Abstract
Objective
Despite evidence for hippocampal abnormalities in elderly depression, it is unknown whether these changes are regionally specific. This study used three-dimensional mapping techniques to identify regional hippocampal abnormalities in early- and late-onset depression. Neuropsychological correlates of hippocampal morphology were also investigated.
Method
With high-resolution magnetic resonance imaging, hippocampal morphology was compared among elderly patients with early- (N=24) and late-onset (N=22) depression and comparison subjects (N=34). Regional structural abnormalities were identified by comparing distances, measured from homologous hippocampal surface points to the central core of each individual’s hippocampal surface model, between groups.
Results
Hippocampal volumes differed between depressed patients and comparison subjects but not between patients with early- and late-onset depression. However, statistical mapping results showed that regional surface contractions were significantly pronounced in late-compared to early-onset depression in the anterior of the subiculum and lateral posterior of the CA1 subfield in the left hemisphere. Significant shape differences were observed bilaterally in anterior CA1–CA3 subfields and the subiculum in patients in relation to comparison subjects. These results were similar when each disease group was separately compared to comparison subjects. Hippocampal surface contractions significantly correlated with memory measures among late- but not early-onset depressed patients or comparison subjects.
Conclusions
More pronounced regional volume deficits and their associations with memory in late-onset depression may suggest that these patients are more likely to develop cognitive impairment over time than individuals with early-onset depression. Mapping regional hippocampal abnormalities and their cognitive correlates may help guide research in defining risk profiles and treatment strategies.
Altered hippocampal morphology has an important role in the pathophysiology of elderly depression. Studies using magnetic resonance imaging (MRI) have reported hippocampal volume decreases, sometimes unilateral, in elderly patients with major depression relative to healthy comparison subjects (1–5), although negative reports exist (6–8).
Of importance, major depression, especially in the elderly, may be considered a heterogeneous disorder because in a subset of patients, the first episode occurs only late in life. This subgroup, frequently referred to as having late-onset depression, exhibits certain unique clinical, biological, and neuroimaging characteristics. For example, patients with late-onset depression are less likely to have psychiatric comorbidity (9) or a family history of depression (9, 10) but are more likely to have medical comorbidity (11) compared with groups with early-onset depression. Some evidence links late-onset depression to greater cognitive deficits (12) and increased risk of dementia (13, 14); other studies relate a lifetime history of depression to a higher risk of dementia (15–17).
Illness duration rather than age itself may predict hippocampal volumes in depressed subjects (18). Reduced hippocampal volumes have been associated with a longer course of illness in elderly depression (2). On the other hand, some studies dichotomized elders by age at illness onset and found hippocampal volume reductions more pronounced in late-onset than early-onset depression (1, 4, 5). Prior evidence thus suggests that age of onset and perhaps also illness duration influence hippocampal morphology in depression. It is also possible that these two measures are themselves correlated. However, because the magnitude and direction of the relationship between age of onset (or biological age in general) and illness duration are solely dependent on study group characteristics and the timing of subject examination, these two variables may be considered to constitute independent hypotheses.
Genotype may be important because smaller hippocampal volumes in late-onset depression have been linked to the long variant of the promoter region of the serotonin transporter gene (8). Indeed, subjects homozygous for the long allele genotype may have greater vascular risk factors and an enhanced vulnerability to stress-induced neurotoxic effects of glucocorticoids, which may both lead to structural hippocampal abnormalities (8, 19, 20). Moreover, the short allele has been found to moderate the influence of stressful life events on the development of depression through mechanisms that may affect hippocampal volume (21).
In elderly subjects, age at onset of depression may be particularly important in accounting for changes in hippocampal morphology. Focusing on hippocampal changes in relation to this critical variable may also help explain how depression could emerge as a risk factor for dementia. The regional specificity of hippocampal abnormalities may deserve special attention. Computational image analysis methods have several advantages over traditional volumetric approaches. They can isolate local, as well as global, differences in brain morphology as potentially associated with neuropathology. One prior study employed computerized methods in young adults with major depression, showing hippocampal shape alterations in the absence of significant volume reductions that were pronounced in the subiculum (22).
In elderly depression, however, the question of regional specificity of hippocampal volume reductions has not been addressed in the literature. The aim of our study was to use a region-of-interest technique and a hippocampal three-dimensional radial atrophy mapping approach (23, 24) to assess subregional structural deformations in depressed elders. Specifically, we set out to examine whether late-onset depression may exhibit greater regional changes in hippocampal morphometry than early-onset depression. We also addressed possible relationships between hippocampal morphology and neuropsychological measures of memory in a subgroup of the subjects included in the present study.
