Abstract
ErbB oncogenes drive the progression of several human cancers. Our study shows that in human carcinoma (A431) and glioma (U373) cells, the oncogenic forms of epidermal growth factor receptor (EGFR; including EGFRvIII) trigger the up-regulation of tissue factor (TF), the transmembrane protein responsible for initiating blood coagulation and signaling through interaction with coagulation factor VIIa. We show that A431 cancer cells in culture exhibit a uniform TF expression profile; however, these same cells in vivo exhibit a heterogeneous TF expression and show signs of E-cadherin inactivation, which is coupled with multilineage (epithelial and mesenchymal) differentiation. Blockade of E-cadherin in vitro, leads to the acquisition of spindle morphology and de novo expression of vimentin, features consistent with epithelial-to-mesenchymal transition. These changes were associated with an increase in EGFR-dependent TF expression, and with enhanced stimulation of vascular endothelial growth factor production, particularly following cancer cell treatment with coagulation factor VIIa. In vivo, cells undergoing epithelial-to-mesenchymal transition exhibited an increased metastatic potential. Furthermore, injections of the TF-blocking antibody (CNTO 859) delayed the initiation of A431 tumors in immunodeficient mice, and reduced tumor growth, vascularization, and vascular endothelial growth factor expression. Collectively, our data suggest that TF is regulated by both oncogenic and differentiation pathways, and that it functions in tumor initiation, tumor growth, angiogenesis, and metastasis. Thus, TF could serve as a therapeutic target in EGFR-dependent malignancies.
Introduction
The epidermal growth factor receptor (EGFR) family plays a key role in normal development and oncogenesis (1). Thus, activation, overexpression, amplification, or mutations of various members of this family (especially EGFR/ErbB1/HER1, and ErbB2/HER-2/neu) are associated with several types of human malignancies (1) including breast, head and neck, non–small cell lung, prostate cancer (2), and malignant glioma (3). In the latter case, both EGFR gene amplification and expression of the activated mutant known as EGFRvIII are responsible for constitutive activation and alteration of EGFR signaling (3). EGFR and ErbB2 oncogenes contribute to tumor progression by altering the intrinsic (e.g., mitogenic, invasive) and angiogenic properties of cancer cells, such as up-regulating vascular endothelial growth factor (VEGF) expression (4–6). The role of the resulting vascular changes is highlighted by perpetual angiogenic remodeling of tumor blood vessels and vascular-dependent tumor growth—now an attractive therapeutic target (7, 8), perivascular nesting of cancer stem cells (9), activation of the coagulation system (cancer coagulopathy; ref. 10), and metastasis. The latter process is often associated with both procoagulant events and proinvasive cellular changes described as epithelial-to-mesenchymal transitions (EMT; ref. 11). This term refers to the morphologic and functional changes of epithelial cancer cells whereby they transiently acquire markers of mesenchymal differentiation (e.g., vimentin), lose some of their epithelial features (e.g., E-cadherin), and assume a spindle shape and highly motile/invasive characteristics (11). EMT is believed to be controlled by interactions between oncogenic and growth factor pathways [e.g., ras and transforming growth factor-β (TGF-β)/SMAD4, WNT, Hedgehog, Notch, Snail, and Twist; ref. 12] and contributes to the more aggressive and metastatic behavior of several cancer cell types (11, 13), and to the cancer cell “stemness” (14).
One poorly understood class of events that occurs at the tumor-vascular boundary is the up-regulation of tissue factor (TF) by both cancer cells and the vascular endothelium (15, 16). TF is a transmembrane protein that interacts with coagulation factor VIIa (FVIIa), whereby it initiates blood coagulation (17). This interaction also triggers intracellular signals, which are primarily mediated by G protein–coupled protease-activated receptors (PAR; ref. 18) in concert with adhesion molecules and several other factors (19). Interestingly, TF expression by cancer cells has been linked to several aspects of tumor progression, including coagulopathy (10), angiogenesis (10), invasion (20), and metastasis (20–22). In addition, TF up-regulation by numerous types of cancer cells has been directly related to loss of tumor suppressor genes (e.g., p53 or PTEN; refs. 6, 23) and activation of oncogenes (e.g., mutant K-ras; ref. 6).
Here, we show that EGFR and EGFRvIII oncogenes drive a robust, constitutive and uniform up-regulation of TF in tumor cells derived from human squamous (epithelial) cell carcinoma and malignant glioma, respectively. We present evidence that the chronic effect of oncogenic EGFR on epithelial cancer cells is modulated by their multilineage differentiation in vivo, whereby they undergo loss of E-cadherin function and acquire a heterogeneous expression of vimentin and TF. Furthermore, blockade of E-cadherin in cultured cancer cells similarly leads to the expression of vimentin and changes in cell shape reminiscent of EMT. This transition resulted in increased TF expression and gave rise to cells with a highly metastatic phenotype. Such cells were also hypersensitive to FVIIa stimulation (TF-dependent signaling), as measured by an increased VEGF production. Finally, inhibition of TF in vivo led to reduced VEGF production, lower vascular density and delayed growth of tumors in immunodeficient mice. Thus, we propose that oncogene (EGFR)–driven expression of TF is modulated by EMT-like changes and that these events participate in tumor initiation, growth, angiogenesis, and metastasis. Hence, targeting TF and/or TF-stimulating influences may have therapeutic value at least in certain tumor contexts.
Materials and Methods
Cell culture and treatments
Human malignant glioma cell lines U373 and U373vIII were maintained in DMEM (HyClone) supplemented with 10% tetracycline-free fetal bovine serum (Invitrogen), 200 μg/mL of hygromycin (Roche), and 50 μg/mL of geneticin (Life Technologies/Invitrogen). A431 human squamous cell carcinoma line (American Type Culture Collection) was cultured in DMEM supplemented with 5% fetal bovine serum. Cells were serum-starved overnight prior to treatments with small molecule inhibitors (20 μmol/L AG1478, 10 μmol/L CAPE, 50 μmol/L Y27632; Biomol Research Laboratories), TGFα (50 ng/mL; R&D Systems), SHE78-7 (2 μg/mL; Zymed Laboratories, Inc.), and recombinant FVIIa (10 mmol/L; Enzyme Research Laboratories; ref. 6).
TF promoter assays
Cells were transiently transfected with hTF-pGL2 construct containing 1.2 kb of the human TF 5′-untranslated region (24), or pGL2 control vector using Lipofectamine 2000 (Invitrogen) along with the LacZ-pcDNA3.1 vector, as a control for transfection efficiency. Cells were treated for 24h posttransfection with 50 ng of TGFα or 20 μmol/L of AG1478, as indicated. Promoter activity was determined using the luciferase assay system (Promega) and TD20/20 luminometer (Turner Designs; ref. 6). Values were normalized to β-galactosidase activity.
