Abstract
Endogenous opioids are integral in modulating drug reward, but it is believed these may act through several mechanisms including hypothalamic-pituitary-adrenocortical (HPA) and dopamine pathways. This study was developed to examine how nicotine dependence alters endogenous opioid regulation of prolactin response, a peripheral marker of dopaminergic activity. Smokers and nonsmokers completed two sessions during which placebo or 50 mg of naltrexone was administered, using a double-blind, counterbalanced design. Blood samples and mood measures were obtained during a resting absorption period, after exposure to two noxious stimuli (cold pressor and thermal pain), and during an extended recovery period. Opioid blockade increased prolactin response, indicating an inhibitory effect of the endogenous opioid system on prolactin, possibly mediated by reduced stimulatory effects of dopamine on this hormone. These responses were attenuated in smokers relative to nonsmokers. There was also gender disparity in prolactin response, with women showing a stronger response to endogenous opioid modification than men regardless of smoking status. The attenuated effects of opioid blockade may reflect dysregulated opiodergic and dopaminergic effects. Results extend previous reports showing blunted opioid regulation of the HPA response in dependent smokers.
Keywords: prolactin, smoking, opioids, dopamine, reward
Introduction
Nicotine is a stimulant that promotes release of pro-opiomelanocortin (POMC), a precursor of the rewarding endogenous opioid beta-endorphin (Chretien and Seidah, 1981). Stressful challenges stimulate the release of this hormone as well (Arnsten et al., 1985). Furthermore, beta-endorphin acts to increase dopamine concentrations (Moore et al., 1987), which have rewarding effects in the nucleus accumbens (Tupala and Tiihonen, 2004) and contributes to drug dependence (Cannon et al., 2004; Di Chiara et al., 2004; Bassareo et al., 2007). Prolactin is a hormone produced by acidophils in the anterior pituitary gland, and it is inhibited by dopaminergic neurons in the arcuate nucleus of the hypothalamus (Freeman et al., 2000). Prolactin may therefore be a useful marker for dopaminergic tone (Ben-Jonathan and Hnasko, 2001).
Both dopamine and endogenous opiate systems are likely to be altered by chronic exposure to nicotine (Ahmed and Koob 2005;Kreek and Koob 1998; Scott et al., 2007). Nicotine directly stimulates the dopaminergic pathway (Fu et al., 2001; Benwell et al. 1990; Rose and Corrigall 1997; Salokangas et al. 2000). It is unclear whether the mechanism of opioid effect on prolactin is direct or mediated via the dopaminergic pathway, and how this may be changed in chronic smokers in response to stress. Given that central dopamine is unable to cross the blood-brain-barrier, a peripheral marker for beta-endorphin and dopamine changes such as prolactin could be useful in investigating the opioid-dopaminergic interactions in nicotine addiction.
There is evidence that opioids potentiate dopamine production (Mathon et al., 2005), as mice without mu opioid receptors have lower dopamine levels. Furthermore, there is evidence that opioids may stimulate dopamine transmission in the nucleus accumbens (Mathon et al., 2005; Khachaturian et al., 1982). We would then expect that naltrexone, an opioid antagonist, would increase prolactin. Furthermore, in animal studies prolactin shows feedback inhibition of its own release by activating tuberoinfundibular dominergic neurons (Moore et al., 1987) with absence of this feedback in the nigrostriatal dopaminergic neurons of the hypothalamus (Moore et al., 1987). Also notably, the interactions between prolactin and dopamine may have variation dependent on gender and age (Grunder et al., 1999; Demarest et al., 1987).
Studies show that nicotine addiction results in a blunted prolactin response to the dopamine antagonist bromocriptine, suggesting the possibility that chronic exposure to nicotine desensitizes dopamine receptors, reduces dopamine turnover, or decreases their count in the nigrostriatal pathways (Netter et al., 2002). These changes may lead to reduced prolactin response to opiate blockade among smokers. To this end, and based on the studies reviewed above, we examined the extent to which nicotine dependence influences opiate modulation of prolactin release by measuring prolactin response to opiate receptor antagonist, naltrexone. Based on previous studies from our and other laboratories using similar pharmacological tests, we expected that responses to opioid blockade would be attenuated in smokers relative to nonsmokers.