Methods and Materials
Subjects
The subjects included 46 elderly patients with major depression and 34 elderly comparison subjects (Table 1). The cohort with depression was subdivided into those with early-onset depression (N=24) and late-onset depression (N=22), with onset of the first major depressive episode after age 60 as a cutoff, which is in line with previous studies (4, 25) and our own work (26) (Table 2). The Structured Clinical Interview for DSM-IV (SCID) was performed to assess the diagnostic status of the patients. All patients met DSM-IV criteria for major depressive disorder and had a 17-item Hamilton Depression Rating Scale (27) score of 15 or greater. Information on prior episodes and the age of onset was obtained from patients and caregivers. All patients were free of psychotropic medications for at least 2 weeks before imaging. Twenty-three patients (eight with early-onset depression and 15 with late-onset depression) were drug naive, and 23 patients had taken an antidepressant at some time (16 with early-onset depression and seven with late-onset depression). The antidepressants included selective serotonin reuptake inhibitors (paroxetine, fluoxetine), serotonin and norepinephrine reuptake inhibitors (venlafaxine), and bupropion at standard doses. None of the patients included in the study reported exposure to antidepressant for more than 6 months. Exclusion criteria were the following: 1) another major psychiatric illness; 2) alcohol or drug dependence; 3) neurologic illness, including stroke, transient ischemic attacks, and dementia; 4) illness or medication precluding cognitive testing; and 5) metal in the body precluding MRI. A neuropsychiatric examination and the SCID for normal subjects were administered to all comparison subjects to rule out current or past psychopathology. All subjects underwent the Mini-Mental State Examination (28). A subset of subjects (N=43) also completed two neuropsychological tests: the California Verbal Learning Test (29) and the Rey-Osterrieth Complex Figure (30) (Table 3).
TABLE 1.
Demographic and Clinical Characteristics of Participants With and Without Depression
| Variable | Comparison Subjects (N=34) |
Subjects With Depression (N=46) |
Analysis | ||||
|---|---|---|---|---|---|---|---|
| N | N | χ2 | df | p | |||
| Gender (female/male) | 19/15 | 34/12 | 2.84 | 1 | 0.09 | ||
| Mean | SD | Mean | SD | Test | df | p | |
| Age (years) | 72.38 | 6.93 | 71.10 | 7.66 | t=0.76 | 78 | 0.45 |
| Mini-Mental State Examination score | 29.48 | 0.79 | 28.68 | 1.45 | t=2.86 | 75 | 0.01 |
| Age of onset (years) | — | 51.43 | 22.89 | — | — | — | |
| Hamilton Depression Rating Scale score | — | 17.73 | 3.29 | — | — | — | |
| Cardiovascular risk factor score | 11.19 | 6.68 | 11.00 | 5.36 | t=0.14 | 73 | 0.89 |
| Total brain volume (cm3) | 1286.93 | 138.72 | 1295.68 | 129.61 | t=0.29 | 78 | 0.77 |
| Right hippocampal volume (mm3) | 1308.45 | 245.62 | 1127.53 | 231.12 | F=16.14 | 1, 75 | 0.001 |
| Left hippocampal volume (mm3) | 1272.75 | 257.65 | 1116.10 | 222.00 | F=12.31 | 1, 75 | 0.001 |
TABLE 2.
Demographic and Clinical Characteristics of Participants With and Without Depression Dichotomized for Age of Depression Onset
| Subjects With Depression | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Comparison Subjects (N=34) | Early-Onset (N=24) | Late-Onset (N=22) | Comparison Versus Early Onset |
Comparison Versus Late Onset |
Early Versus Late Onset |
|||||||||
| N | N | N | χ2 | df | p | χ2 | df | p | χ2 | df | p | ||||
| Gender (female/male) | 19/15 | 20/4 | 14/8 | 3.65 | 1 | 0.06 | 0.09 | 1 | 0.77 | 1.40 | 1 | 0.24 | |||
| Mean | SD | Mean | SD | Mean | SD | F | df | p | F | df | p | F | df | p | |
| Age (years) | 72.38 | 6.93 | 68.00 | 5.83 | 74.50 | 8.09 | 6.38 | 1, 56 | 0.04 | 1.09 | 1, 54 | 0.30 | 9.87 | 1, 44 | 0.01 |
| Mini-Mental State Examination score | 29.48 | 0.79 | 28.72 | 1.61 | 28.64 | 1.33 | 5.38 | 1, 53 | 0.02 | 5.53 | 1, 53 | 0.01 | 0.04 | 1, 42 | 0.84 |
| Age of onset (years) | — | 33.25 | 16.05 | 71.27 | 7.23 | — | — | — | — | — | — | ||||
| Previous episodes of depression | — | 4.80 | 4.13 | 0.50 | 0.90 | — | — | — | — | — | — | ||||
| Hamilton Depression Rating Scale score | — | 17.33 | 2.63 | 18.13 | 3.77 | — | — | — | — | 0.76 | 1, 44 | 0.39 | |||
| Total brain volume (cm3) | 1286.93 | 138.72 | 1273.98 | 137.13 | 1318.28 | 119.91 | 0.11 | 1, 56 | 0.75 | 0.76 | 1, 54 | 0.39 | 1.29 | 1, 44 | 0.26 |
| Cardiovascular risk factor score | 11.19 | 6.68 | 9.36 | 4.57 | 12.64 | 5.69 | 1.24 | 1, 51 | 0.27 | 0.68 | 1, 51 | 0.41 | 4.42 | 1, 42 | 0.04 |
| Left hippocampus volume (mm3) | 1272.75 | 257.65 | 1159.72 | 162.09 | 1068.67 | 268.85 | 8.01 | 1, 53 | 0.02 | 8.39 | 1, 51 | 0.02 | 1.65 | 1, 41 | 0.62 |
| Right hippocampus volume (mm3) | 1308.44 | 245.61 | 1152.04 | 210.61 | 1100.78 | 253.85 | 5.79 | 1, 53 | 0.06 | 6.86 | 1, 51 | 0.04 | 0.45 | 1, 41 | 0.51 |
TABLE 3.