Flow cytometric detection of cell surface TF
Tumor cells were dissociated with an enzyme solution (540 units/mL collagenase III, 600 units/mL hyaluronidase, 2 mg/mL collagenase IV, and 2 mg/mL collagenase I). Cells were stained with sheep antibody against human TF (Affinity Biologicals) and Alexa Fluor 488 donkey anti-sheep secondary antibody (Molecular Probes) and analyzed (FACScalibur; BD Biosciences; ref. 6). In each experiment, several individual tumor preparations were compared with the corresponding cultured cells.
Western blotting and ELISA
Western blotting was performed as described previously (6). Membranes were probed with the following primary antibodies: rabbit anti-human TF IgG (American Diagnostica), rabbit anti-EGFR IgG (Cell Signaling), monoclonal KL1 pan cytokeratin (Novus Biological, Inc.), mouse anti-human E-cadherin (Zymed), monoclonal antibody V9 vimentin (Abcam), and monoclonal β-actin for loading control (Sigma-Aldrich). Horseradish peroxidase–conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Upstate) were used prior to enhanced chemiluminescence detection (Amersham Biosciences). The IMUBIND TF ELISA kit (American Diagnostica) and human VEGF Quantikine ELISA kit (R&D) were used to quantify secreted TF and VEGF levels (respectively) in conditioned medium (6).
Immunostaining
For human nuclei staining, tissue samples were sequentially cryoprotected in 15% and 30% sucrose and frozen in Tissue Tek Cryo-Oct Compound (Sakura Finetek). Cryosections (10 Am in thickness) were fixed in 4% paraformaldehyde and stained overnight, 4°C with mouse anti-human nuclei monoclonal antibody (Chemicon International), followed by Alexa Fluor 488 goat anti-mouse (Molecular Probes). All other tissue staining was performed on 4-μm paraffin sections which were incubated overnight at 4°C with the respective primary antibodies following heat antigen retrieval; except for cytokeratin (Novus Biological, Inc.) staining which required proteolytic digestion prior to a 60 min incubation with the prediluted antibody at room temperature. The following antibodies were used: sheep anti-human TF antibody (Affinity Biologicals), green fluorescent protein (GFP) monoclonal antibody (BD Biosciences), goat anti-mouse endoglin/CD105 antibody (R&D Systems), human vimentin (Abcam), and VEGF (Santa Cruz Biotechnology, Inc.), followed by their respective secondary Alexa Fluor antibodies (Molecular Probes). Blood vessels were counted from at least 20 fields/tumor at 200× magnification and VEGF staining was quantified using SigmaScan Pro 5 software from approximately 10 fields/tumor at 400× magnification (Axioskop 2, Zeiss). Cultured cells were grown on coverslips, fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X and stained with sheep anti-human TF or vimentin. Nuclei were stained for 5 min at room temperature with 4′,6-diamidino-2-phenylindole (DAPI; Sigma Aldrich).
TF activity assay
TF procoagulant (factor Xa generation) assay was performed as described previously (6). Cells were treated for 48 h with SHE78-7 (2 μg/mL) followed by a short incubation (1 h) with 10 mmol/L of hydrogen peroxide prior to assay. TF activity was normalized to total protein.
Tumor analysis
A431 cells were injected s.c. (1 × 105, 2 × 106, or 16 × 106 in different experiments), in 0.2 mL of PBS into yellow fluorescent protein (YFP)/severe combined immunodeficiency (SCID) mice (5). Tumor volume was calculated as described previously (6). CNTO 859 antibody (Centocor, Inc.), which reacts with human, but not with mouse TF, was given via i.p. injections at 200 μg/mouse. Mice inoculated with 1 × 105 A431 cells on day 0 were treated for 7 consecutive days (1–7 days postinjection) with CNTO 859. Alternatively, 16 × 106 A431 cells were mixed with 500 μg of CNTO 859 prior to injection followed with weekly CNTO 859 injections (200 μg). Animal studies were conducted according to protocols approved by the Institutional Animal Care Committees at McMaster and McGill Universities and in accordance with the guidelines of the Canadian Council of Animal Care.
Experimental metastasis
A single cell suspension (2 × 105 in 0.2 mL PBS) of A431 cells or SHE78-7–treated (2 μg/mL, 48 h) A431 cells were injected into the warmed lateral tail veins of YFP/SCID mice. Mice were sacrificed 6 weeks after injection, lungs were excised, and nodules were counted.
Data analysis
All experiments were conducted at least twice with similar results, although in most instances, the data represent a much larger number of independent repeats. All data points were generated from at least triplicate measurements in each experiment. The results of representative assays were expressed as mean ± SD. Animal experiments included at least five mice per group. Whenever appropriate, a Student's t test was used and P < 0.05 was used as the threshold of statistical significance.
Results
Oncogenic EGFR up-regulates TF in human cancer cells
TF up-regulation has been directly related to several transforming events in cancer (6). In order to assess this relationship more fully, we analyzed U373 malignant glioma cells, its variant engineered to express EGFRvIII (U373vIII), and A431 squamous cell carcinoma cells, which endogenously express oncogenic EGFR. Both U373vIII and A431 cells depend on oncogenic EGFR for growth as tumors in SCID mice (4).7
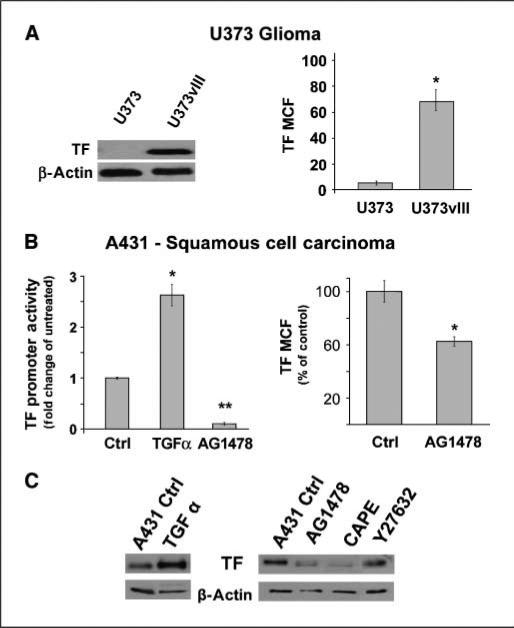
In our hands, U373 cells are indolent and express extremely low levels of TF. In contrast, their EGFRvIII-expressing isogenic counterparts (U373vIII) produce copious amounts of TF transcript and protein, both of which can be suppressed with pharmacologic inhibitors of EGFR (Fig. 1A; data not shown). A431 cells constitutively express TF protein, transcript, and promoter activity (Fig. 1B; data not shown) and can be further stimulated to increase TF expression by treatment with TGFα, an EGFR agonist (Fig. 1B and C). Conversely, TF levels are decreased in these cells upon incubation with AG1478, a small molecule EGFR inhibitor (Fig. 1B and C). The stimulating effect of EGFR on TF expression in A431 cells could be obliterated by treatment with CAPE, the small molecule inhibitor that blocks nuclear translocation of nuclear factor κB (NFκB), but not (minimally) by Y27632 an inhibitor of Rho kinase (Fig. 1C), or (somewhat surprisingly; ref. 17) by agents targeting MEK, ERK, or p38 (data not shown). Collectively, these results suggest that oncogenic/mutant EGFR consistently up-regulates TF in different types of cancer cells, with at least some contribution of NFκB-mediated signaling.
