SUMMARY
Heparan sulfate proteoglycans (HSPGs) are central modulators of developmental processes likely through their interaction with growth factors, such as GDNF, members of the FGF and TGFβ superfamilies, EGF receptor ligands and HGF. Absence of the biosynthetic enzyme, heparan sulfate 2-O-sulfotransferase (Hs2st) leads to kidney agenesis. Using a novel combination of in vivo and in vitro approaches, we have reanalyzed the defect in morphogenesis of the Hs2st−/− kidney. We observed that, while the ureteric bud (UB) forms from all Hs2st−/− Wolffian ducts, about two-thirds do not branch at all, and the remainder stop after the first branching event. Utilizing assays that separately model distinct stages of kidney branching morphogenesis, we found that the Hs2st−/− UB is able to undergo branching and induce mesenchymal-to-epithelial transformation when recombined with control MM, and the isolated Hs2st null UB is able to undergo branching morphogenesis in the presence of exogenous soluble pro-branching growth factors when embedded in an extracellular matrix, indicating that the UB is competent in and of itself. This is in contrast to the prevailing view that the defect underlying the renal agenesis phenotype is due to a primary role for 2-O sulfated HS in UB branching. Unexpectedly, the mutant MM was also fully capable of being induced in recombination experiments with wild-type tissue. Thus, both the mutant UB and mutant MM tissue appear competent in and of themselves, but the combination of mutant tissues fails in vivo and, as we show, in organ culture. We hypothesized a 2OS-dependent defect in the mutual inductive process, which could be on either the UB or MM side, since both progenitor tissues express Hs2st. In light of these observations, we specifically examined the role of the HS 2-O sulfation modification on the morphogenetic capacity of the UB and MM individually. We demonstrate that early UB branching morphogenesis is not primarily modulated by factors that depend on the HS 2-O sulfate modification; however, factors that contribute to MM induction are markedly sensitive to the 2-O sulfation modification. This data suggests that key defect in Hs2st null kidneys is the inability of MM to undergo induction either through a failure of mutual induction or a primary failure of MM morphogenesis. This results in normal UB formation but affects either T-shaped UB formation or iterative branching of the T-shaped UB (possibly two separate stages in collecting system development dependent upon HS). This appears to be the first example of a defect in the MM preventing advancement of early UB branching past the first bifurcation stage, one of the limiting steps in early kidney development.
Keywords: kidney development, branching morphogenesis, metanephric mesenchyme, heparan sulfate
INTRODUCTION
Heparan sulfate proteoglycans (HSPG) are critical to many developmental and physiologic processes. HSPGs, which are abundantly present in the extracellular milieu as a component of cell surface-associated and basement membrane proteoglycans, are modular molecules made up of a protein core coupled to a uniquely sulfated heparan sulfate (HS) chain. HSPGs are involved in growth factor signaling on a variety of levels. While the protein core appears to have some role in these processes, it is believed that the sulfated HS chains modulate growth factor gradients as well as receptor binding and signaling (Baeg et al., 2004; Friedl et al., 2001; Fujise et al., 2003; Kamimura et al., 2004; Reich-Slotky et al., 1994; Shah et al., 2004; Steer et al., 2004). The tremendous diversity in HS chain sulfation pattern is generated during biosynthesis (Esko and Lindahl, 2001). HS glycosaminoglycans (GAGs) are characterized by a variable number of repeating dissacharide units of glucosamine alternating with uronic acid residues which can be modified through N-sulfation, N-acetylation and O-sulfation by at least 16 biosynthetic enzymes. The sequence of modification is strictly governed: N-sulfation, mediated through the N-deacetylase/N-sulfotransferase (NDST) family of enzymes followed by epimerization of glucuronic acid to iduronic acid by C-5 epimerase and then sulfation at the 2-O position of iduronic acid by heparan sulfate 2-O sulfotransferase (Hs2st) and finally 6-O sulfation and 3-O sulfation (reviewed in (Esko and Lindahl, 2001)). Although the enzymatic modifications occur sequentially, some of them do not go to completion, creating unique HS chain sulfation patterns.
Multiple lines of in vitro and in vivo evidence have shown that HS is required for normal kidney development. The permanent mammalian kidney, or metanephros, arises through an outpouching of the Wolffian duct (WD). This epithelial outpouching, termed the ureteric bud (UB), is induced through signals emanating from a region of adjacent intermediate mesoderm, termed metanephric mesenchyme (MM). Through a series of mutual induction steps, the UB undergoes elongation and branching morphogenesis to form the collecting system of the metanephros. Simultaneously, the MM is induced to undergo a mesenchymal-to-epithelial transition, proceeding through a series of well-defined morphologic structures, from comma- to S-shaped bodies; these structures eventually form the more proximal portion of the nephron, from the epithelial glomerulus to the distal tubule (Shah et al., 2004).
Branching morphogenesis in the kidney is regulated by a variety of heparin-binding morphogens known to regulate kidney development including glial cell-line derived neurotrophic factor (GDNF), members of the fibroblast growth factor (FGF) and TGFβ superfamilies, as well as HGF, EGF, pleiotrophin (PTN) and heregulin (HRG), suggesting that the HS-growth factor interaction is important during kidney branching morphogenesis (Lyon et al., 1997; Ornitz, 2000; Qiao et al., 2001; Qiao et al., 1999; Rickard et al., 2003; Sakurai et al., 2001; Sakurai et al., 2005; Ishibe et al., 2009). In vitro perturbation of GAG sulfation or proteolytic degradation of HS in whole embryonic kidney and isolated UB organ culture results in disruption of normal UB branching morphogenesis, an essential process for formation of the kidney (Lelongt et al., 1988; Davies et al., 1995; Platt et al., 1990; Steer et al., 2004). Moreover, in vivo studies have demonstrated that HS sulfation pattern is of central importance to kidney development as null mutation of the HS biosynthetic enzymes Hs2st or C-5 epimerase in mice results in a renal agenesis phenotype (Bullock et al., 1998; Li et al., 2003). However, despite the clear role of HS and HS sulfation pattern in kidney development, a key question remains. Is the defect in kidney development in the mutant mice intrinsic to the UB or MM? In the original description of the Hs2st null mutant, it was suggested that UB branching is primarily affected by lack of HS 2-O sulfation (Wilson et al., 2002); however this hypothesis has not yet been further investigated.
Here, we sought to clarify the role of HS 2-O sulfation in kidney development by first analyzing Hs2st−/− tissues in vitro. We attempted to localize the defect in kidney development in the Hs2st knockout mouse to the UB or the MM and unexpectedly found that both the UB and MM are competent to undergo branching morphogenesis and mesenchymal-to-epithelial transition when induced by wild-type MM and UB, respectively. We then utilized established models of in vitro kidney development to investigate the role of 2-O sulfation in the binding of factors that modulate the various stages of kidney development. We found that factors regulating isolated UB branching morphogenesis are, in fact, not sensitive to the 2-O sulfation modification while factors modulating early MM induction appear to be most vulnerable to 2-O sulfation dependent effects. This represents the first demonstration of a defect in the transition from the first dichotomous branching step to the early UB branching stage by virtue of a defect in mutual inductive signaling that primarily resides in the MM (despite itself being capable of being normally induced). Moreover, the data support the view that distinct growth factor interactions with variably sulfated HS play an important role in driving the stages of kidney development.
MATERIALS AND METHODS
Materials
Heparinoids (heparin and 2-O-desulfated heparin) were obtained from Neoparin (Alameda, CA). Tissue culture media were obtained from Invitrogen (Carlsbad, CA) and fetal calf serum obtained from Biowhittaker (East Rutherford, NJ). Transwell filters (0.4-μm pore size) were obtained from Costar (Corning, NY). Growth factor-reduced Matrigel was obtained from BD Biosciences (San Diego, CA). Recombinant human TGF-β2, BMP-2, BMP-4, rat glial-cell-line-derived neurotrophic factor (GDNF), FGF1, FGF2, FGF7 and FGF10 were from R&D systems (Minneapolis, MN). FITC-conjugated or Rhodamine-conjugated Dolichos biflorus lectin (DB) was obtained from Vector Laboratories (Burlingame, CA). The primary antibody against E-cadherin [mouse monoclonal, 1:1000] was from Transduction Laboratories (San Jose, CA); secondary antibodies were from Jackson Immunoresearch Laboratories (West Grove, PA) or Invitrogen. All other reagents and chemicals, unless otherwise indicated, were from Sigma (St. Louis, MO).
Generation of knockout mice
The mouse strain used was Hs2stTgNSt125NIMR on a mixed C57Bl/6/129Ola background, a gift from Catherine Merry (Bullock et al., 1998). Embryonic day 1 was designated the day when the vaginal plug was first observed. Whole embryonic kidney and isolated WD rudiments were isolated at E12 and isolated UB and MM were obtained at E12 for recombination experiments. Embryonic mice were genotyped as previously described (Bullock et al., 1998). The care and use of animals described in this study conform to the procedures of the laboratory's Animal Protocol approved by the Animal Subjects Program of the University of California, San Diego.
