More than a century ago, Armand Trousseau first described an association between cancer and the coagulation system [1,2]. Later it was discovered that tumor cells release procoagulant microvesicles (often referred to as microparticles) into the culture medium that may be responsible for activation of the coagulation system [3]. The procoagulant protein tissue factor (TF) is expressed by a variety of tumors. Importantly, levels of TF expression increase with advanced cancer stage and high levels are associated with an increased mortality [4–7]. In glioblastoma cells, TF expression is induced by hypoxia and activation of the epidermal growth factor receptor [8,9]. One reason for the increased mortality may be that cancer patients have a high rate of venous thromboembolism. For instance, 11.1% of brain cancer patients have a thrombotic event within 1 year of diagnosis [10]. Indeed, tumor cells release TF-positive microparticles into the blood in mouse models and in cancer patients, and these microparticles may be responsible for triggering venous thrombosis [11–14]. Activation of coagulation by tumor cell TF also enhances pulmonary metastasis in a fibrin-dependent manner [15,16]. Finally, tumor cell TF enhances tumor growth and angiogenesis [6,17]. An earlier study found that overexpression of TF in Meth-A sarcoma cells increased tumor growth and angiogenesis in mice [18]. More recently, Rak and colleagues [19] showed that a selective decrease in TF expression reduced the growth of human colorectal cancer cells and angiogenesis in severe combined immunodeficiency mice.
Ixolaris is a tick salivary protein that has two Kunitz-like domains that are similar to the Kunitz domains found in tissue factor pathway inhibitor. In this issue of the Journal of Thrombosis and Haemostasis, Carneiro-Lobo et al. [20] demonstrate that inhibition of the TF–factor (F)VIIa complex with Ixolaris decreases the growth of human glioblastoma tumors (U87-MG) in nude mice without increasing bleeding [20]. Moreover, the inhibitor reduced vascular endothelial growth factor (VEGF) expression and angiogenesis. There are two limitations of the study. First, U87-MG cells were injected subcutaneously rather than intracranially. Orthotopic xenografts are more physiological models of tumorigenesis, and in the case of gliomas it is unlikely that systemic administration of Ixolaris would gain access to the brain. Second, Ixolaris inhibits both the TF–FVIIa complex and activation of FX by the intrinsic tenase complex. Therefore, it is unclear if the effects of Ixolaris are as a result of inhibition of the TF–FVIIa complex and/or a reduction in levels of the downstream coagulation proteases FXa and thrombin (Fig. 1).
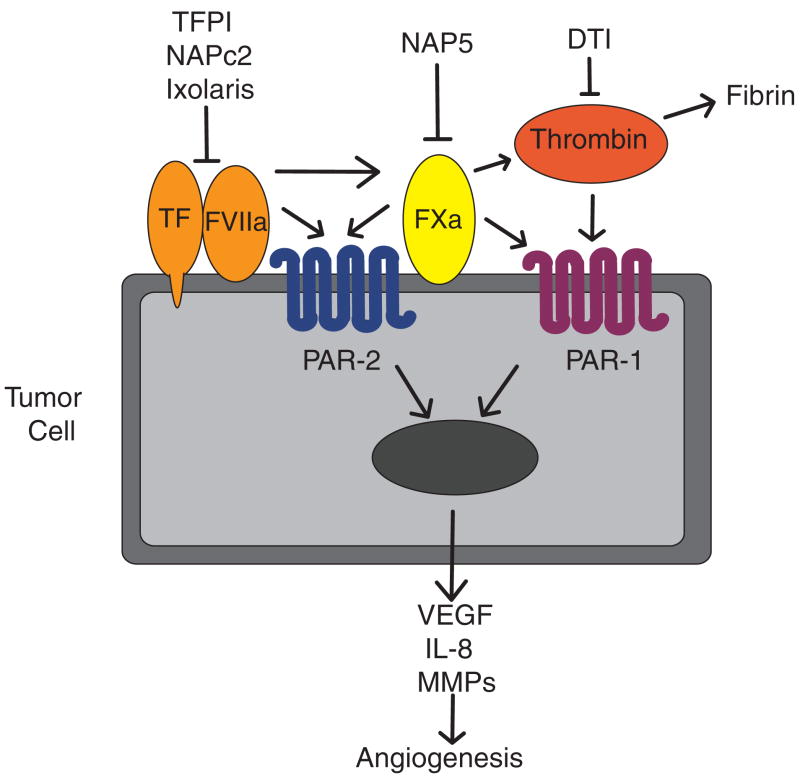
Fig. 1.