Figure 1.
Oncogenic EGFR up-regulates TF in human glioblastoma and squamous cell carcinoma cells. A, Western blot and flow cytometry analysis showing that expression of the constitutively activated EGFRvIII in indolent U373 cells leads to significant expression (*, P < 0.05) of total TF protein and cell surface levels (U373vIII). B, regulation of TF by EGFR in A431 SSC is shown by fold change in TF promoter activity following EGFR stimulation (TGFα, 50 ng, 24 h; *, P < 0.05) or inhibition (AG1478, 20 μmol/L, 24 h; **, P < 0.005) and by flow cytometry which reveals reduced levels of cell surface TF following EGFR inhibition (AG1478, 20 μmol/L, 48 h; *, P < 0.05), as compared with controls. C, Western blot showing that treatment with the EGFR agonist TGFα (50 ng, 48 h) increases TF expression, whereas pharmacologic inhibition of EGFR (AG1478, 20 μmol/L, 48 h) in A431 cells causes a reduction in total TF protein as does inhibition of NFκB nuclear translocation (CAPE, 10 μmol/L, 48 h). The effects of Rho kinase inhibitor (Y27632) in this assay are minimal. MCF, mean channel fluorescence.
Modulation of EGFR-dependent TF up-regulation by the tumor microenvironment
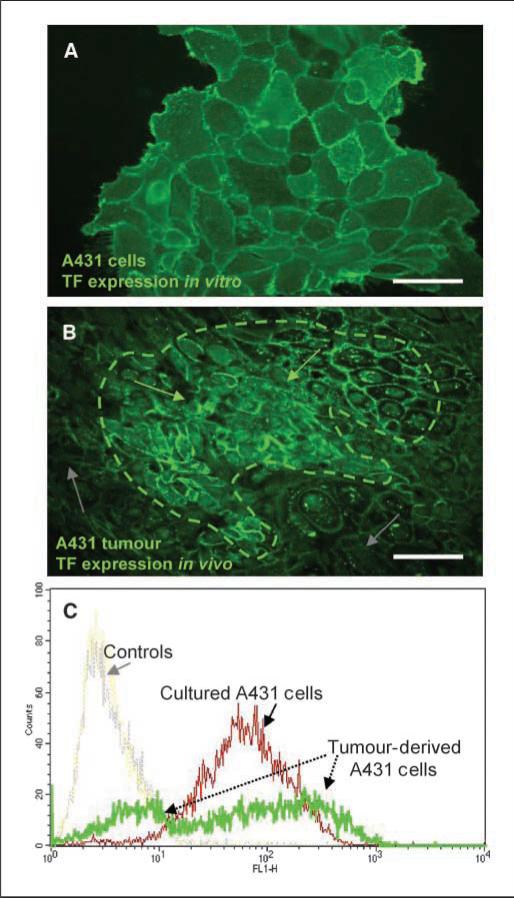
Although the constitutive activation of EGFR leads to a relatively uniform TF expression in cultured A431 cells, tumors composed of the same cells exhibit a considerable diversity in TF levels (Fig. 2A–C). Thus, A431 xenografts stain heterogeneously for human TF antigen, with areas of intense positivity interspersed between regions with minimal, or absent TF staining (Fig. 2B). Moreover, flow cytometry analysis of A431 cells recovered from entire individual tumors reveals a much broader diversity of TF levels (multiple peaks in high and low expression ranges), than is observed in cultured A431 cells (Fig. 2C). Although a degree of heterogeneity may exist within cultured A431 cell populations (25), these results suggest that host factors and/or the tumor microenvironment may modulate oncogene-driven TF expression within the tumor.
Figure 2.
Modulation of the EGFR-dependent TF up-regulation by the tumor microenvironment. A, TF immunofluorescent staining of A431 cells in culture (green). B, 2 × 106 A431 cells were injected into immunodeficient (SCID) mice and the resultant tumors were sectioned and stained for TF expression (green). Tumor sections show differential TF expression patterns, i.e., regions with high TF expression (dotted region, green arrows) and regions with low TF expression (gray arrows). C, tumor-derived cells and culture-maintained cells were stained for cell surface TF expression and analyzed by flow cytometry. A uniform distribution peak is observed in cultured cells indicative of their homogenous TF expression (as seen in A). In contrast, those cells derived from tumors show a heterogeneous mixture of both TF-positive and TF-negative populations (in B). Bars, 100 μM.
The in vivo diversification of TF levels is not unique to A431 cells, as we have observed similar changes in the case of glioma and colorectal carcinoma xenografts, even with cells of clonal origin (ref. 6; data not shown). However, it is also possible that the prominent negative peak in the A431 tumor profile could originate from host (mouse) cells that might be present in the tumor digests and fail to react with anti-human TF antibody. In order to address this, we routinely gated tumor-derived cells according to the forward and side scatter characteristics of cultured A431 cells, and performed additional staining of A431 tumors. For instance, A431 cells were injected s.c. into YFP/SCID mice harboring a constitutively expressed YFP transgene (5). The established tumors were stained for human nuclear antigen (human tumor cell marker) and/or YFP/GFP (host cell marker). As shown in Fig. 3A, nearly the entire A431 tumor mass is composed of cells positive for human nuclear antigen (A431) with only minimal infiltration (<10%) of host-derived, YFP-positive stroma. Therefore, we surmised that heterogeneous expression of TF in vivo is due to the modulation of the EGFR-TF pathway by the tumor microenvironment.
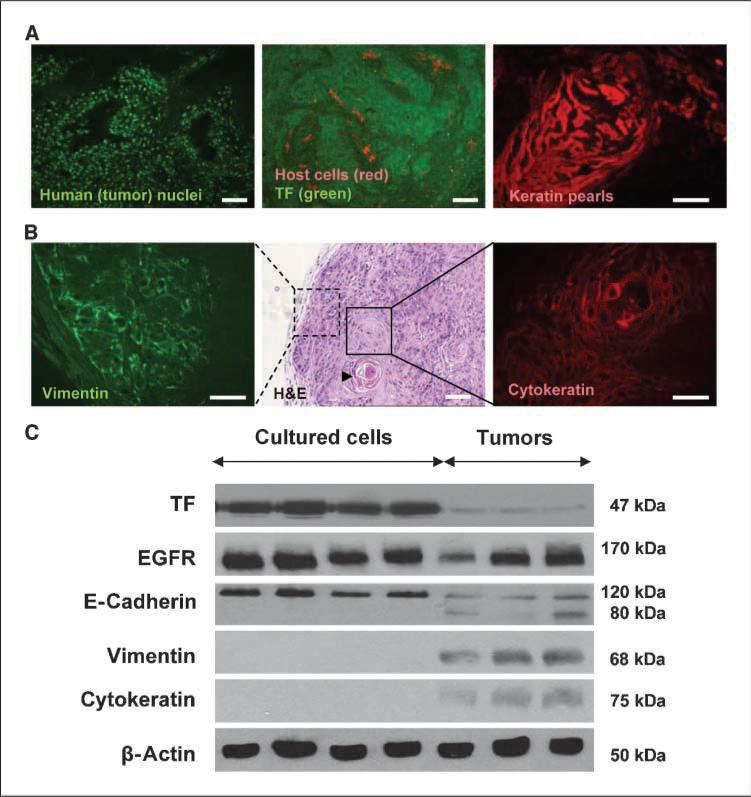
Figure 3.