Demographic and Clinical Characteristics of Neuropsychological Participants With and Without Depression Dichotomized for Age of Depression Onset
| Variable | Comparison Subjects (N=15) |
Subjects With Depression |
Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Early-Onset (N=15) | Late-Onset (N=13) | ||||||||
| N | N | N | χ2 | df | p | ||||
| Gender (female/male) | 10/5 | 13/2 | 8/5 | 2.52 | 2 | 0.80 | |||
| Mean | SD | Mean | SD | Mean | SD | Test | df | p | |
| Age (years) | 70.60 | 7.64 | 68.20 | 5.87 | 73.08 | 8.68 | F=1.54 | 2, 40 | 0.24 |
| Mini-Mental State Examination score |
29.73a | 0.59 | 28.80 | 1.66 | 28.77b | 1.01 | F=3.15 | 2, 40 | 0.05 |
| Education (years) | 15.47 | 2.59 | 14.93 | 2.81 | 15.46 | 2.37 | F=0.20 | 2, 40 | 0.82 |
| Age of onset (years) | — | 35.87 | 17.13 | 70.00 | 7.57 | — | — | — | |
| Previous episodes of depression | — | 5.57 | 4.39 | 0.43 | 1.13 | — | — | — | |
| Hamilton Depression Rating Scale score |
— | 17.33 | 2.60 | 18.18 | 3.94 | t=0.76 | 1, 44 | 0.39 | |
| Total brain volume (cm3) | 1280.25 | 106.83 | 1262.07 | 111.86 | 1302.72 | 130.09 | F=0.43 | 2, 40 | 0.66 |
| California Verbal Learning Test trial 1 score |
5.60 | 1.59 | 6.13 | 1.76 | 4.92 | 1.84 | F=1.70 | 2, 40 | 0.20 |
| California Verbal Learning Test long delay score |
9.46 | 3.48 | 7.93 | 2.78 | 7.31 | 2.25 | F=1.67 | 2, 40 | 0.20 |
| Rey-Osterrieth Complex Figure copy score |
33.23 | 2.35 | 31.40 | 3.70 | 31.93 | 3.16 | F=1.35 | 2, 40 | 0.27 |
| Rey-Osterrieth Complex Figure recall score |
13.60 | 6.19 | 11.70 | 5.77 | 12.00 | 5.85 | F=0.44 | 2, 40 | 0.65 |
Significant difference between comparison subjects and early-onset depression patients (p≤0.05).
Significant difference between comparison subjects and late-onset depression patients (p≤0.05).
The patients were recruited through local newspapers and radio advertisements and through referrals from the geriatric psychiatry ambulatory care programs at the University of California, Los Angeles, Medical Center. The comparison subjects were recruited from the community through newspapers and radio advertisements. The study was approved by the University of California, Los Angeles, Human Subjects Protection Committees; all subjects provided written informed consent before beginning the study procedures. This group was included in a prior study of structural abnormalities in subregions of the prefrontal cortex (31), as well as specific patterns of gray matter abnormalities with cortical pattern-matching methods (26, 32).
Image Data Acquisition and Analysis
All subjects were studied with MRI performed on a 1.5-T Signa Scanner (GE Medical Systems, Milwaukee) with a coronal T1- weighted spoiled gradient/recall acquisition in the steady state (GRASS) with the following parameters: TR=20 msec, TE=6 msec, flip angle=45×, 1.4-mm slice thickness without gaps, field of view=22 cm, number of excitations=1.5, matrix size=256×192 mm, in-plane resolution=0.86×0.86 mm. Each image volume was corrected for magnetic field inhomogeneities (33) and resampled into 1-mm isotropic voxels and placed into the standard coordinate system of the ICBM-305 average brain (34). At the same time, image volumes were corrected for head tilt and alignment with a three-translation and three-rotation rigid-body transformation (without scaling) (35). This procedure corrects for differences in head alignment between subjects to ensure that measurements are not influenced by different brain orientation. In addition, each brain volume was tilted by 30× along the long axis of the hippocampus to allow for unbiased anatomical decisions for hippocampal delineation. Hippocampi were traced on contiguous oblique coronal slices following a detailed, well-established protocol with high intrarater and interrater reliability (intraclass correlation coefficient >0.88) (23, 36). To assist interrater reliability, tracings included the hippocampus proper, the dentate gyrus, and the subiculum. Region-of-interest volumetric data were extracted and analyzed statistically.
To identify regional changes in hippocampal morphology, we used surface-based anatomical mesh modeling methods that allow the matching of equivalent hippocampal surface points, obtained from manual tracings, across subjects and groups. To match the digitized points representing the hippocampus surface traces in each brain volume, the manually derived contours were transformed into three-dimensional parametric surface mesh models with normalized spatial frequency of the surface points within and across brain slices, as previously detailed (23, 24, 36). This step ensures precise comparison of anatomy between subjects and groups at each hippocampal surface point. To quantify local changes in hippocampal morphology representing local volume changes, a three-dimensional medial curve was defined along the long axis of the hippocampus for each hippocampal surface model, and radial distance measures (i.e., the distance from homologous hippocampal surface points to the central core of the individual’s hippocampal surface model) were estimated. These values were used to generate individual four-dimensional maps in which the fourth dimension represents the local distance from the center of the hippocampus to each point on the hippocampal surface. These distance maps can be combined across subjects to produce group average distance maps, and radial distances may be compared at each anatomically homologous hippocampal surface point to provide maps showing regional hippocampal surface deformation or expansion between the groups.