Methods
Participants
Participants included both nonsmokers and smokers who were not interested in smoking cessation and were otherwise free from any medical or psychiatric problems. Participants also were excluded if they were not within +/− 30% of Metropolitan Life Insurance norms, were pregnant, had any regular use of prescribed or over-the-counter medication excluding contraceptives, or had current opiate use or dependence. Smokers were included if they smoked 10 or more cigarettes per day for the past two years. Nonsmokers were included if they had never smoked over the last two years and had smoked fewer than 100 cigarettes over their lifetime. This paper includes a sample of 37 participants who had completed blood samples available to be assayed for prolactin and were drawn from a larger study focusing on opioid and neuroendocrine functions in smokers (al’Absi et al., 2008). The study was approved by the Institutional Review Board of the University of Minnesota, and participants were also provided with monetary incentive for their participation.
Measures and instruments
Prolactin was assayed using ELISA kits (DSL, Sinsheim, Germany) with a lower sensitivity of 0.14 ng/ml. Participants also provided mood state ratings before each blood sampling. Ratings covered two factors, Positive Affect and Distress, adopted from scales with previous success in similar settings (al’Absi et al., 1998; al’Absi et al., 1994). Each item references a seven-point scale anchored by the end points, “Not at All” and “Very Strong.” Items that cover positive affect included ratings of how cheerful, content, calm/relaxed, happy, in control, and interested the participant felt. Distress items provided ratings of how irritable, anxious/tense, sad/depressed, angry, confused, and impatient the participant felt.
Procedures
Participants were screened according to the criteria listed above, and women completed a pregnancy test. Qualified participants completed questionnaires to assess their medical and smoking history. Participants meeting study criteria attended two laboratory sessions lasting four hours each with at least 72 hours between them. Another pregnancy test was conducted prior to each laboratory session. On both sessions, smokers smoked at their usual rate until they arrived at the laboratory, but they were not allowed to smoke during the sessions. All testing began at approximately 12:00 pm to control for circadian rhythm.
Briefly, each session began with participants completing a questionnaire about mood states as well as a blood draw. Participants then received a capsule containing either 50 mg of naltrexone or placebo. The order of naltrexone and placebo administration was randomly assigned, and the experimenter was blinded to the order. Following administration there was a 60-min rest period to allow peak plasma concentration of naltrexone. Blood samples were collected every 20 minutes following naltrexone or placebo. The cold pressor and thermal pain induction procedures were then administered in a counterbalanced order, and pain stimuli were separated by a 20-minute rest period. After the second pain test, participants rested for 60 minutes. During this period, three blood samples were collected. The state-mood questionnaire was completed with every blood sample, after initial rest, after the pain induction procedures, and after the rest recovery period. A more thorough report of the procedures and other measures including the cardiovascular and pain results can be found elsewhere (al’Absi et al., 2008).
Dependent Variables and Data Analysis
The main variables were prolactin concentrations and mood state measures. Prolactin levels prior to drug administration were compared using a 2 (Smoking Status) × 2 (Sex) × 2 (Drug Condition) analysis of variance (ANOVA), and subsequent analyses covaried for the pre-drug levels and used a 2 (Smoking Status) × 2 (Sex) × 2 (Drug Condition) × 7 (Samples obtained after ingestion of the drug capsule) repeated analysis of covariance (ANCOVA). Prolactin responses to pain challenges were analyzed within each drug condition (placebo or naltrexone) using a 2 (Smoking Status) × 2 (Sex) × 2 (Periods: Before and after exposure to the pain challenges) ANOVA. Analysis of mood data was conducted using a 2 (Smoking Status) × 2 (Sex) × 8 (Periods) ANOVA. We used Wilkes’ Lambda correction to test time effect and to correct for repeated measures when necessary (Vasey and Thayer, 1987).
Results
Smokers and nonsmokers did not differ in age, body mass index, years of education, number of daily alcohol serving, or their level of perceived stress (ps > 0.1; see Table 1). They did not differ in their screening heart rate or blood pressure levels (ps > 0.1). The two groups differed in caffeine intake (p < 0.02) and the their score on the State-Trait Anxiety Inventory (p < 0.02), as shown in Table 1.
Table 1.