Changes in TF expression associated with multilineage differentiation of cancer cells in vivo. A, the majority of cells within the tumor mass were human cancer cells, as determined by staining with an antibody directed against human (tumor) cell nuclear antigen (green). Tumors grown in YFP/SCID mice were double-stained using anti-TF (human cancer cells, green) and anti-GFP (host, red) antibodies. Infiltration of YFP-positive host cells in A431 tumors is minimal (<10%) and many stroma-poor tumor regions remain negative for TF. Immunofluorescence staining of cytokeratin (red) reveals highly keratinized pearl structures within these tumors. B, some tumor cells have lost their epithelial phenotype and express the mesenchymal marker vimentin (green), indicative of cells undergoing EMT. H&E staining depicts which regions of the tumor are likely to express vimentin and undergo EMT (tumor periphery) and those that retain their epithelial phenotype and differentiate along an epithelial pathway (central tumor). Arrowhead, a keratin pearl. Cytokeratin immunofluorescence (red) shows regions of the tumor that have undergone epithelial differentiation. C, comparative Western blot analysis between A431 cells maintained in culture and those grown as tumors. A global decrease in TF protein levels were observed in tumors. Changes in EGFR were unremarkable whereas the expression of E-cadherin is reduced and the extracellular region is cleaved, leaving an 80 kDa protein that is unable to make cell-cell contacts. Consistent with this loss of E-cadherin and epithelial phenotype, some tumor regions express vimentin, whereas other regions of these tumors remain epithelial and express high levels of cytokeratin. Bars, 100 μM.
Multilineage differentiation of cancer cells induced by host microenvironment
We observed that heterogeneous TF expression in A431 tumors was coupled with differential cancer cell morphology. For instance, in certain tumor regions these cells expressed features suggestive of advanced (even terminal) epithelial differentiation, such as cuboid shape, clear cytoplasm, expression of cytokeratin and even formation of keratin pearls (Fig. 3A and B). Elsewhere in the tumor cytokeratin was absent and cells exhibited spindle morphology and mesenchymal markers, such as human vimentin (Fig. 3A and B). Western blotting analysis confirmed the simultaneous expression of antithetical human differentiation markers, such as cytokeratin and vimentin in tumors originating from A431 cells in which neither of these markers is expressed in vitro (Fig. 3C). Although A431 tumors are aggressive and ultimately lethal, these findings may suggest that upon contact with the host microenvironment the tumor initiating (stem) A431 cells (25) undergo a multilineage differentiation, which is coupled with the heterogeneous expression of TF.
It is noteworthy that the overall levels of TF in A431 tumors were much lower than those in cultured A431 cells, in spite of the unremarkable changes in expression of the EGFR oncogene (Fig. 3C). Interestingly, cultured A431 cells express ample amounts of E-cadherin, a marker of epithelial differentiation, whereas in tumors, there is a considerable down-regulation of E-cadherin expression. Moreover, in addition to the 120 kDa band typical of intact and functional E-cadherin, tumor lysates also contain a large proportion of an 80 kDa species usually attributed to proteolytic cleavage (26) and loss of adhesive function of this molecule. This is particularly interesting as E-cadherin is a negative regulator of EGFR signaling (27), as well as tumor invasion and metastasis. Furthermore, E-cadherin is often down-regulated or inactivated during the related process of EMT in epithelial cancers (11). However, whether EMT regulates TF expression in EGFR-driven tumors has not been investigated.
Modulation of EGFR-dependent TF expression by E-cadherin–regulated EMT
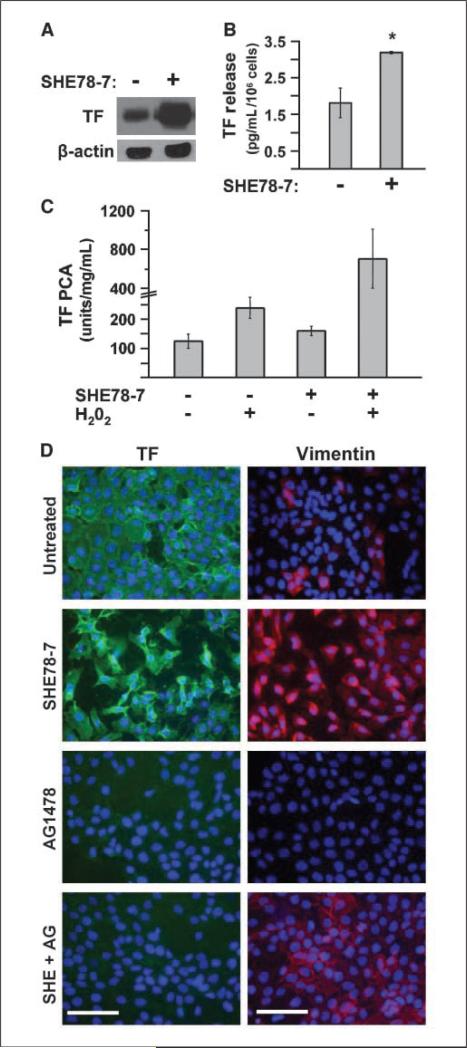
In order to explore whether the loss of E-cadherin activity could explain changes in TF expression among A431 tumor cells in vivo, we used an E-cadherin neutralizing antibody (SHE78-7; ref. 27) to specifically inhibit this molecule on the surface of cultured A431 cells and prevent their homotypic interaction. Remarkably, this treatment led to a robust up-regulation of TF expression and increased shedding of TF-containing microvesicles to the A431-conditioned medium (Fig. 4A and B). Interestingly, A431 cells treated with this reagent alone became only moderately more procoagulant in the TF-dependent coagulation assay (TF-PCA), as did cells incubated with H2O2, a known trigger of TF decryption (28). However, treatment with both agents resulted in 5-fold to 10-fold greater levels of TF-PCA than in the case of control cells (Fig. 4C). Collectively, these results suggest that blockade of E-cadherin results in considerable TF up-regulation, albeit in a predominantly coagulation-deficient (encrypted) form (29).
Figure 4.