Statistical Analyses
Statistical analyses examined whether differences in total hippocampal volume and local changes in hippocampal morphology with radial distance measures were present in patients in relation to healthy comparison subjects and in patients based on age of illness onset. The general linear model was used to assess the overall diagnostic group effect (comparison subjects versus all depressed patients), with covariance for age, sex, and total brain volume. We considered illness duration (from first to most recent episode) as a covariate but discarded this option since a preliminary analysis showed that illness duration and age have a significant negative correlation (r=−0.345, p<0.04). Since changes in brain anatomy are known to occur in association with normal aging processes, it was necessary to remove the variance associated with age from the data before assessing group effects.
Significant overall group effects were followed up by separately examining differences between early-onset depression and comparison subjects, late-onset depression and comparison subjects, and the two disease groups (early-onset versus late-onset depression). Post-hoc Bonferroni corrections were used to control for the potentially inflated type I error associated with performing multiple tests.
To evaluate regional differences in hippocampal volume as indexed by measures of hippocampal radial distance, the same statistical model was used at equivalent hippocampal surface locations in three dimensions. Specifically, we first assessed the overall diagnostic group effect and then applied follow-up tests in which each group was compared with the other. Age, gender, and total brain volume were again included as covariates. Uncorrected two-tailed probability values were mapped onto the averaged hippocampal surface models of the entire group and displayed in three dimensions. Since statistical tests were applied at each of the hippocampal surface points, the hippocampal surface maps were subjected to permutation-based statistics with a threshold of p<0.05 to ensure that the overall pattern of effects in the surface-based maps could not have been observed by chance alone (23, 24). In particular, for permutations, we randomly assigned the subjects to diagnostic groups 100,000 times, performing statistical tests at each hippocampal surface point for each random assignment. With a threshold of p<0.05 and age, gender, and total brain volume as covariates, the number of significant results between patients and comparison subjects and between the two disease groups in the actual data was compared to the number of significant results produced during the permutation testing to produce a corrected overall significance value for each map.
Neuropsychological data were available for a subgroup of the subjects (early-onset depression: N=15; late-onset depression: N=13; comparison subjects: N=15). Since the group size was small and age and education did not differ among these subgroups, Spearman’s rank correlation analyses were used to correlate neuropsychological measures and hippocampal volumes within each diagnostic subgroup. To control for potentially inflated type I error by examining the relationship between four neuropsychological measures and the left and right hippocampal volumes separately, Bonferroni correction was applied, and the new threshold of significance was set at p<0.013. Finally, linear regression analyses were performed to assess relationships between neuropsychological measures and regional changes in hippocampal surface morphology within each diagnostic group. Since these relationships were again assessed pointwise across the hippocampal surface, permutation testing was again used to confirm the overall significance of results.
Results
Subjects
Demographic characteristics did not differ significantly between subjects with depression (N=46) and comparison subjects (N=34) overall. Mini-Mental State Examination scores were significantly lower in depressed subjects overall (Table 1) and in subjects with early-onset or late-onset depression in relation to comparison subjects separately (Table 2). When the cohort with depression was subdivided into those with early-onset and late-onset depression (Table 2), the subjects with early-onset depression also differed significantly from the comparison group in age, and significant age differences were present between the two disease groups. The subjects with late-onset depression showed a significantly higher cardiovascular risk factor score than the subjects with early-onset depression. There was no significant difference in total brain volume or sex distribution among the groups. The subgroup for whom neuropsychological measures were available did not show significant differences in memory performance (Table 3). Mini-Mental State Examination scores were significantly lower in subjects with late-onset and early-onset depression in relation to comparison subjects in the subgroup (Table 3).
Hippocampal Volumes
Table 1 shows means and standard deviations for hippocampal volume measures obtained for the depressed and comparison groups separately. After we applied traditional volumetric analyses, an overall diagnostic group effect was observed, with depressed patients showing significant hippocampal reductions in both the right and left hemispheres (Table 1). Follow-up analyses (Table 2) showed that patients with early-onset depression exhibited hippocampal volume reduction in the left hemisphere and close to significance in the right hemisphere, in relation to comparison hippocampal volume effects that were more pronounced when patients with late-onset depression were related to comparison subjects; subjects with late-onset depression showed significant reductions in hippocampal volumes bilaterally. Patients with early-onset depression did not differ significantly from those with late-onset depression for right or left hippocampal volumetric measures.
Surface Mapping
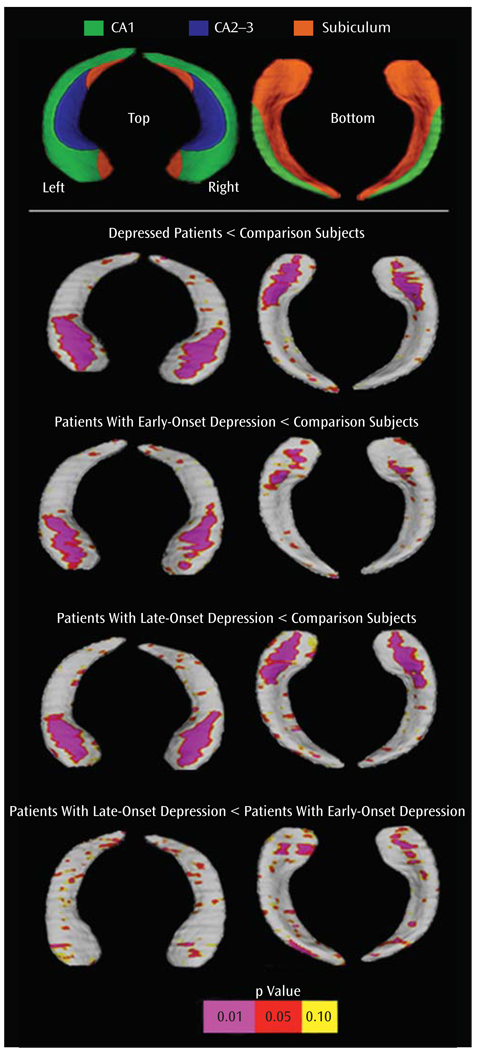
Figure 1 shows the results of statistical tests performed at each hippocampal surface location comparing diagnostic groups in three dimensions (lower panels). Significant reductions in radial distances, used to index regional reductions in hippocampal volume, are referenced by the color bar showing probability values obtained from each hippocampal surface location. These statistical maps show pronounced bilateral volume reductions in anterior CA1-CA3 subfields and the subiculum in depressed patients in relation to comparison subjects (second panel). Permutation testing confirmed the overall significance of these regional results (corrected p values: right, p=0.003; left, p=0.001).