Subject Characteristics
| Variables | Nonsmokers | Smokers | p |
|---|---|---|---|
| Age (years) | 20.2 (1.3) | 24.1 (1.4) | NS |
| Height | 1.7 (0.02) | 1.8 (0.02) | NS |
| Weight | 71.2 (2.3) | 76.8 (2.4) | NS |
| BMI | 24.7 (0.7) | 24.9 (0.7) | NS |
| Education (years) | 14.3 (0.4) | 14.9 (0.4) | NS |
| Alcohol | 0.77 (0.1) | 1.0 (0.1) | NS |
| Caffeine | 0.98 (0.5) | 3.12 (0.5) | 0.01 |
| STAI | 33.0 (1.8) | 42.0 (1.9) | 0.01 |
| PSS | 16.7 (1.2) | 18.5 (1.2) | NS |
Note, means (standard error of the mean); NS = not significant; BMI = body mass index; STAI = State-Trait Anxiety Inventory, PSS = Perceived Stress Scale. Distress1 = initial level of distress
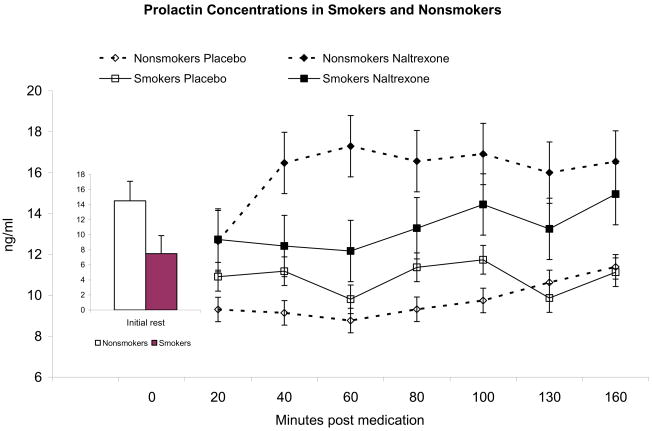
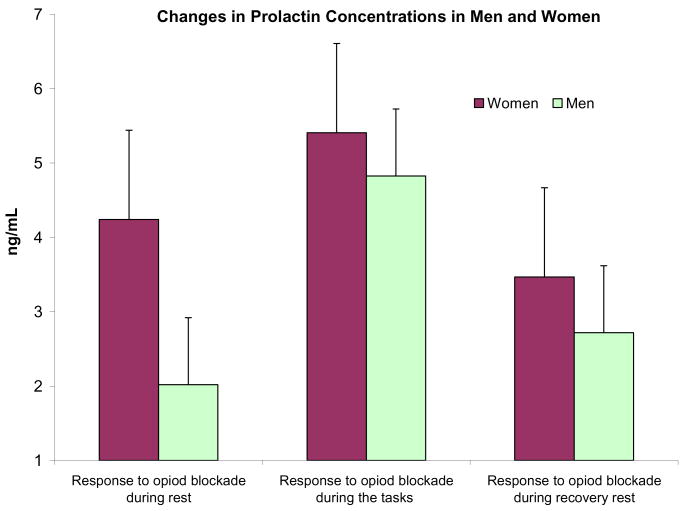
Prolactin levels prior to drug administration on both days did not differ between between men and women, and there was only a trend of lower prolactin levels in smokers relative to nonsmokers (F (1, 35) = 3.09, p = 0.09). Pharmacologic blockade with naltrexone was associated with increased prolactin production as evidenced by a main effect of Drug Condition (F (1, 32) = 26.7, p < 0.0001). Smokers showed an attenuated prolactin release in response to the opioid blockade (F (1, 32) = 7.02, p < 0.01; Figure 1). There was a Smoking status × Drug × Period interaction (F (6,27) = 3.56, p < 0.01; η2 = 0.44) reflecting greater difference between smokers and nonsmokers during the rest period prior to initiating the stressful pain task. This reflects greater prolactin production in response to opioid blockade in nonsmokers relative to smokers. With regard to gender differences, women exhibited a stronger response to endogenous opioid blockade than men (F(1, 32) = 7.57, p < 0.01), especially during the rest periods before exposure to the noxious stimuli (see Figure 2), as demonstrated by a significant Gender × Drug Condition × Period interaction (F(6, 27) = 2.74, p = 0.03; η2 = 0.38)).
Figure 1.
Prolactin concentrations following naltrexone or placebo exposure. The shaded rectangle indicates the time when the two tasks (cold pressor and thermal pain) were presented. Prolactin showed significant increases in response to opioid blockade (p < 0.01), but responses tended to be attenuated in smokers than in nonsmokers (p < 0.01). Inset figure depicts the average from both sessions of the pre-drug levels in smokers and nonsmokers.