Culture of Isolated Whole Embryonic Kidneys
Embryonic kidneys from gestational day 12 mice or 13.5 Sprague-Dawley rat embryos were isolated and applied to the top of Transwell filters placed within individual wells of a 12-well tissue culture dish. The isolated kidneys were cultured (37° C, 5% CO2, 100% humidity) in DMEM/F12 media supplemented with 10% fetal calf serum and varying concentrations of heparin compound as indicated in the text. Kidneys were cultured for 3–4 days, then fixed in 4% paraformaldehyde and stained as indicated in the figure legend. All cultures were performed in triplicate with n≥3 rudiments per sample.
Hs2st expression analysis
Microarray gene expression data for e11.5 mesenchyme, e11.5 ureteric bud, e15.5 uninduced mesenchyme (peripheral blastema) and e15.5 ureteric bud tip were obtained from the GUDMAP consortium database (www.gudmap.org). Genespring GX 7.3 was used to obtain the expression values for the probes for Hs2st for each of the four tissue types. Normalized intensity for each of the probes was graphed. Statistical analysis was carried out using ANOVA.
Isolation and Culture of Wolffian ducts
The entire urogenital tract was isolated from timed pregnant Sprague Dawley rats at e13.5 or mice at e 12. Kidney rudiments were removed and the Wolffian duct (WD) was isolated and stripped of adjacent tissues except for a thin layer of surrounding intermediate mesodermal cells as previously described (Maeshima et al., 2006). Isolated WDs were placed on 0.4μm pore size Transwell filters and cultured at the air-medium interface. Cultures were carried out at 37° C in a fully humidified 5% CO2 atmosphere in the presence of BSN-CM supplemented with 10% FCS, and GDNF (125ng/ml) in the case of rat WD or DMEM/F12 supplemented with 10% FCS, GDNF (125ng/ml), and FGF1 (125ng/ml) in the case of mouse WD. All cultures were performed in triplicate with n≥3 rudiments per sample.
TUNEL Apoptosis Assay
TUNEL labeling of DNA strand breaks was performed in whole kidney rudiments. Kidneys were fixed in 1% paraformaldehyde for 30 min at RT and rinsed in PBS. TUNEL staining was then performed according to the manufacturer's instructions (Intergen, Purchase, NY). Counterstaining with Lotus lectin preceded mounting in Fluoromount. Samples were evaluated by scanning laser confocal microscopy.
Generation of BSN conditioned media
The metanephric mesenchyme-derived cell line (BSN cells) was cultured in DMEM/F12 supplemented with 10% fetal calf serum at 37°C in an atmosphere of 5% CO2. Conditioned medium was collected after incubation with serum-free DMEM/F12 for 3–4 days. After collection, the conditioned media was concentrated approximately five fold, and the buffer was changed to fresh DMEM/F12 media using the Ultrasette filtration device (5K MWCO; Pall; San Diego, CA).
Isolation and Culture of UB
Isolated UB cultures were performed as previously described (Qiao et al., 1999) with slight modifications. Briefly, E13 rat or E11 mouse kidneys were lightly digested with trypsin and the UBs separated from the MM using fine-tipped needles. The UBs were suspended in Transwell filters within a matrix containing growth factor-reduced Matrigel diluted 1:1 with DMEM/F12 media. The isolated UBs were then cultured in BSN-CM supplemented with 10% FCS, 125 ng/ml GDNF and 125–375 ng/ml FGF1 and varying concentrations of heparinoid as indicated in the text. UBs were allowed to grow for 6–7 days. All cultures were performed in triplicate with n≥3 rudiments per sample.
Recombination of Hs2st null and wild-type tissues
Hs2st null UB and MM and wild-type UB and MM were obtained from E12 embryos. Hs2st null tissues were identified by kidney rudiments that failed to undergo branching morphogenesis and were subsequently confirmed via PCR genotyping. UB and MM were isolated as described above. For mix-and-match experiments, Hs2st null UBs were recombined with wild-type MM and vice-versa on the day of isolation on a Transwell filter and cultured in DMEM/F12 with 10% FCS and the culture was carried out for 5–7 days at 37°C, 5% humidified CO2.
Recombination of cultured UB and fresh MM
Cultured isolated rat UBs that were grown for 6–7 days were cleanly separated from as much of the surrounding matrix as possible and placed on top of a Transwell filter in close proximity to freshly isolated E13 rat MM. DMEM/F12 with 10% FCS was added to the well and the culture was carried out at 37°C, 5% humidified CO2 as previously described (Qiao et al., 1999; Steer et al., 2002). All cultures were performed in triplicate with n≥2 replicates per experiment.
Immunohistochemistry and Confocal Analysis
Isolated UB grown in extracellular matrix gels or whole cultured kidneys were fixed in 4% paraformaldehyde (EM Sciences, Fort Washington, PA) for 30 min at room temperature and then washed with PBS. UBs were dissected from the Transwell insert and excess extracellular matrix gel was removed. Immunohistochemistry was performed as previously described (Meyer et al., 2004). Peanut agglutinin (PNA) lectin staining after tissue incubation with neuraminidase was carried out as previously described (Qiao et al., 1999). Specimens were examined by scanning laser confocal microscopy (Nikon D-Eclipse C1). Images were processed with Photoshop software (Adobe, San Jose, CA).
Image Analysis and Statistics
Geometric and quantitative measurement of the tissues was performed using Image Pro Plus. UB tips within cultured embryonic kidneys were visualized by staining with fluorescein-conjugated Dolichos biflorus lectin (1:100). Each assay was performed in at least triplicate and data are presented as mean values + S.D. The Anova single factor test was applied to data from the experiments. A p value of <0.05 was accepted to indicate statistical significance.
RESULTS
Whole Hs2st null kidney rudiments fail to develop in vitro, indicating an intrinsic kidney defect
In the absence of the HS biosynthetic enzyme, Hs2st, kidney agenesis results with 100% penetrance (Bullock et al., 1998) suggesting that HS modifications are critical for the formation of the kidney. During our analysis, we noted that approximately one-third of the mutant kidney rudiments had undergone the first bifurcation step to form the T-shaped UB at e12.5 (Fig. 1), although branching is significantly delayed compared to wild-type. This finding is in contrast to the originally reported phenotype of the Hs2st null mouse (Bullock et al., 1998). Despite the initial UB branching that occurred, renal agenesis persisted; thus as an initial step to determine the specific location affected by loss of Hs2st (UB or MM), we first confirmed that the primary defect is intrinsic to the kidney by performing whole embryonic kidney culture using Hs2st−/− E12 kidney rudiments. Consistent with the in vivo phenotype, these embryonic kidneys did not undergo branching morphogenesis or nephron induction (irrespective of the presence of UB bifurcation) (Fig. 2G,H) whereas wild-type (Fig. 2A,B) and heterozygous (Fig. 2D,E) rudiments grew normally. This result confirmed that the defect in kidney development is fundamental to the kidney and is not due to extrinsic HS-mediated factors. TUNEL staining on the embryonic kidney rudiments demonstrated there was little apoptosis noted in either UB or MM in wild-type and heterozygous kidneys isolated at E11.5 (Fig. 2C,F). Similarly, there was little apoptosis in knockout kidneys (Fig. 2I). This result suggests that the underlying mechanisms leading to the failure of kidney development likely occur in the events that occur after UB formation (UB branching, MM induction) or via early non-apoptotic events.
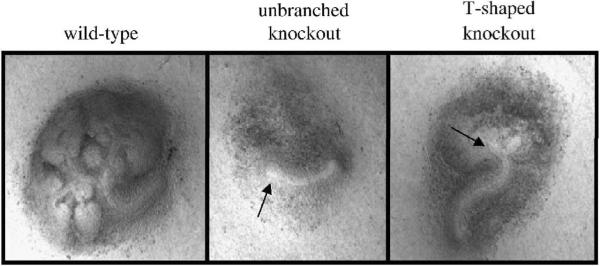
Figure 1.
Phenotypic variations of Hs2st null kidneys. At e12.5, two different ureteric bud (UB) phenotypes are noted amongst Hs2st−/− embryonic kidneys. The most common phenotype is that of an unbranched UB (approximately two-thirds of kidneys analyzed) and the other is of a UB that has undergone the first dichotomous branching step (T-shaped, approximately one-third). Despite the fact that the UB has branched to a T-shape, overall branching is significantly delayed compared to wild-type and renal agenesis occurs at a 100% penetrance rate.