Contribution of tissue factor (TF), coagulation proteases and protease-activated receptors (PARs) to tumor angiogenesis. Formation of the TF–factor (F)VIIa complex on the surface of tumor cells activates the coagulation system. Cleavage of PAR-2 by FVIIa or FXa induces the expression of various pro-angiogenic proteins, including vascular endothelial growth factor (VEGF), interleukin-8 (IL-8) and matrix metalloproteinases (MMPs). Activation of PAR-1 by thrombin or FXa induces a similar set of genes. Various anticoagulants target different proteases of the coagulation cascade, such as direct thrombin inhibitors (DTIs).
Most of the in vitro studies on TF–FVIIa signaling have been performed using a human keratinocyte cell line and MDA-MB-231 human breast cancer cells [21,22]. In MDA-MB-231 cells, the TF–FVIIa complex activates protease-activated receptor-2 (PAR-2) and induces the expression of several pro-angiogenic mediators, such as VEGF, interleukin-8 (IL-8) and chemokine (C-X-C motif) ligand 1 (CXCL1) [22–24]. This led to the notion that TF expression by tumor cells enhances tumor growth in vivo by activation of PAR-2 (Fig. 1). As noted by Carneiro-Lobo et al. [20], MDA-MB-231 cells express very high levels of TF in comparison to U87-MG glioblastoma cells. We analyzed TF expression in an array database [25] and found that MDA-MB-231 cells express much higher levels of TF than 99 different primary breast tumor samples of varying stages and grades (T. McEachron, F. Church, N. Mackman, unpublished data). The results indicate that MDA-MB-231 cells may not be the best breast tumor model for studying TF-related signaling events.
The hypothesis that tumor cell TF enhances tumor growth in vivo has been tested in a variety of mouse models. One study showed that inhibition of the TF–FVIIa complex with NAPc2, a nematode anticoagulant protein, decreased tumor growth and angiogenesis of B16 melanoma cells and Lewis lung carcinoma cells [26]. NAPc2 also inhibited the growth of colorectal tumors in mice [27]. In contrast, specific inhibition of FXa using the nematode anticoagulant protein NAP5 did not reduce tumor growth [26]. In other studies, a humanized anti-TF antibody called CNTO859 inhibited growth of MDA-MB-231 tumors and human epithelial tumors in immunodeficient mice [9,28]. These studies demonstrate that inhibition of TF reduces tumor growth in a variety of mouse models.
Other studies have focused on the mechanism by which TF contributes to tumor growth. Importantly, a monoclonal antibody called 10H10, which inhibits TF–FVIIa signaling without affecting its procoagulant activity, reduced tumor growth and angiogenesis of both MDA-MB-231 and melanoma (m24Met cells) [29]. Moreover, inhibition of PAR-2 but not PAR-1 decreased the growth of the MDA-MB-231 xenografts [29]. Finally, in a genetically engineered mouse model of adenocarcinoma, tumors developed more slowly in PAR-2−/− mice compared with tumors in either wild-type mice or PAR-1−/− mice [30]. However, tumors isolated from PAR-2−/− mice exhibited the same growth rate in wild-type mice as those isolated from control mice [30]. Although these studies support a role of TF and PAR-2 in tumor growth in different mouse models, further studies are required to determine if the TF–FVIIa–PAR-2 signaling pathway is necessary for the growth of a wide variety of tumor types.
The paper by Carneiro-Lobo used a human glioblastoma tumor cell line [20]. Importantly, several studies have shown that thrombin plays a prominent role in the growth of gliomas by increasing VEGF expression in both human and rat glioma cell lines [31,32]. In addition, intracerebral infusion of argatroban, a specific thrombin inhibitor, reduced tumor growth in a C6 glioma model [33]. These results indicate that thrombin, possibly via PAR-1 signaling, plays a role in the growth of gliomas in vivo (Fig. 1).