Figure 2.
Changes in prolactin concentrations obtained by subtracting first sample and placebo levels from respective levels after naltrexone administration. The three readings are averages of samples collected during rest, during the challenges, and during the recovery period.
Additional analysis was conducted using caffeine intake and anxiety score as covariates. The analysis showed that pharmacologic blockade with naltrexone was associated with increased prolactin production as evidenced by a main effect of Drug Condition (F (1, 30) = 4.74, p < 0.05). There was a Smoking Status × Drug × Period interaction (F (6, 25) = 3.03, p = 0.02; η2 = 0.42) reflecting greater difference between smokers and nonsmokers during the rest period prior to initiating the pain tasks. Gender differences were also found with women exhibiting a stronger response to endogenous opioid blockade than men, especially during the rest periods before exposure to the noxious stimuli, as demonstrated by a significant Gender × Drug Condition × Period interaction (F (6, 25) = 2.71, p = 0.04; η2 = 0.39).
Distress was increased in all groups during the pain tests, as indicated by the period main effect (F (7, 28) = 4.55, p < 0.001; η2 = 0.53), but there was no effect of the opioid blockade (F < 1). While nonsmoking women reported greater distress in both conditions than nonsmoking men, the opposite pattern was found among smokers as evidenced by a significant Smoking State × Sex interaction (F (1, 34) = 5.17, p < 0.05). Positive affect was reduced following the pain procedures as evidenced by a main effect of period (F (7, 28) = 7.95, p <0.001; η2 = 0.66). No effect of naltrexone or difference between men and women was found on the positive affect scale (Fs < 1).
Discussion
Our study showed significant increases in prolactin levels in response to opioid blockade, although this response was significantly attenuated in smokers relative to nonsmokers. The results indicate that the endogenous opioid system exerts an inhibitory effect on prolactin production, although it is not clear whether this effect is direct and/or mediated by effects of endogenous opioids on the dopaminergic system. It is possible that smoking alters opioid regulation of prolactin at one or both of these systems.
The endogenous opiate system may affect the release of dopamine (Marinelli 2007). Endogenous opiate neurons, such as β-endorphin producing neurons, project to the ventral tegmental area and the nucleus accumbens (Khachaturian et al., 1984), which are important structures in the dopamine production. Dopamine directly modifies prolactin production with increased dopamine transmission leading to inhibition of prolactin production (Moore et al. 1987). It is therefore possible that opioid blockade removed opioid stimulation of dopamine, leading to the disinhibition of prolactin release; the result is an increase in prolactin production. The attenuated prolactin response to opioid blockade in smokers suggests blunted opioid regulation of dopamine or dysregulated dopaminergic-prolactin interactions in these participants.
We recognize that other pathways also influence prolactin release and may be affected by chronic nicotine exposure. In addition to the inhibitory effects of dopaminergic transmission, other factors have stimulatory effects including estradiol, serotonin, and gamma-Aminobutyric acid (GABA) (Ben-Jonathan and Hnasko, 2001). Hypothalamic factors such as thyrotropin-releasing hormone (Thomas et al., 1986) and gonadotropin-releasing hormone (Denef and Andries, 1983) also stimulate prolactin release. Peptides such as tachykinins and vasoactive intestinal peptide (VIP) also play a stimulatory role (Debeljuk and Lasaga, 2006). Finally, additional spinal reflex activated during nursing may stimulate prolactin release (Grosvenor and Mena, 1971; Lkhider et al., 1997).
It is difficult to determine if the differences in prolactin response to naltrexone were a risk factor for smoking, or a result of long-term effects of nicotine addiction on dopaminergic transmission or prolactin production. Prolactin response is predictive of addictive tendencies (Patkar et al., 2002), and this may be due to its relationship with dopamine levels as described above. In animal studies, administration of cocaine led to a reduction in circulating prolactin levels, suggesting increased dopaminergic transmission (Mantsch et al., 2000). The short-term presence of prolactin abnormalities in blood following cocaine withdrawal show it can be a marker of acute withdrawal (Satel et al., 1991; Buydens-Branchey et al., 1999), and a trend for prolactin dysregulation has been observed with smoking withdrawal (Pickworth et al., 1996). Consistent results from other studies suggest that prolactin dysregulation has a direct relationship with craving and withdrawal (Lee et al., 2005; Reuter and Hennig, 2003). This, combined with previous studies demonstrating the usefulness of measuring peripheral hormonal activity to assess dopaminergic sensitivity (Reuter and Hennig, 2003), indicates that this hormone may be a useful biomarker to consider when investigating stress-related neurobiological dysregulation associated with nicotine dependence.