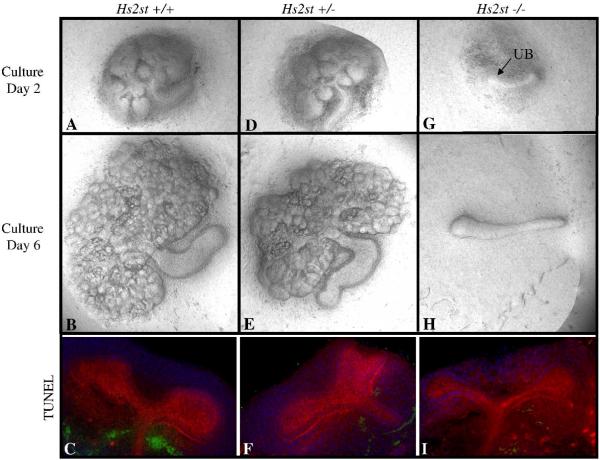
Figure 2.
Embryonic kidney culture and TUNEL staining of kidney rudiments obtained from Hs2st heterozygous matings. Phase contrast micrographs of embryonic day 12 kidneys isolated and cultured in vitro for 6 days. Genotyping was performed following isolation. Both wild-type (A,B) and heterozygous (D,E) kidney rudiments display normal growth and branching patterns on culture day 2 and culture day 6. In contrast, knockout rudiments show no morphogenesis beyond outgrowth of the ureteric bud at culture day 2 (G; arrow points to the ureteric bud) and by culture day 6, the metanephric mesenchyme has degenerated although the ureter remains (H). All images at 10X. TUNEL staining in whole embryonic kidneys. Confocal micrographs of isolated wild-type (A), heterozygous (B) and knockout (C) embryonic day 11.5 kidneys stained for apoptotic cells (green), lotus lectin (red) and DAPI (blue). There is no significant difference in apoptosis between embryonic kidneys that express Hs2st (C,F) and embryonic kidneys that are deficient in Hs2st (H).
Hs2st is expressed in both the UB and MM during early development
During renal development, Hs2st is initially expressed in both UB and MM compartments but subsequently becomes localized to the undifferentiated mesenchyme (Bullock et al., 1998). We sought to confirm these finding using the GUDMAP expression database (www.gudmap.org). GUDMAP was developed as a repository for high resolution analyses to define gene expression throughout kidney development and to correlate the boundaries of gene expression with anatomic domains; the database has now been used to show differences in gene expression within specific kidney cell types and has demonstrated excellent correlation with previously published gene expression data in the UB and MM (Brunskill et al., 2008). We confirmed that the expression of Hs2st is present at e11.5 and is not significantly different between the e11.5 UB and MM (Fig. 3). Consistent with the original report, Hs2st expression was significantly increased in the uninduced MM (peripheral blastema) versus the UB tip at e15. From this expression data one cannot determine where the primary defect occurs since kidney developmental failure occurs early in kidney organogenesis and Hs2st is expressed in both locations at this stage. In addition, because HS proteoglycans are present either as cell surface molecules or as components of the basement membrane, which both cell types potentially contribute to the establishment thereof, the precise location that is affected by loss of HS 2-O sulfation cannot necessarily be determined by the cellular expression of the enzyme. It has been postulated that a primary defect in UB branching may be the responsible mechanism of renal agenesis because UB branching does not occur in the absence of Hs2st (Wilson et al., 2002); however, the eventual localization of Hs2st to the mesenchymal compartment might suggest that the defect underlying the renal agenesis phenotype resides in the MM. Whatever the mechanism, the knockout data suggests that Hs2st is somehow involved in the progression from the UB budding to the iterative UB branching stage of kidney development.
Figure 3.
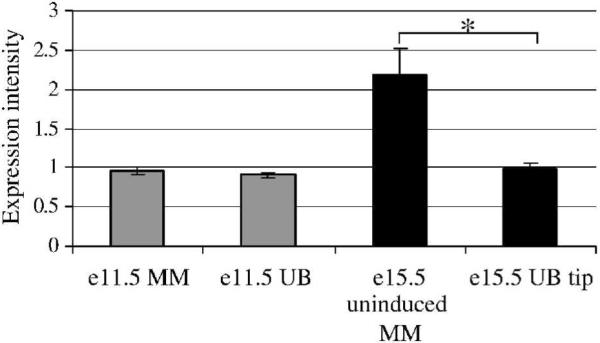
Hs2st expression in ureteric bud (UB) and metanephric mesenchyme (MM) is quantitatively similar at e11.5. Hs2st expression was analyzed using the GUDMAP database (www.gudmap.org). At e11.5, expression of Hs2st was quantitatively similar in the UB and MM while at e15.5, Hs2st expression is significantly higher in uninduced MM (peripheral blastema) vs. the UB tip. Average ± SD, n=3, *p<0.05 compared to e15.5 UB tip.
In vitro kidney epithelial morphogenesis is normal in the absence of Hs2st
In order to resolve the above issues regarding the location of the defect, and to isolate the stage at which kidney development fails, we utilized the mutant kidney rudiments in well-established in vitro assay systems, which are presumed to recapitulate the steps of kidney development based on morphology and gene expression (Qiao et al., 1999;Stuart et al., 2001; Stuart et al., 2003; Maeshima et al., 2006; Rosines et al., 2007; Stuart et al., 2001; Tsigelny et al., 2008). Briefly, a model of kidney collecting system morphogenesis has been proposed in which the processes leading to nephron formation consist of discrete and functionally separable “stages”, some of which overlap in vivo (Monte et al., 2007; Sampogna and Nigam, 2004; Rosines et al., 2007; Nigam and Shah, 2009; Tsigelny et al., 2008; Shah et al., 2004). These stages include UB outgrowth from the WD, formation of the “T-shaped” UB, early UB branching, late branching, and branching termination and tubule maintenance. In vitro and in vivo studies suggest that these stages of kidney collecting system development are governed by a unique set of factors and that disruption of these processes leads to stage-specific defects (renal agenesis, diminished nephron number, cystic kidney disease). It is further hypothesized that specific growth factor-HS interactions play key roles in the transition from one stage to the next (Monte et al., 2007; Nigam and Shah, 2009; Sampogna and Nigam, 2004; Shah et al., 2004; Steer et al., 2004). These in vitro systems enable the separate modeling of these processes related to development of the kidney collecting system through branching (Bush et al., 2004; Maeshima et al., 2006; Qiao et al., 2001; Qiao et al., 1999; Rosines et al., 2007).
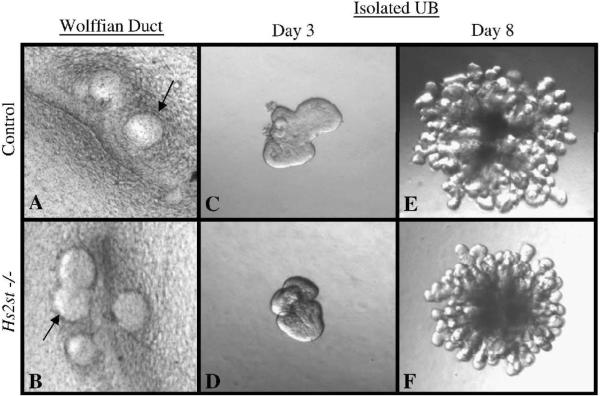
We first attempted to elucidate whether the underlying developmental processes involved in UB formation (specifically: morphogenesis, proliferation, the ability to deliver and receive reciprocal inductive signals) are intact in the absence of 2-O sulfated HS using an in vitro isolated WD culture system that models prospective MM signaling to the WD to form the UB (Maeshima et al., 2006). Consistent with the in vivo phenotype, the isolated WD from Hs2st null mice underwent budding similar to non-knockout counterparts (Fig. 4A,B) suggesting that the early morphogenetic processes are not dependent upon HS 2-O sulfation. This is in contrast to embryonic kidney growth (in which the UB and MM are also both present) where development fails (Fig. 2G,H).
Figure 4.
Epithelial morphogenesis occurs in Hs2st−/− Wolffian ducts (WD)and isolated ureteric buds (UB). A, B: Phase contrast micrographs of embryonic day 12 WD isolated and cultured in vitro for 3 days in DMEM/F12 supplemented with 125ng/ml GDNF and 125ng/ml FGF1. Genotyping was performed following isolation. Isolated WDs from control and knockout rudiments show similar levels of epithelial budding suggesting that the Hs2st null WD is competent to form a ureteric bud under these conditions. Arrows point to areas of budding from the WD. C–F: Phase contrast micrographs of UBs isolated from E11 kidney rudiments, suspended in a 3-dimensional extracellular matrix and cultured in the presence of BSN conditioned media and 125ng/ml GDNF and 125ng/ml FGF1. Isolated UBs from control (C,E) and knockout (D,F) rudiments show essentially the same pattern of growth and branching suggesting that the Hs2st null UB is competent to undergo branching morphogenesis under these conditions. All images at 10×.
