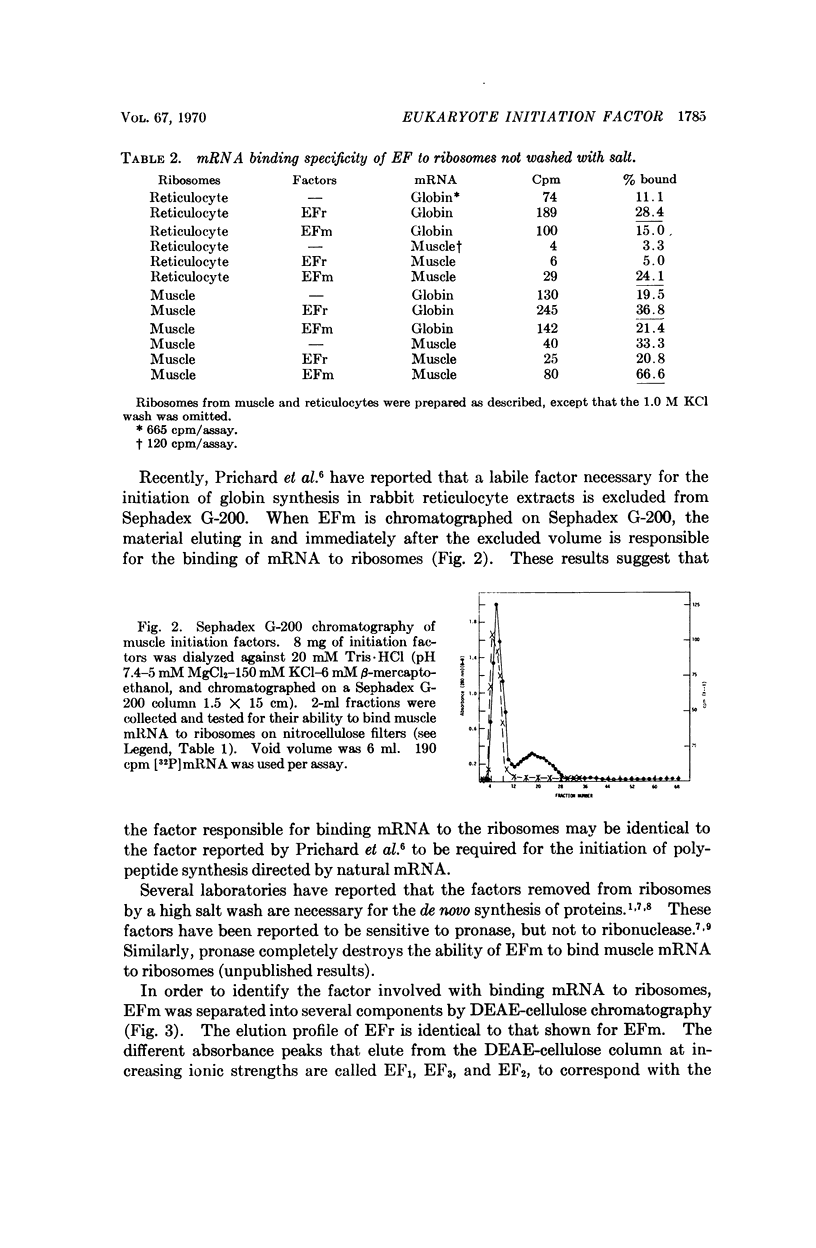
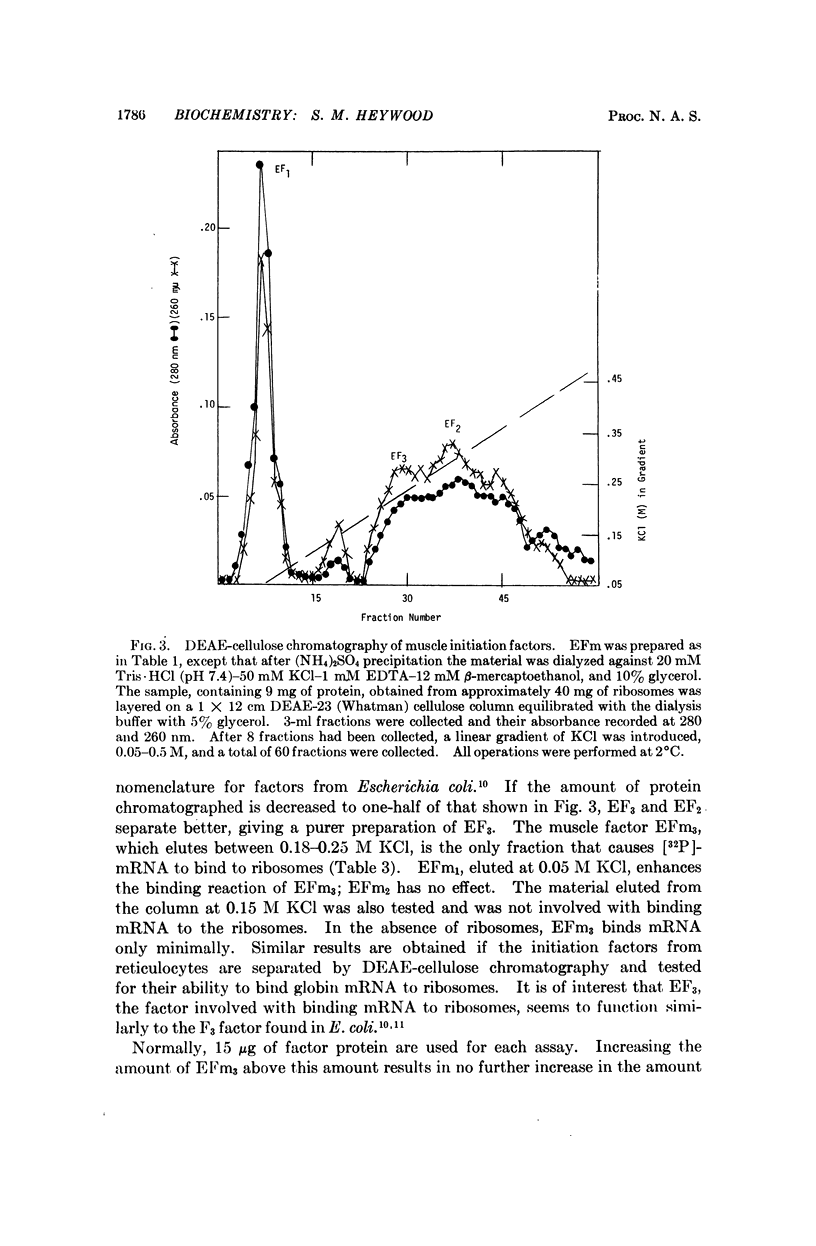
Abstract
It has been shown previously that ribosomal factors removed from chick muscle ribosomes by a high salt wash are required for both the binding of muscle mRNA to ribosomes and mRNA-directed synthesis of myosin on reticulocyte ribosomes. These factors can be separated into several components on DEAE-cellulose. A factor eluting between 0.18 and 0.25 M KCl is responsible for binding mRNA to the ribosome. This binding factor shows specificity in the recognition of mRNA, as has been demonstrated by the preferential binding of muscle and globin mRNA to ribosomes which contain their respective binding factors. Similarly, globin mRNA is preferentially translated when reticulocyte factors are present on the ribosome.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen B. B. A factor converting monoribosomes into polyribosomes during protein synthesis in vitro. Biochem J. 1968 Nov;110(2):231–236. doi: 10.1042/bj1100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S. K., Rudland P. S. Control of translation by T4 phage: altered binding of disfavoured messengers. Nature. 1970 May 30;226(5248):820–823. doi: 10.1038/226820a0. [DOI] [PubMed] [Google Scholar]
- Godin C., Kruh J., Dreyfus J. C. Reconstituted reticulocyte ribosomes active in hemoglobin synthesis. Biochim Biophys Acta. 1969 May 20;182(1):175–179. doi: 10.1016/0005-2787(69)90532-2. [DOI] [PubMed] [Google Scholar]
- Heywood S. M. Formation of the initiation complex using muscle messenger RNAs. Nature. 1970 Feb 21;225(5234):696–698. doi: 10.1038/225696a0. [DOI] [PubMed] [Google Scholar]
- Heywood S. M., Nwagwu M. Partial characterization of presumptive myosin messenger ribonucleic acid. Biochemistry. 1969 Sep;8(9):3839–3845. doi: 10.1021/bi00837a050. [DOI] [PubMed] [Google Scholar]
- Heywood S. M. Synthesis of myosin on heterologous ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:799–803. doi: 10.1101/sqb.1969.034.01.091. [DOI] [PubMed] [Google Scholar]