It is important to consider the host of endocrine factors interacting in states of acute emotional dysregulation. This could be especially important in the context of examining the role of stress in addictive processes. In previous protocol examining the role of stress in smoking relapse, we have found that attenuated cortisol response predicts relapse (al’Absi et al., 2005). Other studies have shown a parallel response between cortisol and prolactin with opioid blockade, which could be viewed as a form of pharmacological stress (Reuter and Hennig, 2003). The nature of the challenge is likely to determine its effectiveness in activating prolactin release (Van de Kar and Blair, 1999). To this end, our pain tests failed to evoke a prolactin response in this study, but their use was not specifically intended to invoke a stress response, but instead to measure effects of opioid blockade on nociception. It is worth noting that other stress hormones (e.g., ACTH and cortisol) show appreciable responses to similar challenges (al’Absi et al., 2008).
Although the differences between men and women found in this study were intriguing, these results should be cautiously interpreted in light of the small sample size. We note, however, a biological hypothesis to examine these differences. Estrogen levels increase prolactin expression by inhibiting dopaminergic release (Ben-Jonathan and Hnasko, 2001), and therefore this may account for the gender differences. Effects of estrogens on prolactin release may occur at both the hypothalamus and pituitary level. This is supported by evidence indicating that prolactin elevation in response to estrogen may be influenced by stress, and this effect is mediated partially by increased dopaminergic activity (Kam et al., 2000). The interaction of estrogen, prolactin, and dopaminergic transmission under conditions of stress is a promising line of inquiry to investigate previous reported sex difference in the role of stress in various addictive process (al’Absi, 2007).
This study was limited by the focus on a young and relatively homogenous group of participants. Women were included only during the follicular phase of their menstrual cycle, potentially limiting the potential generalizations of the results. As indicated earlier, we can not discern whether the actions of naltrexone on prolactin were direct or mediated via dopamine. Additional studies could further elucidate the exact mechanisms of dysregulated prolactin response to opioid blockade in terms of examining the interaction of both the opioid system and dopaminergic transmission in nicotine dependence. This may be pursued using multiple pharmacological challenges, such as dopamine antagonist bromocriptine or fluphenazine, and opioid antagonists, such as naltrexone..
In conclusion, this study showed attenuated prolactin response to opioid blockade among smokers. The results extend previous studies showing that nicotine dependence is associated with attenuated opioid modulation of the HPA axis (al’Absi et al., 2008). This dysregulation may also play a role in the previously observed blunted responses to stress among dependent smokers (al’Absi et al., 2005). The results suggest long-term changes in the interaction between endogenous opioid and dopaminergic systems that may contribute to effects of nicotine addiction. Furthermore, our data suggest the possibility of using prolactin as a peripheral marker of endogenous opioid and dopamine systems in studies that evaluate the interaction of these systems in nicotine dependence.