We then investigated if HS 2-O sulfation plays a role in the next stage of kidney development, robust UB branching, by analyzing MM-independent UB branching. Initially we applied an established in vitro model system of isolated UB branching to the Hs2st null UB to determine if the UB devoid of 2-O sulfated HS would undergo branching morphogenesis in the presence of exogenous 3-dimensional extracellular matrix and growth factors. This well characterized assay, in which the UB is induced to undergo robust iterative branching morphogenesis in the absence of contact with mesenchyme, has been fruitfully employed in the purification of key morphogenetic factors that affect UB branching (Bush et al., 2004; Sakurai et al., 2001; Sakurai et al., 2005; Steer et al., 2004). Hs2st−/− UBs were isolated at E11 and cultured in the presence of media conditioned by MM-derived cells plus 125ng/ml GDNF and 125ng/ml FGF1. Under these conditions, mutant UBs proliferated and branched in a pattern similar to control UBs (Fig 4C–F). These findings indicate that the mutant UB is competent to undergo branching morphogenesis when cultured in a suitable matrix environment and in the presence of branch stimulatory factors (matrix and growth factors presumably supplied by wild-type MM) and, notably, that the failure of kidney development in the Hs2st mutant mice is not likely to be intrinsic to the UB.
Hs2st−/− MM is capable of inducing UB branching morphogenesis and undergoing mesenchymal-to-epithelial transformation in vitro
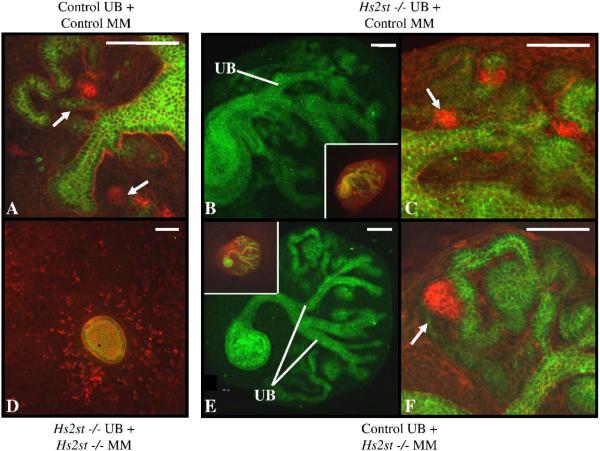
The above results suggest that the epithelial component of the Hs2st null kidney rudiment is competent to undergo branching morphogenesis. We then sought to test if MM inductive capacity was intact by performing “mix-and-match” experiments between mutant and wild-type/heterozygous tissues (Fig 4). UBs and MMs were isolated at e11.5, and mutant tissue was visually identified through the absence/delay of UB branching. Tissue genotype was later confirmed via PCR. Consistent with the above results demonstrating UB competence, control MM was able to induce branching morphogenesis of Hs2st−/− UB's, and these mutant UBs were able to induce mesenchymal-to-epithelial transition of the control MM (Fig 5B) suggesting that the UB's transformative and inductive properties are intact even in the absence of Hs2st within the UB; thus it appears that factors that modulate early UB branching are either not dependent upon 2-O sulfated HS or their effects are mediated through a non-cell autonomous mechanism. Surprisingly, a similar result was noted when Hs2st−/− MM was recombined with control UB (Fig 5C) indicating that MM lacking 2-O sulfated HS is also competent to undergo mesenchymal-to-epithelial transition in the presence of wild-type HS provided by the UB.
Figure 5.
Mix-and-match recombination culture between Hs2st knockout and control tissues. Ureteric buds (UB) and metanephric mesenchyme (MM) were isolated from E12 kidney rudiments. Knockout tissue was visually identified by lack of UB branching morphogenesis and tissue genotype was later confirmed via PCR. Confocal micrographs of recombined tissues stained with E-cadherin (green) and peanut agglutinin (red indicates podocytes) outline epithelial (green- UB and MM derived) and mesenchymal (red) structures. Images show lack of mutual induction in knockout UB recombined with knockout MM (D) and UB branching and mesenchymal-to-epithelial transformation in control (wild-type) UB recombined with control (wild-type) MM (A), knockout UB recombined with control (heterozygous) MM (B,C), and control (heterozygous) UB recombined with knockout MM, and (E,F). B,E: Epithelial structures in the recombined tissue, UB branches are noted emanating from the common ureter Insets show tissue with both green and red channels displayed. C,F: High power images of B,E showing pre-glomerular structures (arrows-podocytes) derived from the MM. Scale bars 100μm.
Together, these results demonstrate that, in isolation (WD and UB) or in the presence of wild-type HS provided by the UB (MM), each Hs2st−/− progenitor compartment is competent to undergo its specified morphogenetic program suggesting that 2-O sulfated HS is not required for these intrinsic events. Interestingly, we noted a difference in inductive capacity when UB and MM were obtained from kidney rudiments that had not undergone the first UB bifurcation event. Unlike the UB and MM from Hs2st−/− embryonic kidneys that had a T-shaped UB, neither UB nor MM obtained from kidney rudiments with an unbranched UB was able to induce their respective control tissue. Thus, it appears that these separate stages of kidney development (formation of the T-shaped UB and iterative UB branching) have varying sensitivity to 2-O sulfated HS, a topic worthy of further study. These results suggest that factors that modulate early iterative UB branching are most sensitive to this HS modification.
The different stages of embryonic kidney development demonstrate varying sensitivity to the heparin 2-O sulfation modification
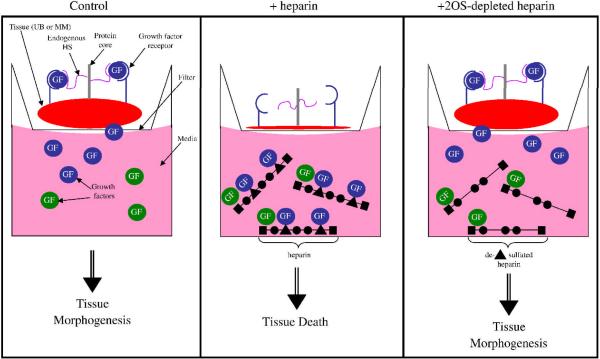
Given the above results demonstrating that each progenitor tissue of the Hs2st null kidney rudiment is intrinsically capable of undergoing its specified morphogenetic program, it seems reasonable to posit that the mechanism underlying the renal agenesis phenotype is due to altered HS modulation of growth factor activity, since HS is primarily involved in the binding of growth factors in developing tissues (Garner et al., 2008; Iwao et al., 2009; Makarenkova et al., 2009; Steer et al., 2004). We therefore sought to clarify the role of 2-O sulfated HS on growth factors involved in embryonic kidney development through a competition assay (schematized in Fig. 6). In this assay, heparin or 2OS-depleted heparin are added to the media in which the tissue of interest is cultured. 2OS-depleted heparin is synthetically modified so that only 6-O- and N- sulfate groups on the glucosamine moieties remain. Since heparin avidly binds many factors, heparin (which contains 2-OS moieties), within the media, competitively binds factors away from endogenous tissue HS (either at the cell surface or in the extracellular matrix). Thus, we compared the effect of adding heparin versus adding 2OS-depleted heparin at various concentrations, using tissue morphogenesis as a read out (Fig 6). In the case of this assay, the heparin compounds exogenously applied to the media are not meant to substitute for endogenous HS but rather are used to elucidate the HS binding requirement for factors within the media that are potentially important to kidney development. A titration curve was performed to determine if a differential effect between heparin and 2OS-depleted heparin occurred at each stage. If there is a concentration at which the addition of heparin to the media results in inhibition of tissue morphogenesis, and the addition of 2OS-depleted heparin does not, it can be assumed that the missing 2-O sulfation modification is important to the binding of at least one or more factors important to the morphogenetic event. Conversely, if the addition of 2OS-depleted heparin also inhibits tissue morphogenesis to the same degree as heparin does, even at low concentrations, then the sulfation modification is not necessary for the binding of factor(s) critical to morphogenesis as they are still bound to the 2OS-depleted heparin within the media (see Fig. 6, middle panel).
Figure 6.
Schematic of the effect of heparin and 2OS-depleted heparin in the in vitro culture system. Under control conditions (far left panel), growth factors present within the media and secreted by the tissue of interest (embryonic kidney, ureteric bud or metanephric mesenchyme) are captured by heparan sulfate (HS) present on the cells and within the extracellular matrix of the tissue which results in tissue morphogenesis (blue circles represent growth factors necessary for morphogenesis while the green circles are not critical but may be supportive for morphogenesis). Middle panel: When fully sulfated heparin (with 2-O sulfated residues, represented by the triangle) is added to the media it binds a variety of growth factors. The heparin competitively binds factors away from endogenous HS, thereby trapping the factors within the media, resulting in tissue death. When 2OS-depleted heparin is added to the media (far left panel), growth factors that depend on the 2-O sulfated moiety for binding (blue circles), will be free in the media and able to bind to endogenous HS while those that do not depend on the 2-O sulfated moiety for binding (green circles) will continue to be trapped in the media. In this schematic, the blue growth factors are critical for tissue morphogenesis and are then able to induce morphogenesis.