It is somewhat surprising that there are few reports of bleeding in studies using anticoagulants to treat tumor-bearing mice. The one exception is that specific inhibition of FXa using the anticoagulant protein NAP5 resulted in high mortality rates as a result of intraperitoneal hemhorrage [26]. One possibility is that tumor-bearing mice are hyper-coagulable because of the presence of TF-positive microparticles in the blood [11,12,19] (J.-G. Wang, T. McEachron, N. Mackman, unpublished data). These procoagulant microparticles may prevent hemorrhage in anticoagulated mice and explain the low incidence of bleeding in these studies.
Inhibiting the TF–FVIIa complex is a potential therapeutic approach to treat multiple solid tumor types. Additional benefits of this approach would be a reduction in metastasis and thrombosis. However, the greatest challenge to targeting TF is to find an efficacious dose of inhibitor that does not cause bleeding in cancer patients.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (HL095096 to N. Mackman) and (F31CA142162 to T. McEachron).
Footnotes
Disclosure of Conflict of Interests
N. Mackman is on the scientific advisory board of Thrombo-targets and is a consultant for Bayer Schering and Daiichi-Sankyo.
References
- 1.Rak J, Yu JL, Luyendyk J, Mackman N. Oncogenes, trousseau syndrome, and cancer-related changes in the coagulome of mice and humans. Cancer Res. 2006;66:10643–6. doi: 10.1158/0008-5472.CAN-06-2350. [DOI] [PubMed] [Google Scholar]
- 2.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–9. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dvorak HF, Van DeWater L, Bitzer AM, Dvorak AM, Anderson D, Harvey VS, Bach R, Davis GL, DeWolf W, Carvalho AC. Procoagulant activity associated with plasma membrane vesicles shed by cultured tumor cells. Cancer Res. 1983;43:4434–42. [PubMed] [Google Scholar]
- 4.Rak J, Milsom C, Magnus N, Yu J. Tissue factor in tumour progression. Best Pract Res Clin Haematol. 2009;22:71–83. doi: 10.1016/j.beha.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RC. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg. 1995;82:1101–4. doi: 10.1002/bjs.1800820831. [DOI] [PubMed] [Google Scholar]
- 6.Schaffner F, Ruf W. Tissue factor and protease-activated receptor signaling in cancer. Semin Thromb Hemost. 2008;34:147–53. doi: 10.1055/s-2008-1079254. [DOI] [PubMed] [Google Scholar]
- 7.Kasthuri R, Taubman M, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27:4834–8. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rong Y, Belozerov VE, Tucker-Burden C, Chen G, Durden DL, Olson JJ, Van Meir EG, Mackman N, Brat DJ. Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein-1 transcriptional activity. Cancer Res. 2009;69:2540–9. doi: 10.1158/0008-5472.CAN-08-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milsom CC, Yu JL, Mackman N, Micallef J, Anderson GM, Guha A, Rak JW. Tissue factor regulation by epidermal growth factor receptor and epithelial-to-mesenchymal transitions: effect on tumor initiation and angiogenesis. Cancer Res. 2008;68:10068–76. doi: 10.1158/0008-5472.CAN-08-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White RH, Chew H, Wun T. Targeting patients for anticoagulant prophylaxis trials in patients with cancer: who is at highest risk? Thromb Res. 2007;120 (Suppl 2):S29–40. doi: 10.1016/S0049-3848(07)70128-7. [DOI] [PubMed] [Google Scholar]
- 11.Yu JL, Rak JW. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J Thromb Haemost. 2004;2:2065–7. doi: 10.1111/j.1538-7836.2004.00972.x. [DOI] [PubMed] [Google Scholar]
- 12.Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost. 2008;6:1517–24. doi: 10.1111/j.1538-7836.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- 13.Tesselaar ME, Romijn FP, van der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 14.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–5. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis JL, Amirkhosravi A. Effect of antihemostatic agents on experimental tumor dissemination. Semin Thromb Hemost. 2002;28:29–38. doi: 10.1055/s-2002-20562. [DOI] [PubMed] [Google Scholar]