- Hsu W. T., Weiss S. B. Selective translation of T4 template RNA by ribosomes from T4-infected Escherichia coli. Proc Natl Acad Sci U S A. 1969 Sep;64(1):345–351. doi: 10.1073/pnas.64.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K., Sabol S., Wahba A. J., Ochoa S. Translation of the genetic message. VII. Role of initiation factors in formation of the chain initiation complex with Escherichia coli ribosomes. Arch Biochem Biophys. 1968 May;125(2):542–547. doi: 10.1016/0003-9861(68)90612-7. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Lingrel J. B. The synthesis of mouse hemoglobin beta-chains in a rabbit reticulocyte cell-free system programmed with mouse reticulocyte 9S RNA. Biochem Biophys Res Commun. 1969 Oct 8;37(2):204–212. doi: 10.1016/0006-291x(69)90720-7. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Schweet R. Isolation of a protein fraction from reticulocyte ribosomes required for de novo synthesis of hemoglobin. Arch Biochem Biophys. 1968 May;125(2):632–646. doi: 10.1016/0003-9861(68)90622-x. [DOI] [PubMed] [Google Scholar]
- Moss B., Ingram V. M. Hemoglobin synthesis during amphibian metamorphosis. I. Chemical studies on the hemoglobins from the larval and adult stages of Rana catesbeiana. J Mol Biol. 1968 Mar 28;32(3):481–492. doi: 10.1016/0022-2836(68)90336-7. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Gilbert J. M., Shafritz D. A., Anderson W. F. Factors for the initiation of haemoglobin synthesis by rabbit reticulocyte ribosomes. Nature. 1970 May 9;226(5245):511–514. doi: 10.1038/226511a0. [DOI] [PubMed] [Google Scholar]
- Revel M., Herzberg M., Greenshpan H. Initiator protein dependent binding of messenger RNA to the ribosome. Cold Spring Harb Symp Quant Biol. 1969;34:261–275. doi: 10.1101/sqb.1969.034.01.032. [DOI] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L. Messenger RNA in avian erythroblasts at the transcriptional and translational levels and the problem of regulation in animal cells. J Cell Physiol. 1968 Oct;72(2 Suppl):181+–181+. doi: 10.1002/jcp.1040720413. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Dube S. K., Rudland P. S. Control of translation of T4 phage: altered ribosome binding at R17 initiation sites. Nature. 1970 May 30;226(5248):824–827. doi: 10.1038/226824a0. [DOI] [PubMed] [Google Scholar]
- Terman S. A. Relative effect of transcription-level and translation-level control of protein synthesis during early development of the sea urchin. Proc Natl Acad Sci U S A. 1970 Apr;65(4):985–992. doi: 10.1073/pnas.65.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]