In vitro organ culture using 2-OS depleted heparin gives results comparable to the Hs2st−/− kidney
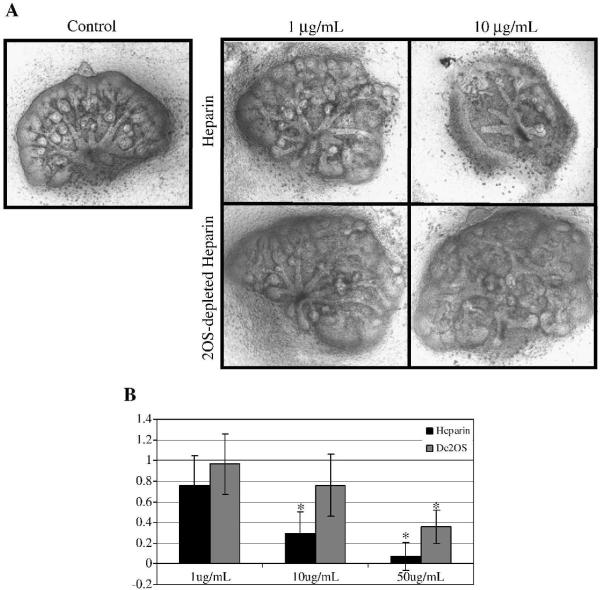
We first analyzed the HS sulfation dependence of factors involved in embryonic kidney culture. It has been previously demonstrated that addition of 10 μg/mL of heparin is the minimum concentration at which heparin, in the media, competitively binds growth factors and results in significant reduction in UB branching morphogenesis in the whole embryonic kidney (Davies et al., 2003). A similar concentration of 2OS-depleted heparin did not significantly suppress UB branching, confirming that the 2-OS sulfation modification is important in the binding of factors involved in kidney development (Fig 7), a finding consistent with the in vivo Hs2st−/− phenotype and organ culture of the mutant whole kidney (Fig 2).
Figure 7.
A: Heparin and 2OS-depleted heparin titration curve on embryonic kidney rudiments. Phase contrast micrographs of e13 rat embryonic kidneys that were cultured in the presence of varying concentrations of heparin and 2OS-depleted heparin for 4–5 days. At a concentration of 10μg/mL, heparin markedly disrupts ureteric bud (UB) branching morphogenesis and mesenchymal induction while a similar dose of 2OS-depleted heparin has little effect on these processes. Images at 10× B: Graphical analysis of the average number of UB tips as a percentage of control. UB tips were counted after staining embryonic kidneys for UB specific Dolichos bifloris (raw data not shown). At higher concentrations (50 μg/mL), addition of heparin and 2OS-depleted heparin inhibits UB branching. Mean ± SD, n>3, *p<0.05 compared to control.
The binding of UB branching factors is independent of heparin 2-O sulfation
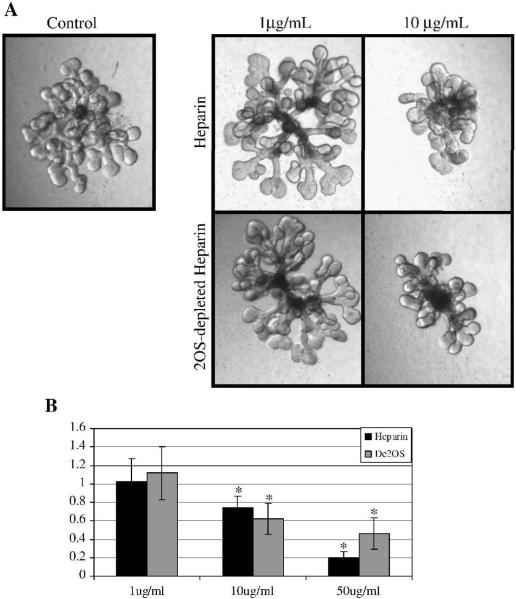
The aforementioned result in organ culture does not differentiate whether the effect is on the UB or MM side. When heparin and 2OS-depleted heparin were added to the media of the cultured isolated UB (in the presence of MM derived soluble growth factors), UB branching was significantly inhibited compared to control at all concentrations tested (Fig 8). This result demonstrates that both heparin and 2OS-depleted heparin competitively bind factors that stimulate isolated UB branching. Thus, the 2-O sulfation modification does not appear to be necessary for binding of these UB branch promoting factors. This is in marked contrast to the results from whole kidney organ culture (in which both UB and MM are present) where there was a significant difference in morphogenesis between kidneys treated with heparin and 2OS-depleted heparin at a concentration of 10 μg/mL (Fig 7, described above). Thus, it appeared that essential factors that stimulate isolated UB branching have similar affinity to 2OS-depleted heparin as to fully sulfated heparin. It therefore seems unlikely that the Hs2st−/− phenotype can be explained by a defect in growth factor induced UB branching. This led us to consider whether the defect lay on the MM side.
Figure 8.
Heparin and 2OS-depleted heparin titration curve on isolated ureteric bud (UB) branching morphogenesis. A: Phase contrast micrographs of UBs isolated from E13 kidney rudiments, suspended in a 3-dimensional extracellular matrix and cultured in the presence of BSN conditioned media and 125ng/ml GDNF and 125ng/ml FGF1 and varying concentrations of heparin and 2OS-depleted heparin. Images at 10×. B: Graphical analysis of the average number of UB tips as a percentage of control. At a concentration of 10μg/mL and higher, both heparin and 2OS-depleted heparin significantly inhibit UB branching indicating that the binding of factors involved in UB branching are not markedly affected by the 2-O sulfate modification. Mean ± SD, n>3, *p<0.05 compared to control.
In vitro epithelialization of the MM depends on heparin 2-O sulfation
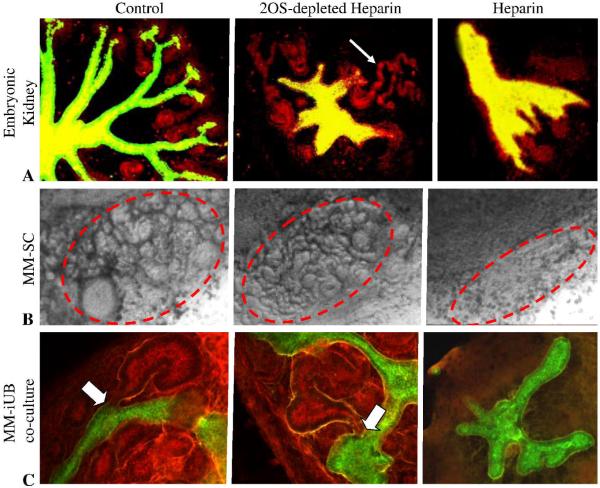
If Hs2st−/− UB branching occurs normally in isolation from the MM (Fig 5) and when recombined with wild-type MM (Fig 4C), while being abnormal in the whole embryonic kidney in vivo and in organ culture (Fig 1)- and if factors that stimulate iterative branching of the wild-type isolated UB in vitro are not dependent upon HS 2-O sulfation (Fig 8)- then it seemed plausible that the defect preventing movement from the UB budding to the iterative branching stage in the Hs2st−/− kidney resides in the inductive capacity of the MM. We investigated this possibility by evaluating the effect of the modified heparin on MM induction. For these experiments, embryonic spinal cord, a known potent inducer of MM, was placed in close contact with freshly isolated MM. Widespread cell death without mesenchymal-to-epithelial transformation was observed in the MM/spinal cord culture (as well as organ culture) in the presence of fully sulfated heparin while MM exposed to 2OS-depleted heparin underwent mesenchymal-to-epithelial transition similar to control (Fig. 9B). Since fully sulfated heparin has 2-O sulfated residues, the marked difference in its effects compared to 2OS-depleted heparin is consistent with a key role for the 2-O sulfate moiety in MM morphogenesis. Thus, these results suggest that the binding of factors that stimulate mesenchymal-to-epithelial transition are sensitive to HS 2-O sulfation and, given the insensitivity of factors that stimulate isolated UB branching to the same modification (Fig. 8), supports the view that the main defect in the Hs2st−/− kidney lies in the role of HS to modulate growth factor activity in the MM.
Figure 9.