Neutralization of E-cadherin potentiates EGFR-driven TF expression and induces EMT-like phenotype. A, TF Western blot showing that treatment of A431 cells with SHE78-7 antibody (2 μg/mL, 48 h) leads to a considerable increase (*, P < 0.05) in total TF protein. B, TF ELISA showing that inhibition of E-cadherin (SHE78-7, 2 μg/mL, 48 h) stimulates the release of TF microparticles into conditioned medium. C, although SHE78-7 treatment increases TF procoagulant activity, a proportion of TF on the surface of A431 cells is still encrypted (or inactive). Cell surface TF can be decrypted following a short treatment with hydrogen peroxide (H2O2, 10 mmol/L, 60 min), which can be augmented following inhibition of E-cadherin with SHE78-7 (2 μg/mL, 48 h). D, TF immunofluorescence (green) on A431 cells in culture shows that although inhibition of E-cadherin leads to an increase in TF expression, regulation of TF in A431 SSC is dependent on EGFR activation. Thus, inhibition of EGFR causes a down-regulation in TF protein levels even when E-cadherin signaling is blocked. Vimentin immunofluorescence (red) shows that normally, A431 SSC is largely vimentin-negative. However, loss of epithelial phenotype by blocking E-cadherin coincides with a massive increase in vimentin expression, which is independent of EGFR signaling. Inhibition of EGFR and/or E-cadherin in A431 cells was accomplished by treatment with AG1478 (20 μmol/L, 24 h) and/or SHE78-7 (2 μg/mL, 48 h), respectively. DAPI (blue). Bars, 100 μM.
Although the effects of SHE78-7 antibody on TF levels are intriguing, it is unclear whether these effects are accompanied by EMT-like processes and whether they are EGFR-dependent (27) or EGFR-independent in nature. A431 cells cultured in the presence of SHE78-7 undergo a profound change in cell shape, assume mesenchymal (spindle) morphology, and express vimentin (Fig. 4D), hallmarks of EMT. As expected, these changes are accompanied by an increase in TF immunostaining (Fig. 4D). Importantly, this induction of TF in the presence of SHE78-7 was still dependent on EGFR activation, as it was totally obliterated by the EGFR inhibitor AG1478. In contrast, SHE78-7–induced expression of vimentin persisted in the presence of AG1478. Taken together, these findings suggest that E-cadherin suppresses/modulates the stimulating effects of EGFR on TF expression, and this can be alleviated by EMT-like changes.
The functional significance of TF expression for EGFR-driven tumorigenesis
Our observations suggest that oncogenic EGFR drives the expression of TF in A431 tumors; however, in vivo this influence becomes restricted to a subset of cancer cells. This restricted TF expression is likely due to several factors including the onset of multilineage differentiation, perturbations in E-cadherin signaling, and possibly other events. Because these changes lower the overall TF levels in tumors (versus cultured cells) the question arises as to whether this receptor still affects tumor formation. To address this, we injected various numbers of cancer cells into immunodeficient (SCID) mice and subjected them to treatment with the TF-blocking antibody CNTO 859, the antitumor effects of which have already been described (25, 30). Despite the restricted and heterogeneous TF expression in A431 tumors, this treatment resulted in striking antitumor effects (Fig. 5). Thus, the s.c. injection of threshold tumorigenic numbers of A431 cells (105) followed by only 1 week of CNTO 859 administration (10 mg/kg) led to a near-complete suppression of tumor initiation, an effect that lasted up to 5 weeks posttreatment termination, at which point, control tumors had already reached critical sizes (Fig. 5A). This experimental design is often used to detect the presence of tumor-initiating (stem) cells (31), and in our case, it may suggest that TF neutralization interferes with their tumor-triggering activity in the A431 population, as has recently been postulated (25).
Figure 5.
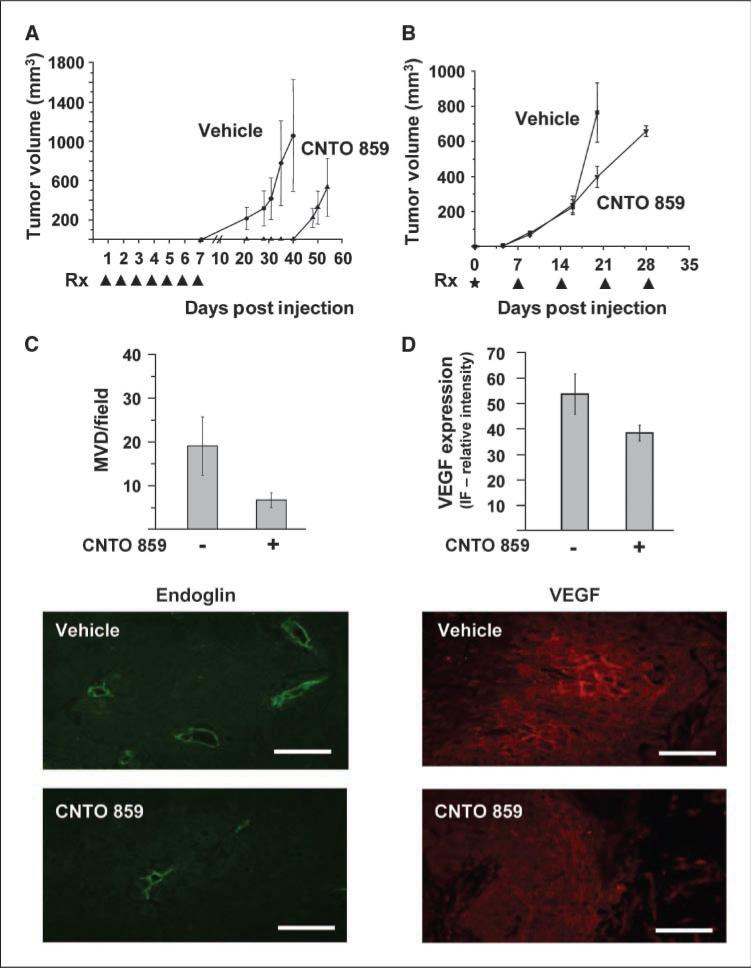
Targeting TF expression inhibits tumor initiation, growth, and angiogenesis. A, tumor growth initiation and tumor growth are severely delayed following early inhibition of TF. A431 cells (1 × 105) were injected s.c. into YFP/SCID mice and mice were treated on days 1 to 7 postinjection (arrows, Rx) with CNTO 859 (200 μg/mouse) or vehicle. B, treatment with CNTO 859 antibody reduces tumor growth rate and angiogenesis. In a second experiment, a large number of A431 cells (16 × 106) were mixed with 500 μg of CNTO 859 prior to injection and mice were injected with CNTO 859 (200 μg/mouse) or vehicle every 7 d until tumors reached a volume of 600 mm3. C, bar graph shows that there are fewer blood vessels in CNTO 859–treated tumors than in vehicle-treated controls. Vessels were counted from at least 20 fields per tumor (magnification, ×200). Immunofluorescence of blood vessels using endoglin/CD105 antibody (green). D, quantification of VEGF produced by tumors treated with CNTO 859 is reduced compared with vehicle-treated tumors. VEGF immunofluorescence (red). Bars, 100 μM.