Acknowledgments
During this study Dr. al’Absi was funded by National Institute of Health grants CA88272 and DA016351. We thank Clemens Kirschbaum, Ph.D., of Dresden University in Germany for help in assaying the prolactin samples and to Deanna Ellestad for help with the data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–90. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59 doi: 10.1016/j.ijpsycho.2005.10.010. Epub. [DOI] [PubMed] [Google Scholar]
- al’Absi M. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–17. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Lovallo WR, McKey BS, Pincomb GA. Borderline hypertensives produce exaggerated adrenocortical responses to mental stress. Psychosom Med. 1994;56:245–50. doi: 10.1097/00006842-199405000-00011. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Lovallo WR, McKey B, Sung BH, Whitsett TL, Wilson MF. Hypothalamic–pituitary–adrenocortical responses to psychological stress and caffeine in men at high and low risk for hypertension. Psychosom Med. 1998;60:521–527. doi: 10.1097/00006842-199807000-00021. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Hatsukami D, Westra R. Blunted opiate modulation of hypothalamic-pituitary-adrenocortical activity in men and women who smoke. Psychosom Med. 2008;70:928–35. doi: 10.1097/PSY.0b013e31818434ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Berridge C, Segal DS. Stress produces opioid-like effects on investigatory behavior. Pharmacol Biochem Behav. 1985;22:803–9. doi: 10.1016/0091-3057(85)90531-3. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MN, Di Chiara G. Differential impact of pavlovian drug conditioned stimuli on in vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology (Berl) 2007;191:689–703. doi: 10.1007/s00213-006-0560-7. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–63. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–56. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Smoking-associated changes in the serotonergic systems of discrete regions of human brain. Psychopharmacology (Berl) 1990;102:68–72. doi: 10.1007/BF02245746. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hudson J, Rothman M, Fergeson P, McKernin C. Serotonergic function in cocaine addicts: prolactin responses to sequential D,L-fenfluramine challenges. Biol Psychiatry. 1999;4:1300–6. doi: 10.1016/s0006-3223(98)00268-6. [DOI] [PubMed] [Google Scholar]
- Cannon CM, Bseikri MR. Is dopamine required for natural reward. Physiol Behav. 2004;81:741–8. doi: 10.1016/j.physbeh.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Chretien M, Seidah NG. Chemistry and biosynthesis of pro-opiomelanocortin. ACTH, MSH’s, endorphins and their related peptides. Mol Cell Biochem. 1981;34:101–127. doi: 10.1007/BF02354864. [DOI] [PubMed] [Google Scholar]
- Debeljuk L, Lasaga M. Tachykinins and the control of prolactin release. Peptides. 2006;27(11):3007–19. doi: 10.1016/j.peptides.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Demarest KT, Moore KE, Riegle GD. Acute restraint stress decreases tuberoinfundibular dopaminergic neuronal activity: evidence for a differential response in male versus female rats. Neuroendocrinology. 1985;41:504–10. doi: 10.1159/000124227. [DOI] [PubMed] [Google Scholar]
- Denef C, Andries M. Evidence for paracrine interaction between gonadotrophs and lactotrophs in pituitary cell aggregates. Endocrinology. 1983;112:813–822. doi: 10.1210/endo-112-3-813. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Brower VG, Sharp BM. Norepinephrine secretion in the hypothalamic paraventricular nucleus of rats during unlimited access to self-administered nicotine: An in vivo microdialysis study. J Neurosci. 2001;21:8979–89. doi: 10.1523/JNEUROSCI.21-22-08979.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosvenor CE, Mena F. Effect of Suckling upon the Secretion and Release of Prolactin from the Pituitary of the Lactating Rat. J Anim Sci. 1971;32:115–136. [PubMed] [Google Scholar]
- Grunder G, Wetzel H, Schlosser R, Anghelescu I, Hillert A, Lange K, Hiemke C, Benkert Neuroendocrine response to antipsychotics: effects of drug type and gender. Biol Psychiatry. 1999;45:89–97. doi: 10.1016/s0006-3223(98)00125-5. [DOI] [PubMed] [Google Scholar]