Effects of heparin and 2OS-depleted heparin on mesenchymal-to-epithelial transformation. A: A: Confocal micrographs of embryonic kidney cultured for 4 days in the absence (control) or presence of 100 μg/ml 2OS-depleted heparin or heparin. Note that addition of heparin results in generalized tissue death (A, far right panel) while addition of 2OS-depleted heparin (A, middle panel) inhibits UB branching but mesenchymal induction continues to take place at UB tips that do form (D. biflorus lectin (green) and E-cadherin (red); arrow point to induced MM structures) B: Phase contrast micrographs of metanephric mesenchyme (MM) isolated from E13 rat kidney co-cultured with spinal cord. MM was cultured for 5 days in the absence (control, far left panel) or presence of 100 mg/ml heparin (B, far right panel) or 2OS-depleted heparin (B, middle panel). Circled areas highlight MM that has undergone mesenchymal-to-epithelial transition. C: Effects of 2OS-depleted and fully sulfated heparin on mutual induction between the isolated UB and MM. Confocal micrographs of isolated UB cultured for 7 days then recombined with freshly isolated MM and further cultured for 5 days in the absence (control) or presence of 100 μg/ml heparin (C, far left panel) or 2OS-depleted heparin (C, middle panel) (D. biflorus (green) and peanut agglutinin (red) outline UB derived (green) and mesenchymal (red) structures). Note the absence of induced MM structures including comma and s-shaped bodies in the heparin treated tissues and apparently normal MM induction in the presence of 2OS-depleted heparin (arrowheads point to induced MM structures; arrows in higher magnification images (D) point to connecting segments between isolated UB and MM derived tissues).
MM inductive capacity for iterative UB branching morphogenesis depends on HS 2-O sulfation
Even though factors stimulating mesenchymal-to-epithelial transformation in the MM were highly sensitive to the heparin 2-O sulfation modification (Fig 9B), it is worth re-emphasizing here that the Hs2st−/− MM could normally induce wild-type iterative UB branching (and, as described, the mutant UB could induce wild-type MM to undergo normal mesenchymal-to-epithelial transition and epithelialization, Fig 5) despite the failure of Hs2st−/− kidney formation in vivo and in vitro (Fig 2). This suggested that the key role for HS 2-O sulfation lay in its role in enabling MM inductive capacity for movement of the UB from the T-shaped phase to the iterative branching phase of kidney development. Furthermore, it could be argued that spinal cord is a surrogate for the natural inducer.
Therefore, we sought to address whether the natural inducer- by way of the UB/MM recombination assay- gives consistent results. In other words, do the factors that modulate inductive signals from the UB to the MM that stimulate mesenchymal-to-epithelial transition require heparin 2-O sulfation for binding? This assay takes advantage of the ability of isolated UBs cultured or “developed” for 7 days and freshly isolated MM to undergo mutual induction (Qiao et al., 1999; Steer et al., 2002; Rosines et al., 2007; Shah et al., 2009). Under control conditions (Fig. 9C), freshly isolated MM was able to induce elongation and tapering of cultured isolated UB branches into the MM which in turn, was induced to form comma and S-shaped bodies as occurs in vivo during the iterative UB branching phase of kidney development (Qiao et al., 1999; Shah et al., 2009). These segments eventually connected to form nascent nephron units. In the presence of fully sulfated heparin, widespread cell death was observed within the MM and no recombination (mesenchymal-to-epithelial transition plus connection of UB and MM structures) took place (Fig 9C, far right panel). This is consistent with the results already described for whole kidney organ culture (Fig 9A). Normal recombination, with iterative UB branching, occurred in the presence of 2OS-depleted heparin (despite the fact that isolated UB branching was inhibited by 2OS-depleted heparin) with connecting segment formation (white arrows in Fig. 9C). This data supports the view that MM induction is dependent upon factors that bind to 2-O sulfated HS. Thus, the defect in the Hs2st null mouse leading to renal agenesis mainly resides within the MM's ability to induce progression of the UB from the budding stage to the iterative branching stage of nephrogenesis.
DISCUSSION
We have utilized a novel combination of in vivo and in vitro approaches to provide the first clear example of a defect in the transition of the ureteric bud (UB) from the first dichotomous branching step of kidney development to the iterative branching phase as a consequence of a primary MM defect; this arises from inadequate 2-O sulfation of heparan sulfate (HS) moieties. This step is one of the limiting steps in early kidney collecting system development, the other being the UB budding event which is not, as we show, affected by the lack of HS 2-O sulfation in vitro or in vivo.
Heparan sulfate is a glycosaminoglycan abundantly present on the cell surface, extracellular matrix and within the basement membrane. Disruption of HS synthesis leads to embryonic lethality in mice (Lin et al., 2000; Stickens et al., 2005), while disruption of HS sulfation has a variety of developmental consequences on multiple different organ systems (Bullock et al., 1998; Fan et al., 2000; Grobe et al., 2005; Li et al., 2003; Shworak et al., 2002). The severe kidney phenotypes associated with null mutation of Hs2st and C5 epimerase highlight the centrality of HS sulfation modifications to this developmental process. Here, we have re-examined the in vivo phenotype and employed several in vitro assays that recapitulate the stages of kidney development to isolate the defect in the Hs2st null mouse that results in renal agenesis. Quite surprisingly, when Hs2st null progenitor tissues (Wolffian duct (WD), UB and metanephric mesenchyme (MM)) are cultured either in isolation, as with the WD or UB or in the presence of wild-type HS, as with the MM, each is capable of undergoing its specified developmental program; however, when combined with tissue carrying mutant HS (either as embryonic kidney culture or in UB-MM co-culture), these processes fail. That each can develop in isolation suggests that the downstream intracellular signaling pathways are intact and that it is likely the extracellular environment that is unfavorable to kidney development.
To our knowledge, such a set of results has never been reported. It has previously been suggested that the primary target for 2-O sulfated HS is to primarily modulate UB branching (Wilson et al., 2002). By using variably sulfated heparin derivatives we are able to demonstrate that UB branching is actually not modulated by factors that depend on 2-O sulfate HS, although factors modulating mesenchymal-to-epithelial transition in the MM are sensitive to the 2-O sulfation modification. Taken together these results demonstrate that the early cessation of kidney development in the Hs2st−/− mouse is not the result of an intrinsic defect within the UB but rather, most likely a primary disturbance in the signaling of MM induction in vivo and in vitro, and possibly also (at least in vitro) a failure of MM morphogenesis.
One of the unexpected findings of this study was that in approximately 30% of embryos, the UB had undergone the first dichotomous branching event. We can only speculate that the reason for the discrepancy in our observed phenotype from that which was originally reported (Bullock et al., 1998) is due to the laboratory timing of embryonic development, which can vary from experiment to experiment and lab to lab by half a day, thereby leading to somewhat different results. Irrespective of the reason, the finding suggests another possible role for 2-O sulfated HS: modulation of factor(s) that stimulate the unbranched UB to undergo the first dichotomous branching step.
Another surprising finding was the ability of mutant UB and MM to undergo their morphogenetic programs in the presence of wild-type HS (Fig 5). This result would seem to discount cell surface HS, either on the UB or MM itself, as a primary modulator of these processes. It is possible, however, that shedding of cell surface HSPGs in response to extracellular cues (Selleck, 2006) provides a repository of HS-associated growth factor within the matrix that may be important to the inductive process; this mechanism would provide an explanation of why wild-type HS from either the UB or MM is adequate to drive the system in vitro.
Alternatively, it has been proposed that HS presented in trans to a growth factor receptor can compensate for loss of cell surface HSPGs, such that local, cell-autonomous deficiencies in HS production can be fully rescued by adjacent cells, thereby acting cell-nonautonomously (Kramer and Yost, 2002). This concept has recently been proven in vascular morphogenesis. Complete loss of N-sulfated HS in the basement membrane between endothelial cells and mural cells abrogates VEGFR signaling in endothelial cells (Stenzel et al., 2009). In the absence of endothelial cell-derived HS, N-sulfated HS produced by mural cells is sufficient to support VEGFR signaling in endothelial cells (Jakobsson et al., 2006) illustrating that HS and growth factor receptors can interact in trans in developmental processes. Hs2st itself is expressed in both the UB and MM (Bullock et al., 1998), and HS is found throughout the kidney, but is present at highest concentrations in the basement membrane that lies between the UB and MM (Davies et al., 1995). It may be that HS present within the basement membrane is necessary for high affinity growth factor-growth factor receptor interactions which transmit inductive signals to the UB. Alternatively, these interactions may be part of an autocatalytic loop that functions to amplify growth factor signals (Monte et al., 2007; Nigam and Shah, 2009). In the case of the Hs2st null mouse, it appears that appropriately sulfated HS from either the UB or MM “in trans”, can compensate for lack in the other compartment. These “in trans” 2-O sulfated HS may be important in the growth factor-dependant inductive UB-MM crosstalk necessary for early UB branching, and our cumulative data argue that this is where the defect resides (on the MM side); thus explaining the renal agenesis that occurs in the absence of Hs2st.