Interestingly, the requirement for TF is not limited to circumstances involving tumor initiation and latency. For instance, when an excess of A431 cells (16 × 106) was inoculated into SCID mice, tumors emerged rapidly in both control and CNTO 859–treated animals. However, tumor growth rate was markedly lower in mice receiving the antibody treatments (Fig. 5B). We reasoned that this may be due to interference with TF-dependent regulation of tumor angiogenesis (6), including production of VEGF (32). Indeed, both microvascular density and the expression of VEGF, as determined by staining for CD105/endoglin and VEGF antigens, respectively, were visibly reduced in A431 tumors exposed to CNTO 859 relative to their vehicle-treated controls (Fig. 5C and D).
EMT sensitizes tumor cells to TF-dependent induction of VEGF expression and promotes metastasis
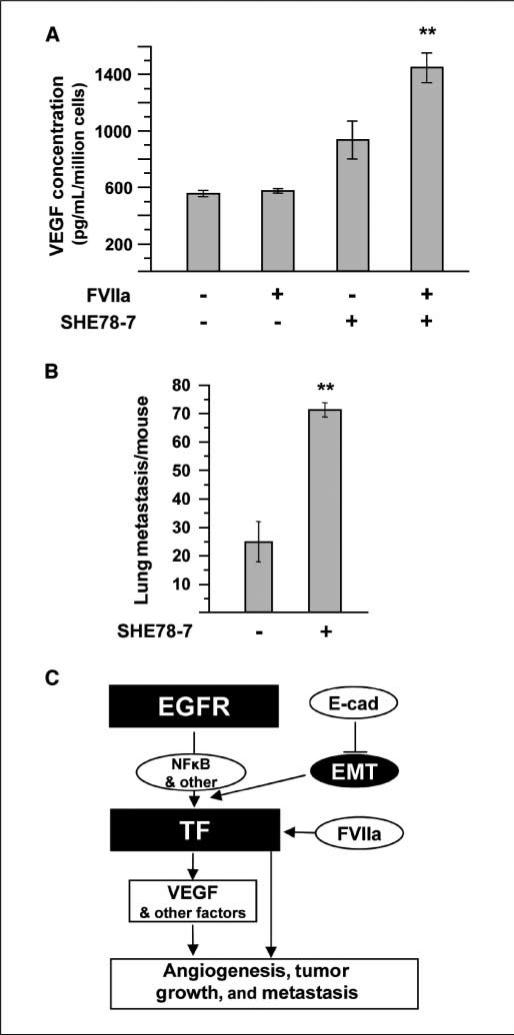
Our data suggest that a subset of cancer cells expressing TF may disproportionately contribute to the aggressiveness and VEGF expression of A431 tumors. Such an effect would likely occur upon contact with FVIIa, the key TF ligand and inducer of TF signaling (18, 29). However, in our hands, A431 cells produced significant levels of VEGF irrespective of FVIIa addition (Fig. 6A). We reasoned that these conditions might not accurately capture the effects of FVIIa in vivo, where TF expression and signaling could be affected by proteolysis and disruption of E-cadherin signaling (and EMT-like events). Interestingly, disruption of E-cadherin by treatment with SHE78-7 antibody led to a marked up-regulation of VEGF production. This effect was significantly enhanced in the presence of the recombinant FVIIa. This event is attributable to TF signaling due to the specificity of FVIIa binding and the fact that cell lines deficient for TF do not respond to this treatment (ref. 19; data not shown).
Figure 6.
EMT facilitates VEGF up-regulation by TF/VIIa signaling and metastasis. Medium from A431 cells following activation of TF-dependent signaling or EMT was collected for a VEGF ELISA. A, treatment of cells with activated factor VIIa (FVIIa, 10 nmol/L, 24 h) has little effect on VEGF expression. Inhibition of E-cadherin (SHE78-7, 2 μg/mL, 48 h) leads to a moderate increase in VEGF levels; however, when E-cadherin inhibition is coupled with FVIIa treatment, a significant increase (**, P < 0.005) in VEGF expression is obtained. Inhibition of E-cadherin sensitizes cells to TF/FVIIa-dependent VEGF signaling. B, induction of EMT in A431 cells by treatment with SHE78-7 (2 μg/mL, 48 h) prior to tail vein injection gives rise to a significantly higher (**, P < 0.005) number of experimental lung metastasis than for untreated A431 cells. C, the proposed interrelationship between oncogenic EGFR, E-cadherin, and TF in epithelial cancer cells. In SSC, oncogenic EGFR regulates TF expression, likely mediated in part by the transcription factor, NFκB. However, this EGFR-dependent TF expression can be modulated by various cellular signaling and differentiation pathways that are engaged by cancer cells within different microdomains of the tumor. These include loss of E-cadherin function and subsequent EMT, as well as activation of TF signaling. All of these changes can alter VEGF expression, angiogenesis, tumor growth, and metastasis.
Furthermore, we were able to show that inhibition of E-cadherin and induction of EMT generates A431 cells with a high metastatic potential. Indeed, cells pretreated with the E-cadherin–neutralizing antibody, SHE78-7, prior to tail vein injection gave rise to a significantly higher number of lung nodules than control (untreated) cells. Collectively, our results imply that oncogenic EGFR triggers the up-regulation of TF in cancer cells and that this effect can be modulated by influences, such as E-cadherin function, differentiation and EMT. Although such changes restrict TF expression to a particular cell subset, these cells may play an especially important role in tumor initiation, growth, angiogenesis, metastasis, and/or expression of VEGF, as summarized in Fig. 6B.
Discussion
The effect of oncogenic changes on TF expression adds a new dimension to the linkage between genetic tumor progression, cancer coagulopathy, and angiogenesis (6, 23, 33, 34). However, it does not fully explain the mechanisms of TF regulation by cancer cells and stroma (refs. 15, 16, 51). In this regard, our study contributes several novel findings. We have documented the effect of two different oncogenic alterations affecting EGFR (EGFRvIII mutation and EGFR up-regulation/amplification) on the increased expression of TF in two different types of cancer cells, glioma and epithelial carcinoma. In the latter case, this effect seems to be dependent on intact NFκB signaling. However, it remains to be established whether other known regulatory elements involved in TF gene transcription including AP-1, SP1, Egr-1, and NFAT (35, 36) participate in TF up-regulation downstream of EGFR oncogenes.
The recent study by Rong and colleagues described loss of PTEN tumor suppressor gene as a key event driving TF up-regulation in hypoxic glioma/glioblastoma cells (23, 36). Neither U373 glioma cells, nor their aggressive U373vIII counterparts, expressed PTEN (data not shown), although they profoundly differ in their TF levels, as a result of changes in EGFRvIII expression. Therefore, we postulate that this frequently activated oncogene (37) may represent another critical event in TF regulation in human glioblastoma, a disease known for procoagulant sequelae (23).