FIGURE 1.
Hippocampal Maps Showing Depressed Patients and Comparison Subjectsa
a Panel 1: schematic representation of the hippocampal subfields is mapped onto the hippocampal surface. Definitions are based on Duvernoy and Bougouin (1998). Lower panels: statistical maps compare hippocampal surface deformation/expansion between elderly depressed patients (N=46) and comparison subjects (N=34), between early-onset depressed patients (N=24) and comparison subjects, between late-onset depressed patients (N=22) and comparison subjects, and between early-onset depressed patients and late-onset depressed patients. Group sizes were early-onset depression (N=24), late-onset depression (N=22), and comparison subjects (N=34). For all statistical maps, the color bar encodes the probability values for the observed effects.
Figure 1 also shows that a similar pattern of bilateral regional reductions appears when subjects with late-onset depression and comparison subjects are compared (panel 4) and, to a lesser extent, between subjects with early-onset depression and comparison subjects (panel 3). Permutation testing confirmed the overall significance of hippocampal regional effects between each disease group and the comparison group (corrected p values: patients with early-onset depression versus comparison subjects: right, p<0.02; left, p<0.02; patients with late-onset depression versus comparison subjects: right, p=0.006; left, p=0.001). Comparisons between the two disease groups showed that patients with late-onset depression had more pronounced local volume reductions concentrated in anterior aspects of the subiculum, as well as lateral-posterior aspects of the CA1 subfield (panel 5). Permutation analyses showed that the regional patterns of hippocampal volume reduction were significant for the left hemisphere and just less than significant for the right hemisphere (corrected p values: right, p<0.08; left, p<0.05). Surface mapping did not reveal any surface expansions between groups.
Cognitive Correlations
Significant positive associations between cognitive performance and hippocampal volumes were observed among patients with late-onset depression but not among patients with early-onset depression or comparison subjects. Hippocampal volumes were associated with performance on the two nonverbal tests (Rey-Osterrieth Complex Figure copy: right, r=0.72, p=0.006; left, r=0.60, p<0.04; Rey-Osterrieth Complex Figure recall: right, r=0.58, p<0.05; left, r=0.60, p<0.04). The association between the right hippocampal volume and the Rey-Osterrieth Complex Figure copy score survived Bonferroni correction.
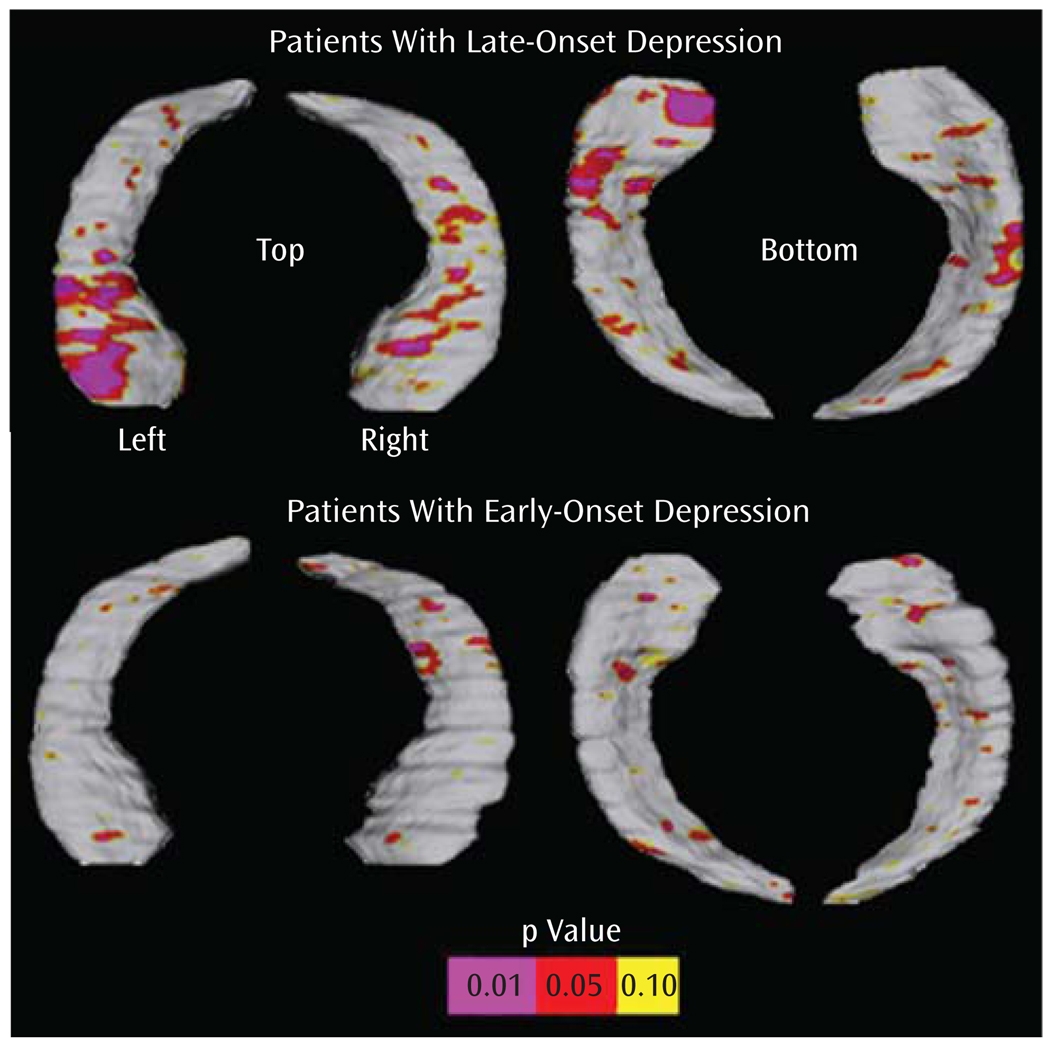
Significant positive correlations, confirmed by permutation tests, were observed between both verbal and nonverbal tests and hippocampal surface morphology within the late-onset depression subgroup but not within patients with early-onset depression or comparison subjects (permutation-corrected p values, late-onset depression: California Verbal Learning Test trial 1: right, p=0.04; left, p<0.04; California Verbal Learning Test long delay: right, p=0.16; left, p=0.03; Rey-Osterrieth Complex Figure copy: right, p=0.01; left, p=0.001; Rey-Osterrieth Complex Figure recall: right, p<0.02; left, p<0.02). Figure 2 shows three-dimensional statistical maps correlating the delayed recall score on the California Verbal Learning Test with hippocampal radial atrophy within the late-onset depression and the early-onset depression subgroups.
FIGURE 2.