- Kam K, Park Y, Cheon M, Son G, Kim K, Ryu K. Effects of immobilization stress on estrogen-induced surges of luteinizing hormone and prolactin in ovariectomized rats. Endocrine. 2000;12(3):379–87. doi: 10.1385/ENDO:12:3:279. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, Haber SN, Akil J, Watson SJ. Proopiomelanocortin peptide immunocytochemistry in rhesus monkey brain, Brain Res. Bull. 1984;13:785–800. doi: 10.1016/0361-9230(84)90237-5. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Watson SJ. Some perspectives on monoamine-opioid peptide interaction in rat central nervous system. Brain Res Bull. 1982;(1–6):441–62. doi: 10.1016/0361-9230(82)90154-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–91. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Lee Y, Joe K, Sohn I, Na C, Kee B, Chae S. Changes of smoking behavior and serum adrenocorticotropic hormone, cortisol, prolactin, and endogenous opioid levels in nicotine dependence after naltrexone treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:639–647. doi: 10.1016/j.pnpbp.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Lkhider M, Delpal S, Le Provost F, Ollivier-Bousquet M. Rat prolactin synthesis by lactating mammary epithelial cells. FEBS Lett. 1997;401:117–122. doi: 10.1016/s0014-5793(96)01450-0. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Effects of cocaine self-administration on plasma corticosterone and prolactin in rats. J Pharmacol Exp Ther. 2000;294:239–47. [PubMed] [Google Scholar]
- Marinelli M. Dopaminergic reward pathways and effects of stress. In: al’Absi M, editor. Stress and Addiction. London: Academic Press/Elsevier; 2007. pp. 41–83. [Google Scholar]
- Mathon DS, Ramakers GM, Pintar JE, Marinelli M. Decreased firing frequency of midbrain dopamine neurons in mice lacking mu opioid receptors. Eur J Neurosci. 2005;21:2883–6. doi: 10.1111/j.1460-9568.2005.04123.x. [DOI] [PubMed] [Google Scholar]
- Meta T, Fujihara H, Kawasaki M, Hashimoto H, Saito T, Shibata M, Saito J, Oka T, Tsuji S, Onaka T, Ueta Y. Prolactin-releasing peptide is a potent mediator of stress responses in the brain through the hypothalamic paraventricular nucleus. Neuroscience. 2006;141(2):1069–86. doi: 10.1016/j.neuroscience.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Moore KE, Demarest KT, Lookingland KJ. Stress, prolactin, and hypothalamic dopaminergic neurons. Neuropharmacology. 1987;26:801–8. doi: 10.1016/0028-3908(87)90055-4. [DOI] [PubMed] [Google Scholar]
- Netter P, Toll C, Lujic C, Reuter M, Hennig J. Addictive and nonaddictive smoking as related to responsivity to neurotransmitter systems. Behav Pharmacol. 2002;13:441–9. doi: 10.1097/00008877-200209000-00017. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Hill KP, Sterling RC, Gottheil E, Berrettini WH, Weinstein SP. Serum prolactin and response to treatment among cocaine-dependent individuals. Addict Biol. 2002;7:45–53. doi: 10.1080/135562101200100599. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Baumann MH, Fant RV, Rothman RB, Henningfield JE. Endocrine responses during acute nicotine withdrawal. Pharmacol Biochem Behav. 1996;55:433–7. doi: 10.1016/s0091-3057(96)00114-1. [DOI] [PubMed] [Google Scholar]
- Reuter M, Henniq J. Cortisol as an indicator of dopaminergic effects on nicotine craving. Hum Psychopharmacol. 2003;18:437–46. doi: 10.1002/hup.503. [DOI] [PubMed] [Google Scholar]
- Reuter M, Siegmund A, Netter P. The trigonometric responder approach: a new method for detecting responders to pharmacological or experimental challenges. Pharmacopsychiatry. 2002;35(5):182–9. doi: 10.1055/s-2002-34118. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology (Berl) 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Salokangas RK, Vilkman H, Ilonen T, Taiminen T, Bergman J, Haaparanta M, Solin O, Alanen A, Syvalahti E, Hietala J. High levels of dopamine activity in the basal ganglia of cigarette smokers. Am J Psychiatry. 2000;7:632–4. doi: 10.1176/appi.ajp.157.4.632. [DOI] [PubMed] [Google Scholar]
- Satel SL, Price LH, Palumbo JM, McDougle CJ, Krystal JH, Gawin F, Charney DS, Heninger GR, Kleber HD. Clinical phenomenology and neurobiology of cocaine abstinence: a prospective inpatient study. Am J Psychiatry. 1991;148:1712–6. doi: 10.1176/ajp.148.12.1712. [DOI] [PubMed] [Google Scholar]
- Scaramuzzi R, Downing K, Williamson S, Pollard I. The circulating concentrations of FSH, LH and prolactin in the oestradiol-implanted ovarectomized ewe treated with caffeine. Anim Reprod Sci. 1997;45(4):273–82. doi: 10.1016/s0378-4320(96)01597-7. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S, Zubieta JK. Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology. 2007;32:450–7. doi: 10.1038/sj.npp.1301238. [DOI] [PubMed] [Google Scholar]
- Thomas GB, Cummins JT, Hammond JM, Horton RJ, Clarke IJ. Prolonged secretion of prolactin in response to thyrotrophin-releasing hormone after hypothalamo-pituitary disconnection in the ewe. J Endocrinol. 1986;111(3):433–8. doi: 10.1677/joe.0.1110433. [DOI] [PubMed] [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–47. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology. 1987;24:479–86. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]