The influence of oncogenic pathways on various genes is often expected to be constitutive. However, we observed that in epithelial cells, the EGFR-driven up-regulation of TF is modulated by the residual cancer cell differentiation program and EMT-like events, dependent on E-cadherin ligation. This extends our earlier studies on the regulation of TF by cell adhesion and cell shape (38). These findings are also consistent with the emerging role of E-cadherin in regulating the function of various receptor tyrosine kinases, including their oncogenic effects on cell growth and invasion (39). In this regard, a number of EGFR regulatory targets are known to be affected by E-cadherin ligation/status including the expression of p27/Kip1 cell cycle inhibitor (27), MUC5AC mucin (40), vimentin, MMP-2, uPA, and several other genes (41). In this manner, E-cadherin may affect cellular behavior and gene expression patterns, notably though several signaling mechanisms that include both the canonical β-catenin/Wnt signaling (42), as well as changes in the activity of RhoA GTPases (43) and other pathways (44). In our hands, pharmacologic inhibition of Rho kinase (Y27632) had a minimal effect on EGFR-driven expression of TF in A431 cells. However, this treatment was performed on cells that retained functional E-cadherin, and therefore, the exact role of these intersecting pathways in TF regulation remains to be investigated. Our results do not preclude the importance of RhoA, MEK/Erk, and other pathways in TF regulation by other types of cancer cells.
We report here that E-cadherin disengagement stimulates production of VEGF. Moreover, under these conditions, cancer cells become hypersensitive to further increases in VEGF production under the influence of the TF/VIIa pathway. This may suggest that cancer cells that have retained their E-cadherin function may be protected, at least to some degree, from proangiogenic effects of the extrinsic coagulation cascade. Conversely, loss of E-cadherin or EMT may render certain cancer cell subsets (e.g., metastatic and/or cancer stem cells) highly responsive to TF/FVIIa-mediated angiogenic switching. Such a notion is consistent with our results suggesting that E-cadherin inhibition in A431 cells generates cells with a greater metastatic capacity than those cells with functioning E-cadherin molecules. Furthermore, blocking TF activity in vivo resulted in a down-regulation of VEGF expression, diminished vascularity, and retarded tumor growth. It is noteworthy that treatment of cultured A431 cells with the anti–E-cadherin neutralizing antibody (SHE78-7) leads to an overexpression of TF, although mostly in the encrypted state. Our observation that even under such conditions, FVIIa stimulation leads to a marked increase in VEGF levels, but not in TF-PCA, is consistent with studies that suggest a role of encrypted TF in intracellular signaling via VIIa/TF/PAR-2 cascade (29). It is plausible that expression of genes aside from VEGF may be similarly affected by the interplay between EGFR, E-cadherin, and TF in vivo.
The absolute level of TF expression is often viewed as proportional to the role (contribution) of this receptor in tumor progression (45). However, our experiments suggest a different interpretation and highlight the significance of TF expression by certain subpopulations of cancer cells rather than global levels of this molecule. For instance, the TF decrease in A431 tumors occurs as cancer cells diversify into low and high expressors, the latter of which may become “diluted” in the population. In this regard, our previous study suggests that small subsets of cancer cells expressing markers of cancer-initiating (stem) cells, such as CD133, may also possess an increased expression of TF (25). In this setting, TF could serve as an organizer of pericellular clotting, leading to the formation of a provisional fibrin matrix and to the accumulation/release of growth-regulating influences from platelets and plasma (e.g., growth factors, chemokines, and thrombin), all of which could serve as a provisional “cancer stem cell niche” (25). There is mounting evidence that the formation of such a niche involves elements of angiogenesis, including up-regulation of VEGF (9, 46, 47).
We speculated that obliteration of TF on the surface of cancer stem cells could diminish their tumor-initiating properties (25). Indeed, in the present study, we provide evidence that tumor initiation (take) rate of A431 cells is lowered under conditions in which tumor cell–associated human TF (but not host/mouse TF) is selectively targeted with the CNTO 859 antibody. This is in spite of the global decrease of TF expression in A431 tumors (versus cultured cells).
In A431 tumors, we observed evidence of multilineage differentiation marked by the emergence of terminally differentiated epidermoid cell subsets expressing cytokeratin (or keratin pearls) and largely devoid of specific TF staining. These cells were interspersed with less differentiated cells, including those with mesenchymal characteristics (vimentin expression) and high levels of TF. This could be a functional equivalent of the recently described motile cancer stem cells (48) which harbor features of EMT (14). It is presently unclear what drives the differentiation process of A431 cells that occurs only in vivo and accompanies aggressive tumorigenesis. Although we have been able to recapitulate the generation of vimentin-positive aspects of these tumors in vitro (by blocking E-cadherin), the trigger of terminal epithelial differentiation in vivo remains unclear and this process (marked by cytokeratin expression) does not normally occur in cultured A431 cells. Nonetheless, our data suggest that tumor aggressiveness is clearly influenced by a minority of cancer cells expressing TF. We postulate that TF up-regulation associated with the EMT-like phenotype may represent a feature of tumor-initiating cells (13, 14, 48) and a potentially more informative biomarker than global TF expression itself.
It is presently unclear whether TF has any role in inducing EMT, or is simply a part of a related phenotype. Nonetheless, our experiments with CNTO 598 may suggest that events in which low numbers of (single) cancer cells participate in the formation of overt outgrowths (e.g., during tumor initiation or dissemination) could be more susceptible to TF inhibition than the growth of more established tumors. This may explain why experimental studies on TF-involvement metastasis (events dependent on single/few cancer cells) are largely congruent (21, 22, 49), whereas discordant results have been obtained with experimental “primary” tumor models in which large numbers (millions) of cancer cells are used to trigger tumor formation (22, 32, 50).
In summary, we report a novel linkage between the expression of oncogenic EGFR, E-cadherin, and TF and suggest that TF may play a cell subset–dependent role in cancer. Therefore, we postulate that targeting TF may have therapeutic (e.g., antiangiogenic, antimetastatic) consequences even in tumors in which TF is expressed by only a fraction of tumor (initiating) cells.
Acknowledgments
Grant support: National Cancer Institute of Canada and Canadian Cancer Society (J. Rak). J. Rak is a recipient of the NCIC Scientist Award, and is the Jack Cole Chair in Pediatric Oncology. Infrastructure contribution from the Fonds de la Recherche en santé du Québec is also acknowledged.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734solely to indicate this fact.
We are thankful to our colleagues at the HRC and McGill, and to our families for their continued support and encouragement.
Footnotes
Unpublished data.