Interestingly, neither the UB nor MM isolated from Hs2st null rudiments that had an unbranched UB was able to induce mesenchymal-to-epithelial transformation or branching morphogenesis respectively. Thus, the cascade of inductive signals between the undifferentiated metanephric mesenchyme and epithelial UB that first induce UB formation, then stimulate UB birfurcation and finally bring about iterative UB branching appear to have varying sensitivity to HS sulfation. The first step, while likely to be modulated (in part) by heparin binding growth factors, involves molecules that are seemingly not as dependent upon HS sulfation pattern while the factors that move the unbranched UB towards bifurcation demonstrate a significant sensitivity to the HS 2-O sulfation modification, whereas those that modulate early UB branching are exquisitely sensitive.
HS 2-O sulfation has been demonstrated to be an essential component for high affinity HS binding for many factors important to kidney development including GDNF. While GDNF has been shown to primarily modulate UB branching (Qiao et al., 1999; Shakya et al., 2005), complex interactions that lead to mesenchymal differentiation have not been ruled out. Several studies suggest that GDNF bioactivity demonstrates an unusually high dependence upon the presence of HS 2-O sulfates (Rickard et al., 2003; Davies et al., 2003). GDNF has been shown to be necessary for formation of the UB from the WD and in branch initiation (Sainio et al., 1997), and mice homozygous null for GDNF predominantly display a phenotype of renal agenesis due to lack of formation of the ureteric bud although a small proportion do undergo rudimentary UB branching (Cacalano et al., 1998; Pichel et al., 1996; Schuchardt et al., 1994). Although the expression of GDNF is rapidly downregulated in Hs2st−/− kidney rudiments, the observation that the majority of GDNF deficient mice do not form a ureteric bud suggests that HS 2-O sulfation is not an absolute requirement for GDNF signaling in vivo since the UB forms in the absence of Hs2st (Wilson et al., 2002). Alternatively, a recently described GDNF-independent bypass pathway for UB budding may be operative (Maeshima et al., 2007). This study supports these possibilities since budding and branching occurs normally in Hs2st null WDs and UBs, respectively.
The question then remains, if not GDNF, what are other possible factors involved in the Hs2st null phenotype? One possibility includes members of the Wnt family of secreted molecules, which have been shown to be important during mesenchymal-to-epithelial transition (Schmidt-Ott et al., 2006). Wnt9b, expressed in the WD and its derivatives, has been shown to be a primary modulator of mesenchymal transformation. Knockout of Wnt9b results in renal agenesis with intact ureter formation, a similar phenotype to that seen in Hs2st mutants. Further analysis of Wnt9b null mice reveals that UB invasion of the MM occurs at the appropriate stage and the first branching event is initiated; however mesenchymal induction fails and subsequent UB branching is disrupted (Carroll et al., 2005). Similar to the Hs2st mutant mouse, Wnt9b −/− mice have downregulated GDNF and Wnt11 expression suggesting that the Wnt11-GDNF positive regulation loop is disrupted (Majumdar et al., 2003). In certain respects, the Hs2st−/− kidney can be viewed as the counterpart example to the Wnt9b −/− kidney with the primary defect being on the MM rather than WD/UB side but nevertheless, leading to a similar phenotype. Wnts are known to be dependant upon HS for signaling (Lin, 2004); thus these, along with other heparin binding growth factors known to affect budding and branching (Bush et al., 2004; Maeshima et al., 2006; Sakurai et al., 2001; Sakurai et al., 2005), are candidate signaling pathways that lead to the renal agenesis phenotype.
In summary, this study utilizes a novel combination of in vivo and in vitro analyses to dissect the potential roles of differential HS sulfation during kidney development. Our analyses revealed involvement of Hs2st in the advancement to the iterative UB branching stage as a result of a primary MM defect in 2-O sulfation. The combination of in vivo and in vitro strategies used here adds power to the analysis that neither approach allows on its own. We use this combined approach to show that the failure in kidney development in the Hs2st mutant mouse is likely the result of inhibition of mutual inductive signaling that occurs after WD budding and primarily resides in the MM. This is in contrast to previous assertions suggesting a primary role of sulfated HS in UB branching, rather than MM differentiation (Wilson et al., 2002); of course, this hypothesis was reasonable in that it was based on histology. A corollary of the combined in vivo/in vitro approach lies in the observation that if, as this study suggests, the inductive signal mediated by 2-O sulfated HS can be facilitated by wild-type HS provided by either the UB or MM, then it is unlikely that UB or MM specific knockout of Hs2st would actually result in a kidney phenotype and thus would not help to clarify the mechanism of developmental failure; it was only through the separate modeling of the stages of kidney development in vitro and titration of the activity of factors that are involved in UB and MM morphogenesis in these stages that we were able to identify the defect.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Catherine Merry for the generous gift of the Hs2st null mice and Shamara Closson for assistance with breeding and animal care. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants RO1-DK57286, RO1-DK65831, HL035018 (to S.K.N). M.M. Shah is also supported by a Research Career Award from NIDDK (K08-DK069324). H. Sakurai is also supported by an American Heart Association Scientist Development Award. J.D. Esko is supported by NIH grant GM33063.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baeg GH, Selva EM, Goodman RM, Dasgupta R, Perrimon N. The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev Biol. 2004;276:89–100. doi: 10.1016/j.ydbio.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell. 2008;15:781–91. doi: 10.1016/j.devcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock SL, Fletcher JM, Beddington RS, Wilson VA. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 1998;12:1894–906. doi: 10.1101/gad.12.12.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, Schwesinger C, Qiao J, Nigam SK. TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266:285–98. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–92. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Davies J, Lyon M, Gallagher J, Garrod D. Sulphated proteoglycan is required for collecting duct growth and branching but not nephron formation during kidney development. Development. 1995;121:1507–17. doi: 10.1242/dev.121.5.1507. [DOI] [PubMed] [Google Scholar]
- Davies JA, Yates EA, Turnbull JE. Structural determinants of heparan sulphate modulation of GDNF signalling. Growth Factors. 2003;21:109–19. doi: 10.1080/08977190310001621005. [DOI] [PubMed] [Google Scholar]
- Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001;108:169–73. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Xiao L, Cheng L, Wang X, Sun B, Hu G. Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett. 2000;467:7–11. doi: 10.1016/s0014-5793(00)01111-x. [DOI] [PubMed] [Google Scholar]
- Friedl A, Filla M, Rapraeger AC. Tissue-specific binding by FGF and FGF receptors to endogenous heparan sulfates. Methods Mol Biol. 2001;171:535–46. doi: 10.1385/1-59259-209-0:535. [DOI] [PubMed] [Google Scholar]
- Fujise M, Takeo S, Kamimura K, Matsuo T, Aigaki T, Izumi S, Nakato H. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development. 2003;130:1515–22. doi: 10.1242/dev.00379. [DOI] [PubMed] [Google Scholar]
- Garner OB, Yamaguchi Y, Esko JD, Videm V. Small changes in lymphocyte development and activation in mice through tissue-specific alteration of heparan sulphate. Immunology. 2008;125:420–9. doi: 10.1111/j.1365-2567.2008.02856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe K, Inatani M, Pallerla SR, Castagnola J, Yamaguchi Y, Esko JD. Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development. 2005;132:3777–86. doi: 10.1242/dev.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibe S, Karihaloo A, Ma H, Zhang J, Marlier A, Mitobe M, Togawa A, Schmitt R, Czyczk J, Kashgarian M, Geller DS, Thorgeirsson SS, Cantley LG. Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development. 2009;136:337–45. doi: 10.1242/dev.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao K, Inatani M, Matsumoto Y, Ogata-Iwao M, Takihara Y, Irie F, Yamaguchi Y, Okinami S, Tanihara H. Heparan sulfate deficiency leads to Peters anomaly in mice by disturbing neural crest TGF-beta2 signaling. J Clin Invest. 2009;119:1997–2008. doi: 10.1172/JCI38519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Kreuger J, Holmborn K, Lundin L, Eriksson I, Kjellen L, Claesson-Welsh L. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev Cell. 2006;10:625–34. doi: 10.1016/j.devcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Rhodes JM, Ueda R, McNeely M, Shukla D, Kimata K, Spear PG, Shworak NW, Nakato H. Regulation of Notch signaling by Drosophila heparan sulfate 3-O sulfotransferase. J Cell Biol. 2004;166:1069–79. doi: 10.1083/jcb.200403077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KL, Yost HJ. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell. 2002;2:115–24. doi: 10.1016/s1534-5807(01)00107-1. [DOI] [PubMed] [Google Scholar]