Statistical Maps Showing Significant Relationships Between the Delayed Recall Score on the California Verbal Learning Test and Regional Hippocampal Atrophy Within Late-Onset Depression (N=13) and Early-Onset Depression (N=15)
Discussion
For the first time, to our knowledge, in this report, we used novel computerized surface-based image analysis techniques to isolate regional hippocampal volume deficits in elderly depression based on age at illness onset. The key findings were the following:
Significant surface contractions indexing regional volume deficits in the hippocampal head, possibly corresponding to areas in CA1–CA3 subfields and the subiculum, when the depressed cohort was examined as one group
Similar spatial patterns of significant bilateral surface contractions between patients with late-onset depression and comparison subjects and, to a lesser extent, between patients with early-onset depression and comparison subjects, with minor involvement of the subiculum
Comparison of the two disease groups revealed significant left regional hippocampal reductions in patients with late-onset depression, possibly concentrated in the subiculum and in posterior-lateral aspects of the CA1
Significant relationships between hippocampal deficit patterns and memory performance among patients with late-onset depression but not patients with early-onset depression
Global volume differences were consistent with most earlier studies (1–5), showing smaller hippocampal volumes in elderly depressed patients in relation to comparison subjects, although some reports have shown differences as being more pronounced for the right than for the left hemisphere (1, 2). Our results may support other work that dichotomized subjects by age at illness onset (1, 4, 5) and found greater hippocampal volume reductions in late-onset depression. Indeed, in the present study, traditional volumetric analyses displayed significant differences between each disease group and the comparison group, with greater deficits in patients with late-onset depression versus comparison subjects than patients with early-onset depression versus comparison subjects, but we did not detect significant differences in global hippocampal volume between early-onset and late-onset depression. However, when computational mapping methods were applied, the two disease groups not only differed significantly from the comparison subjects but also from each other. Indeed, when effects are highly localized, maps are likely to detect regional changes in hippocampal structure that volumetric measures may miss (22).
Damage to particular subfields is supported by postmortem studies, revealing complex neuronal abnormalities in the subiculum (37) as well as in distinct layers of hippocampal subfields, with highest changes in the CA1, followed by the CA2 and the CA3 (38).
Factors that may contribute to hippocampal damage include hypercortisolemia and ischemia. Of interest, the CA1 and CA2 subfields appear especially vulnerable to vascular damage (39), which may correspond well with our maps of local volume reductions in both early-onset and late-onset depression and with the hypothesis that especially ischemic small-vessel disease may be implicated in the pathogenesis of elderly depression (40). Our maps may also suggest possible damage to the CA3 subregion. Both the CA2 and CA3 subregions receive the major hypothalamic projection to the hippocampus formation. Since the hippocampus is thought to modulate the secretion of adrenocorticotrophic hormone (41), enhanced vulnerability to damage from cortisol may at least partly reflect aberrant regulatory circuitry involving hippocampal subfields linked to the hypothalamus.