Disclosure of Potential Conflicts of Interest
J. Rak consults for Nuvelo, Inc., and Othera, Inc., in the area of targeting TF in cancer.
References
- 1.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 2.Normanno N, De Luca A, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Huang PH, Mukasa A, Bonavia R, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A. 2007;104:12867–72. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petit AM, Rak J, Hung MC, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Viloria-Petit A, Miquerol L, Yu JL, et al. Contrasting effects of VEGF gene disruption in embryonic stem cell-derived versus oncogene-induced tumors. EMBO J. 2003;22:4091–102. doi: 10.1093/emboj/cdg408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–41. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9(Suppl 1):2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- 9.Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–6. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 10.Rickles FR. Mechanisms of cancer-induced thrombosis in cancer. Pathophysiol Haemost Thromb. 2006;35:103–10. doi: 10.1159/000093551. [DOI] [PubMed] [Google Scholar]
- 11.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 12.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Hugo H, Ackland ML, Blick T, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 14.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209–15. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 16.Milsom C, Yu J, May L, et al. The role of tumor- and host-related tissue factor pools in oncogene-driven tumor progression. Thromb Res. 2007;120(Suppl 2):S82–91. doi: 10.1016/S0049-3848(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 17.Mackman N. Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol. 2005;25:2273–81. doi: 10.1161/01.ATV.0000183884.06371.52. [DOI] [PubMed] [Google Scholar]
- 18.Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005;25:1545–50. doi: 10.1161/01.ATV.0000171155.05809.bf. [DOI] [PubMed] [Google Scholar]
- 19.Versteeg HH, Schaffner F, Kerver M, et al. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111:190–9. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruf W, Fischer EG, Huang HY, et al. Diverse functions of protease receptor tissue factor in inflammation and metastasis. Immunol Res. 2000;21:289–92. doi: 10.1385/IR:36:1:289. [DOI] [PubMed] [Google Scholar]
- 21.Amarzguioui M, Peng Q, Wiiger MT, et al. Ex vivo and in vivo delivery of anti-tissue factor short interfering RNA inhibits mouse pulmonary metastasis of B16 melanoma cells. Clin Cancer Res. 2006;12:4055–61. doi: 10.1158/1078-0432.CCR-05-2482. [DOI] [PubMed] [Google Scholar]
- 22.Palumbo JS, Talmage KE, Massari JV, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133–41. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong Y, Post DE, Pieper RO, Durden DL, Van Meir EG, Brat DJ. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res. 2005;65:1406–13. doi: 10.1158/0008-5472.CAN-04-3376. [DOI] [PubMed] [Google Scholar]
- 24.Mackman N, Morrissey JH, Fowler B, Edgington TS. Complete sequence of the human tissue factor gene, a highly regulated cellular receptor that initiates the coagulation protease cascade. Biochemistry. 1989;28:1755–62. doi: 10.1021/bi00430a050. [DOI] [PubMed] [Google Scholar]
- 25.Milsom C, Anderson GM, Weitz JI, Rak J. Elevated tissue factor procoagulant activity in CD133-positive cancer cells. J Thromb Haemost. 2007;5:2550–52. doi: 10.1111/j.1538-7836.2007.02766.x. [DOI] [PubMed] [Google Scholar]
- 26.Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24:73–6. doi: 10.1016/s0968-0004(98)01343-7. [DOI] [PubMed] [Google Scholar]
- 27.St.Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27KIP1. J Cell Biol. 1998;142:557–71. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–61. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 29.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282:25416–24. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 30.Ngo CV, Picha K, McCabe F, et al. CNTO 859, a humanized anti-tissue factor monoclonal antibody, is a potent inhibitor of breast cancer metastasis and tumor growth in xenograft models. Int J Cancer. 2007;120:1261–7. doi: 10.1002/ijc.22426. [DOI] [PubMed] [Google Scholar]
- 31.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Deng Y, Luther T, et al. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest. 1994;94:1320–7. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tallman MS, Lefebvre P, Baine RM, et al. Effects of alltrans retinoic acid or chemotherapy on the molecular regulation of systemic blood coagulation and fibrinolysis in patients with acute promyelocytic leukemia. J Thromb Haemost. 2004;2:1341–50. doi: 10.1111/j.1538-7836.2004.00787.x. [DOI] [PubMed] [Google Scholar]
- 34.Boccaccio C, Sabatino G, Medico E, et al. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature. 2005;434:396–400. doi: 10.1038/nature03357. [DOI] [PubMed] [Google Scholar]
- 35.Mackman N. Regulation of the tissue factor gene. Thromb Haemost. 1997;78:747–54. [PubMed] [Google Scholar]
- 36.Rong Y, Hu F, Huang R, et al. Early growth response gene-1 regulates hypoxia-induced expression of tissue factor in glioblastoma multiforme through hypoxia-inducible factor-1-independent mechanisms. Cancer Res. 2006;66:7067–74. doi: 10.1158/0008-5472.CAN-06-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–53. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milsom C, Rak J. Regulation of tissue factor and angiogenesis-related genes by changes in cell shape. Biochem Biophys Res Commun. 2005;337:1267–75. doi: 10.1016/j.bbrc.2005.09.187. [DOI] [PubMed] [Google Scholar]
- 39.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23:1739–48. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Schein AJ, Nadel JA. E-cadherin promotes EGFR-mediated cell differentiation and MUC5AC mucin expression in cultured human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1049–60. doi: 10.1152/ajplung.00388.2004. [DOI] [PubMed] [Google Scholar]
- 41.Andersen NF, Standal T, Nielsen JL, et al. Syndecan-1 and angiogenic cytokines in multiple myeloma: correlation with bone marrow angiogenesis and survival. Br J Haematol. 2005;128:210–7. doi: 10.1111/j.1365-2141.2004.05299.x. [DOI] [PubMed] [Google Scholar]
- 42.Perrais M, Chen X, Perez-Moreno M, Gumbiner BM. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell. 2007;18:2013–25. doi: 10.1091/mbc.E06-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mateus AR, Seruca R, Machado JC, et al. EGFR regulates RhoA-GTP dependent cell motility in E-cadherin mutant cells. Hum Mol Genet. 2007;16:1639–47. doi: 10.1093/hmg/ddm113. [DOI] [PubMed] [Google Scholar]
- 44.Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat Rev Mol Cell Biol. 2001;2:887–97. doi: 10.1038/35103068. [DOI] [PubMed] [Google Scholar]
- 45.Nakasaki T, Wada H, Shigemori C, et al. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol. 2002;69:247–54. doi: 10.1002/ajh.10061. [DOI] [PubMed] [Google Scholar]
- 46.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 47.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 48.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 49.Mueller BM, Reisfeld RA, Edgington TS, Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci U S A. 1992;89:11832–6. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toomey JR, Kratzer KE, Lasky NM, Broze GJ., Jr Effect of tissue factor deficiency on mouse and tumor development. Proc Natl Acad Sci U S A. 1997;94:6922–6. doi: 10.1073/pnas.94.13.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu JL, May L, Milsom CC, et al. Contribution of host-derived tissue factor to tumor neovascularization. Arterioscl Thromb Vasc Biol. 2008;28:1975–81. doi: 10.1161/ATVBAHA.108.175083. [DOI] [PMC free article] [PubMed] [Google Scholar]