- Lelongt B, Makino H, Dalecki TM, Kanwar YS. Role of proteoglycans in renal development. Dev Biol. 1988;128:256–76. doi: 10.1016/0012-1606(88)90289-8. [DOI] [PubMed] [Google Scholar]
- Li JP, Gong F, Hagner-McWhirter A, Forsberg E, Abrink M, Kisilevsky R, Zhang X, Lindahl U. Targeted disruption of a murine glucuronyl C5-epimerase gene results in heparan sulfate lacking L-iduronic acid and in neonatal lethality. J Biol Chem. 2003;278:28363–6. doi: 10.1074/jbc.C300219200. [DOI] [PubMed] [Google Scholar]
- Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–21. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- Lin X, Wei G, Shi Z, Dryer L, Esko JD, Wells DE, Matzuk MM. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol. 2000;224:299–311. doi: 10.1006/dbio.2000.9798. [DOI] [PubMed] [Google Scholar]
- Lyon M, Rushton G, Gallagher JT. The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific. J Biol Chem. 1997;272:18000–6. doi: 10.1074/jbc.272.29.18000. [DOI] [PubMed] [Google Scholar]
- Maeshima A, Sakurai H, Choi Y, Kitamura S, Vaughn DA, Tee JB, Nigam SK. Glial Cell Derived Neurotrophic Factor Independent Ureteric Bud Outgrowth from the Wolffian Duct. J Am Soc Nephrol. 2007 doi: 10.1681/ASN.2007060642. [DOI] [PubMed] [Google Scholar]
- Maeshima A, Vaughn DA, Choi Y, Nigam SK. Activin A is an endogenous inhibitor of ureteric bud outgrowth from the Wolffian duct. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–85. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, Patel VN, Lang RA, Mohammadi M. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci Signal. 2009;2:ra55. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TN, Schwesinger C, Bush KT, Stuart RO, Rose DW, Shah MM, Vaughn DA, Steer DL, Nigam SK. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev Biol. 2004;275:44–67. doi: 10.1016/j.ydbio.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Monte JC, Sakurai H, Bush KT, Nigam SK. The developmental nephrome: systems biology in the developing kidney. Curr Opin Nephrol Hypertens. 2007;16:3–9. doi: 10.1097/MNH.0b013e3280118a5a. [DOI] [PubMed] [Google Scholar]
- Nigam SK, Shah MM. How does the ureteric bud branch? J Am Soc Nephrol. 2009;20:1465–9. doi: 10.1681/ASN.2008020132. [DOI] [PubMed] [Google Scholar]
- Ornitz DM. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays. 2000;22:108–12. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–6. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Platt JL, Trescony P, Lindman B, Oegema TR. Heparin and heparan sulfate delimit nephron formation in fetal metanephric kidneys. Dev Biol. 1990;139:338–48. doi: 10.1016/0012-1606(90)90303-z. [DOI] [PubMed] [Google Scholar]
- Qiao J, Bush KT, Steer DL, Stuart RO, Sakurai H, Wachsman W, Nigam SK. Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech Dev. 2001;109:123–35. doi: 10.1016/s0925-4773(01)00592-5. [DOI] [PubMed] [Google Scholar]
- Qiao J, Sakurai H, Nigam SK. Branching morphogenesis independent of mesenchymalepithelial contact in the developing kidney. Proc Natl Acad Sci U S A. 1999;96:7330–5. doi: 10.1073/pnas.96.13.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich-Slotky R, Bonneh-Barkay D, Shaoul E, Bluma B, Svahn CM, Ron D. Differential effect of cell-associated heparan sulfates on the binding of keratinocyte growth factor (KGF) and acidic fibroblast growth factor to the KGF receptor. J Biol Chem. 1994;269:32279–85. [PubMed] [Google Scholar]
- Rickard SM, Mummery RS, Mulloy B, Rider CC. The binding of human glial cell line-derived neurotrophic factor (GDNF) to heparin and heparan sulphate: importance of 2-O-sulphate groups and effect on its interaction with its receptor GFR{alpha}1. Glycobiology. 2003;6:6. doi: 10.1093/glycob/cwg046. [DOI] [PubMed] [Google Scholar]
- Rosines E, Sampogna RV, Johkura K, Vaughn DA, Choi Y, Sakurai H, Shah MM, Nigam SK. Staged in vitro reconstitution and implantation of engineered rat kidney tissue. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0710428105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arumae U, Meng X, Lindahl M, Pachnis V, Sariola H. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development. 1997;124:4077–87. doi: 10.1242/dev.124.20.4077. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Bush KT, Nigam SK. Identification of pleiotrophin as a mesenchymal factor involved in ureteric bud branching morphogenesis. Development. 2001;128:3283–93. doi: 10.1242/dev.128.17.3283. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Bush KT, Nigam SK. Heregulin induces glial cell line derived neurotrophic growth factor-independent, non-branching proliferation and differentiation of ureteric bud epithelia. J Biol Chem. 2005 doi: 10.1074/jbc.M507962200. [DOI] [PubMed] [Google Scholar]
- Sampogna RV, Nigam SK. Implications of gene networks for understanding resilience and vulnerability in the kidney branching program. Physiology (Bethesda) 2004;19:339–47. doi: 10.1152/physiol.00025.2004. [DOI] [PubMed] [Google Scholar]
- Sampogna RV, Nigam SK. Implications of gene networks for understanding resilience and vulnerability in the kidney branching program. Physiology (Bethesda) 2004;19:339–47. doi: 10.1152/physiol.00025.2004. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Lan D, Hirsh BJ, Barasch J. Dissecting stages of mesenchymal-to-epithelial conversion during kidney development. Nephron Physiol. 2006;104:56–60. doi: 10.1159/000093287. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–3. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Selleck SB. Shedding light on the distinct functions of proteoglycans. Sci STKE. 2006;2006:pe17. doi: 10.1126/stke.3292006pe17. [DOI] [PubMed] [Google Scholar]
- Shah MM, Sampogna RV, Sakurai H, Bush KT, Nigam SK. Branching morphogenesis and kidney disease. Development. 2004;131:1449–62. doi: 10.1242/dev.01089. [DOI] [PubMed] [Google Scholar]
- Shah MM, Tee JB, Meyer TN, Meyer-Schwesinger C, Choi Y, Sweeney DE, Gallegos TF, Johkura K, Rosines E, Kouznetsova V, Rose DW, Bush KT, Sakurai H, Nigam SK. The Instructive Role Of Metanephric Mesenchyme On Ureteric Bud Patterning, Sculpting And Maturation. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.00125.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell. 2005;8:65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Shworak NW, HajMohammadi S, de Agostini AI, Rosenberg RD, Enjyoji K, Princivalle M, Christi P, Lech M, Beeler D, Rayburn H, Schwartz JJ, Barzegar S, Post MJ. Mice deficient in heparan sulfate 3-O-sulfotransferase-1: normal hemostasis with unexpected perinatal phenotypes: Normal levels of anticoagulant heparan sulfate are not essential for normal hemostasis. Glycoconj J. 2002;19:355–61. doi: 10.1023/A:1025377206600. [DOI] [PubMed] [Google Scholar]
- Steer DL, Bush KT, Meyer TN, Schwesinger C, Nigam SK. A strategy for in vitro propagation of rat nephrons. Kidney Int. 2002;62:1958–65. doi: 10.1046/j.1523-1755.2002.00694.x. [DOI] [PubMed] [Google Scholar]
- Steer DL, Shah MM, Bush KT, Stuart RO, Sampogna RV, Meyer TN, Schwesinger C, Bai X, Esko JD, Nigam SK. Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney. Dev Biol. 2004;272:310–27. doi: 10.1016/j.ydbio.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Stenzel D, Nye E, Nisancioglu M, Adams RH, Yamaguchi Y, Gerhardt H. Peripheral mural cell recruitment requires cell-autonomous heparan sulfate. Blood. 2009;114:915–24. doi: 10.1182/blood-2008-10-186239. [DOI] [PubMed] [Google Scholar]
- Stickens D, Zak BM, Rougier N, Esko JD, Werb Z. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development. 2005 doi: 10.1242/dev.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart RO, Bush KT, Nigam SK. Changes in global gene expression patterns during development and maturation of the rat kidney. Proc Natl Acad Sci U S A. 2001;98:5649–54. doi: 10.1073/pnas.091110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart RO, Bush KT, Nigam SK. Changes in gene expression patterns in the ureteric bud and metanephric mesenchyme in models of kidney development. Kidney Int. 2003;64:1997–2008. doi: 10.1046/j.1523-1755.2003.00383.x. [DOI] [PubMed] [Google Scholar]
- Tsigelny IF, Kouznetsova VL, Sweeney DE, Wu W, Bush KT, Nigam SK. Analysis of metagene portraits reveals distinct transitions during kidney organogenesis. Sci Signal. 2008;1:ra16. doi: 10.1126/scisignal.1163630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VA, Gallagher JT, Merry CL. Heparan sulfate 2-O-sulfotransferase (Hs2st) and mouse development. Glycoconj J. 2002;19:347–54. doi: 10.1023/A:1025325222530. [DOI] [PubMed] [Google Scholar]