Hippocampal connections are topographically organized along the anterior-posterior axis in addition to the horizontal axis (42). For example, projections to and from the prefrontal cortices occur in a gradient concentrated in anterior hippocampal regions (43). Our prior investigations in elderly depression have shown bilateral gray matter abnormalities in distinct subregions of the prefrontal cortex, including the anterior cingulate, the orbitofrontal cortex, and the gyrus rectus (31, 32). Late-onset depression may be associated with greater cortical atrophy than early-onset depression, especially in temporal and parietal regions (26). This may offer a potential explanation for the more pronounced local volume reductions in both the hippocampal head and tail of subjects with late-onset depression compared to subjects with early-onset depression, as found in the present study.
A key finding of our study was the observation of local volume reductions concentrated in anterior aspects of the subiculum, as well as lateral-posterior aspects of the CA1 subfield in late-onset depression compared with early-onset depression. With computational mapping approaches, CA1 and subiculum involvement were found in patients with mild cognitive impairment who later developed Alzheimer’s disease (36). Patients with a late age of illness onset may therefore be at a higher risk of developing dementia over time. Our exploratory findings of significant interactions between regional hippocampal surface contractions and verbal as well as nonverbal memory performance in late-onset depression but not early-onset depression of a subgroup may further support this view. Three-dimensional maps of cognitive correlates of hippocampal surface morphology were more sensitive for detecting these relationships than correlation of memory scores with hippocampal volumes. Specifically, a strong correlation for the CA1 subfield and the subiculum was observed with the California Verbal Learning Test long delay in the left hemisphere among patients with late-onset depression, which may resemble patterns found in early Alzheimer’s disease (36). Mini-Mental State Examination scores differed significantly between patients with late-onset depression and comparison subjects in the subgroup, yet they remained within the normal range. In addition, patients with late-onset depression did not differ from comparison subjects or patients with early-onset depression in their mean delayed recall scores or any other memory measure. This may imply that regional hippocampal atrophy patterns and their associations with memory performance could become apparent before clinical evidence of cognitive decline. Hippocampal atrophy may therefore be a candidate neurobiological marker for defining risk profiles for dementia conversion. Longitudinal studies are warranted to support this hypothesis by specifically exploring the pathophysiology of structural and neuropsychological changes over time and their relation to treatment and illness course (44). In particular, longitudinal studies may help clarify whether patients with early-onset depression are more amenable to antidepressant treatments than patients with late-onset depression.
The limitations of this study should be noted. Despite the relatively small overall group size and the small size of the subgroups, we were able to detect significant morphological differences between groups, and significant associations between regional hippocampal volume reductions and memory performance, respectively. A larger group will better define hippocampal regions that correlate best with cognitive measures and determine the specificity and sensitivity of our methods in defining risk profiles. In addition, combined MRI and postmortem studies with prospectively gathered clinical information about depressive symptoms and cognitive function would help elucidate the link between changes seen in MRI and an underlying neuropathology (e.g., Alzheimer’s disease) that may cause depression. Without direct pathological validation, the interpretation of the subregional involvement remains arbitrary. The subregional boundaries that we used were similar to those proposed by other research groups (22, 45). Nevertheless, what we interpret as specific regional involvement may reflect changes in another hippocampal region. In addition, study groups were not optimally matched for age and gender. However, our analyses included age, gender, and total brain volume as covariates, thus reducing the possibility of confounding effects.
In conclusion, we mapped hippocampal volume deficits in elderly depression, showing prominent local reductions in CA1–CA3 fields and the subiculum. This study is also the first to our knowledge to show that concentrated volume reductions in the CA1 and the subiculum distinguish late-onset from early-onset depression. In addition, we provided preliminary evidence for a significant association between hippocampal surface contractions and memory performance among patients with late-onset but not early-onset depression. Identifying regional abnormalities in hippocampal structure may thus help dissociate possible neuroanatomic vulnerabilities to illness progression based on age at onset, thereby offering potentially new perspectives for defining appropriate interventions.
Acknowledgments
Supported by NIH through research grants (R01-MH-63674, MH-55115, MH-61567, MH-02043) to Dr. Kumar, the Career Development Award (K01 MH073990) to Dr. Narr, the NIH Roadmap for Medical Research (U54 RR021813) titled Center for Computational Biology, and NIH/NLM resource grant (P41 RR013642) to Dr. Toga. Additional support was provided by the General Clinical Research Center, awarded to the UCLA Medical Center.
Dr. Kumar has been a consultant for Bristol-Myers Squibb. The remaining authors report no competing interests.