- 16.Mueller BM, Ruf W. Requirement for binding of catalytically active factor VIIa in tissue factor-dependent experimental metastasis. J Clin Invest. 1998;101:1372–8. doi: 10.1172/JCI930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rickles FR, Shoji M, Abe K. The role of the hemostatic system in tumor growth, metastasis, and angiogenesis: tissue factor is a bifunctional molecule capable of inducing both fibrin deposition and angiogenesis in cancer. Int J Hematol. 2001;73:145–50. doi: 10.1007/BF02981930. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Deng Y, Luther T, Muller M, Ziegler R, Waldherr R, Stern DM, Nawroth PP. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest. 1994;94:1320–7. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–41. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 20.Carneiro-Lobo TC, Konig S, Machado DE, Nasciutti LE, Forni MF, Francischetti IM, Sogayar MC, Monteiro RQ. Ixolaris, a tissue factor inhibitor, blocks primary tumor growth and angiogenesis in a glioblastoma model. J Thromb Haemost. 2009;7:1855–64. doi: 10.1111/j.1538-7836.2009.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camerer E, Gjernes E, Wiiger M, Pringle S, Prydz H. Binding of factor VIIa to tissue factor on keratinocytes induces gene expression. J Biol Chem. 2000;275:6580–5. doi: 10.1074/jbc.275.9.6580. [DOI] [PubMed] [Google Scholar]
- 22.Albrektsen T, Sorensen BB, Hjorto GM, Fleckner J, Rao LV, Petersen LC. Transcriptional program induced by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J Thromb Haemost. 2007;5:1588–97. doi: 10.1111/j.1538-7836.2007.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hjortoe GM, Petersen LC, Albrektsen T, Sorensen BB, Norby PL, Mandal SK, Pendurthi UR, Rao LV. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood. 2004;103:3029–37. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006;66:307–14. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- 25.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes thatmediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hembrough TA, Swartz GM, Papathanassiu A, Vlasuk GP, Rote WE, Green SJ, Pribluda VS. Tissue factor/factor VIIa inhibitors block angiogenesis and tumor growth through a nonhemostatic mechanism. Cancer Res. 2003;63:2997–3000. [PubMed] [Google Scholar]
- 27.Zhao J, Aguilar G, Palencia S, Newton E, Abo A. rNAPc2 inhibits colorectal cancer in mice through tissue factor. Clin Cancer Res. 2009;15:208–16. doi: 10.1158/1078-0432.CCR-08-0407. [DOI] [PubMed] [Google Scholar]
- 28.Ngo CV, Picha K, McCabe F, Millar H, Tawadros R, Tam SH, Nakada MT, Anderson GM. CNTO 859, a humanized anti-tissue factor monoclonal antibody, is a potent inhibitor of breast cancer metastasis and tumor growth in xenograft models. Int J Cancer. 2007;120:1261–7. doi: 10.1002/ijc.22426. [DOI] [PubMed] [Google Scholar]
- 29.Versteeg HH, Schaffner F, Kerver M, Petersen HH, Ahamed J, Felding-Habermann B, Takada Y, Mueller BM, Ruf W. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111:190–9. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Versteeg HH, Schaffner F, Kerver M, Ellies LG, Andrade-Gordon P, Mueller BM, Ruf W. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer Res. 2008;68:7219–27. doi: 10.1158/0008-5472.CAN-08-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamahata H, Takeshima H, Kuratsu J, Sarker KP, Tanioka K, Wakimaru N, Nakata M, Kitajima I, Maruyama I. The role of thrombin in the neo-vascularization of malignant gliomas: an intrinsic modulator for the up-regulation of vascular endothelial growth factor. Int J Oncol. 2002;20:921–8. [PubMed] [Google Scholar]
- 32.Xu Y, Gu Y, Keep RF, Heth J, Muraszko KM, Xi G, Hua Y. Thrombin up-regulates vascular endothelial growth factor in experimental gliomas. Neurol Res. 2009;31:759–65. doi: 10.1179/174313209X385699. [DOI] [PubMed] [Google Scholar]
- 33.Hua Y, Tang LL, Fewel ME, Keep RF, Schallert T, Muraszko KM, Ho JT, Xi GH. Systemic use of argatroban reduces tumor mass, attenuates neurological deficits and prolongs survival time in rat glioma models. Acta Neurochir Suppl. 2005;95:403–6. doi: 10.1007/3-211-32318-x_82. [DOI] [PubMed] [Google Scholar]