References
- 1.Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 2.Bell-McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF, III, Becker JT. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159:1424–1427. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd AJ, Ferrier IN, Barber R, Gholkar A, Young AH, O’Brien JT. Hippocampal volume change in depression: late- and early-onset compared. Br J Psychiatry. 2004;184:488–495. doi: 10.1192/bjp.184.6.488. [DOI] [PubMed] [Google Scholar]
- 5.Hickie I, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, Wilhelm K, Parker G. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- 6.Pantel J, O’Leary DS, Cretsinger K, Bockholt HJ, Keefe H, Magnotta VA, Andreasen NC. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus. 2000;10:752–758. doi: 10.1002/1098-1063(2000)10:6<752::AID-HIPO1012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 7.Ashtari M, Greenwald BS, Kramer-Ginsberg J, Hu H, Wu M, Patel P, Pollack S. Hippocampal/amygdala volumes in geriatric depression. Psychol Med. 1999;29:629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- 8.Taylor WD, Steffens DC, Payne ME, MacFall JR, Marchuk DA, Svenson IK, Krishnan RR. Influence of serotonin transporter promoter region polymorphisms on hippocampal volumes in late-life depression. Arch Gen Psychiatry. 2005;62:537–544. doi: 10.1001/archpsyc.62.5.537. [DOI] [PubMed] [Google Scholar]
- 9.Lyness JM, Pearson JL, Lebowitz BD, Kupfer DJ. Age at onset of late-life depression. Am J Geriatr Psychiatry. 1994;2:4–8. doi: 10.1097/00019442-199400210-00002. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulos GS, Schultz SK, Lebowitz BD. Late-life depression: a model for medical classification. Biol Psychiatry. 2005;58:283–289. doi: 10.1016/j.biopsych.2005.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salloway S, Malloy P, Kohn R, Gillard E, Duffy J, Rogg J. MRI and neuropsychological deficits in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- 13.Schweitzer I, Tickwell V, O’Brien J, Ames D. Is late onset depression a prodrome of dementia? Int J Geriatr Psychiatry. 2002;17:997–1005. doi: 10.1002/gps.525. [DOI] [PubMed] [Google Scholar]
- 14.Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Boeve BF, Ivnik RJ, Petersen RC, Shane Pankratz V, Rocca WA. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment. Arch Gen Psychiatry. 2006;63:435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 15.Devanand DP, Sano M, Tang MX, Taylor S, Gurland BJ, Wilder D, Stern Y, Mayeux R. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 16.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapp MA, Schnaider-Beeri M, Grossman HT, Sano M, Perl DP, Puohit DP, Gorman JM, Haroutunian V. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63:161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 18.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent depression. J Neuroscience. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whyte EM, Pollock BG, Wagner WR, Mulsant BH, Ferrell RE, Mazumdar S, Reynolds CF., III Influence of the serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158:2074–2076. doi: 10.1176/appi.ajp.158.12.2074. [DOI] [PubMed] [Google Scholar]
- 20.Frodl T, Meisenzahl EM, Zill P, Baghai T, Rujescu D, Leinsinger G, Bottlender R, Schule C, Zwanzger P, Engel RR, Ruprecht R, Bondy B, Reiser M, Moller HJ. Reduced hippocampal volume associated with the long variant of the serotonin transporter polymorphism in major depression. Arch Gen Psychiatry. 2004;61:177–183. doi: 10.1001/archpsyc.61.2.177. [DOI] [PubMed] [Google Scholar]
- 21.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 22.Posener JA, Wang L, Price JL, Gado MH, Province MA, Miller MI, Babb CM, Csernansky JG. High-dimensional mapping of the hippocampus in depression. Am J Psychiatry. 2003;160:83–89. doi: 10.1176/appi.ajp.160.1.83. [DOI] [PubMed] [Google Scholar]
- 23.Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Thompson PM, Hayashi KM, De Zubicaray GL, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer’s disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 25.Alexopoulos GS. In: Clinical and biological findings in late-onset depression, in American Psychiatric Press Review Psychiatry. Tasman A, Goldfinger SM, Kaufmann CA, editors. Washington, DC: American Psychiatric Press; 1990. pp. 249–262. [Google Scholar]
- 26.Ballmaier M, Kumar A, Thompson PM, Narr KL, Lavretsky H, Estanol L, DeLuca H, Toga AW. Localizing gray matter deficits in late-onset depression using cortical pattern matching methods. Am J Psychiatry. 2004;161:2091–2099. doi: 10.1176/appi.ajp.161.11.2091. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental-State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Delis DC, Kramer J, Kaplan E, Ober BA. California Verbal Learning Test (CVLT) Manual. San Antonio, Tex: Psychological Corporation; 1987. [Google Scholar]
- 30.Corwin J, Bylsma FW. Translations of excerpts from Andre Rey’s psychological examination of traumatic encephalopathy and PA Osterrieth’s the Complex Figure Copy Test. Clin Neuropsychol. 1993;7:3–15. [Google Scholar]
- 31.Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- 32.Ballmaier M, Sowell ER, Thompson PM, Kumar A, Narr KL, Lavretsky H, Welcome SE, DeLuca H, Toga AW. Mapping brain size and cortical gray matter changes in elderly depression. Biol Psychiatry. 2004;55:382–389. doi: 10.1016/j.biopsych.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Sled JG, Pike GB. Standing-wave and RF penetration artifacts caused by elliptic geometry: an electrodynamic analysis of MRI. IEEE Trans Med Imaging. 1998;17:653–662. doi: 10.1109/42.730409. [DOI] [PubMed] [Google Scholar]
- 34.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) NeuroImage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- 35.Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration, II: intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 36.Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson AW. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63:693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 37.Rosoklija G, Toomayan G, Ellis SP, Mann JJ, Latov N, Hays AP, Dwork AJ. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 38.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HBM, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2005;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duvernoy HM, Bougouin P. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections With MRI, second completely revised and expanded ed. New York: Springer; 1998. [Google Scholar]
- 40.Lyness JM. The cerebrovascular model of depression in late life. CNS Spectr. 2002;7:712–715. doi: 10.1017/s1092852900008695. [DOI] [PubMed] [Google Scholar]
- 41.Herman JP, Schafer MKH, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypthalamo-pituitary-adrenocortical area. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 43.Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- 44.Sheline YI, Mittler BL, Mintun MA. The hippocampus and depression. Eur Psychiatry. 2002;17:300–305. doi: 10.1016/s0924-9338(02)00655-7. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, Cernansky JG. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. NeuroImage. 2003;20:667–682. doi: 10.1016/S1053-8119(03)00361-6. [DOI] [PubMed] [Google Scholar]