Abstract
To investigate the relationship of HIF1α signaling to oxidative stress, tissue hypoxia, angiogenesis and inflammation, female Fischer 344 rats were irradiated to the right hemithorax with a fractionated dose of 40 Gy (8 Gy × 5 days). The lung tissues were harvested before and at 4, 6, 10, 14, 18, 22 and 26 weeks after irradiation for serial studies of biological markers, including markers for hypoxia (HIF1α, pimonidazole and CA IX), oxidative stress (8-OHdG), and angiogenesis/capillary proliferation (VEGF/CD 105), as well as macrophage activation (ED-1) and cell signaling/fibrosis (NFκB, TGFβ1), using immunohistochemistry and Western blot analysis. HIF1α staining could be observed as early as 4 weeks postirradiation and was significantly increased with time after irradiation. Importantly, HIF1α levels paralleled oxidative stress (8-OHdG), tissue hypoxia (pimonidazole and CA IX), and macrophage accumulation consistent with inflammatory response. Moreover, changes in HIF1α expression identified by immunohistochemistry assay parallel the changes in TGFβ1, VEGF, NFκB and CD 105 levels in irradiated lungs. These results support the notion that oxidative stress and tissue hypoxia might serve as triggering signals for HIF1α activity in irradiated lungs, relating to radiation-induced inflammation, angiogenesis and fibrosis.
INTRODUCTION
The lung is inevitably exposed to radiation during treatment for many tumors in the thoracic region, and radiation-induced lung toxicity remains a critical limiting factor for escalating radiation doses to optimally treat tumors (1). Although the radiation response of the lung has been studied thoroughly in animals and humans, the exact processes leading to late radiation damage are not completely understood.
The prevalent view in the current literature regarding radiation-induced normal tissue injury is that ionizing radiation triggers a cascade of molecular events that begins immediately and continues to promote tissue damage long after the normal tissue was irradiated (2). These molecular events not only are initiated by the generation of reactive oxygen species but also are perpetuated by their continuous production and involvement in direct cellular damage and indirect complex cellular signaling (2, 3). These processes may overpower cellular antioxidant defenses and increase oxidative burden, which perpetuates radiation injury (4–6). The importance of endothelial cell damage as a major contributor to normal tissue injury after irradiation has been investigated (7, 8). Early hypoperfusion due to radiation-induced vascular changes and escalated oxygen consumption, a consequence of increased cellular metabolism, has been ascribed to the generation of tissue hypoxia, which further exacerbates injury (5). Later this response is followed by prominent macrophage infiltration and the production of cytokines and additional reactive species (4, 5, 9). In this manner, hypoxia may continuously amplify a non-healing wound response, characterized by fibrogenesis through TGFβ1 activity and angiogenesis through VEGF production (5, 9).
HIF1α has been described as the major regulator of tissue oxygen homeostasis (10). Elegant studies have shown that hypoxia may not be the only influence on HIF1 activity; conversely, oxidative stress might play an important role in HIF1 activity (11, 12). Recently, we documented that in irradiated lungs, HIF1α activity is associated with increased hypoxia and oxidative stress (9); however, the role of HIF1α in the time course of radiation-induced lung injury is not clearly defined. Identification of molecular pathways in radiation-induced lung injury might provide more effective therapeutic targets to prevent the development of pulmonary damage and result in better therapeutic outcome.
Here we hypothesized that a fractionated radiation regimen in our well-established rodent model of lung injury would lead to activation of the HIF1α pathway. The goal of this study was to determine HIF1α activity after fractionated irradiation and to show how it was related to oxidative stress, tissue hypoxia, angiogenesis and inflammation and their relationships to radiation-induced lung injury.
MATERIAL AND METHODS
Animals
Experiments were performed using 80 female Fischer 344 rats with prior approval from the Duke University Institutional Animal Care and Use Committee. The animals were housed three per cage and were maintained under identical standard laboratory conditions. Food and water were provided ad libitum. Serial studies were performed before and at 4, 6, 10, 14, 18, 22 and 26 weeks after irradiation.
Irradiation
At the time of irradiation all rats weighed between 160 and 170 g to minimize possible variations in lung size. The animals were anesthetized before irradiation with intraperitoneal injection of ketamine (65 mg/kg) and xylazine (4.5 mg/kg) and were placed in a prone position. Hemithoracic radiation was delivered to the right lung with a fractionated dose of 40 Gy (8 Gy × 5 days) at a dose rate of 0.71 Gy/min using 150 kV X rays (Therapax 320, Pantak Inc., East Haven, CT). Lead blocks (12 mm) were used to protect the left thorax and the rest of the body.
Histology
Ten rats each were killed humanely with an overdose of pentobarbital before and at 4, 6, 10, 14, 18, 22, and 26 weeks after irradiation for histopathology and immunohistochemistry. Lungs of five animals from each time were infused by tracheal instillation of a solution containing 10% neutral-buffered formalin, 2% glutaraldehyde, and 0.085 M sodium cacodylate buffer for 25 min for fixation prior to removal of the lung. After removal, the lungs were preserved in 10% formalin for 24 h, and then the different right lung lobes were separated and embedded in paraffin. Then the tissue was cut into 5μm-thick sections with a microtome, stored on slides and stained. Lungs of the remaining five animals per time were snap frozen in liquid nitrogen and then stored at −80°C for Western blot analysis.
Pimonidazole (Hypoxia) Determination
Five animals at each time were administered pimonidazole hydrochloride (Hydroxyprobe-1; Chemicon International Inc., Temecula, CA) at 70 mg/kg (i.p.) 3 h before killing. The tissues were harvested for immunohistochemistry as described previously (13).
Immunohistochemistry
Immunohistochemistry was performed as described elsewhere (5, 13). Briefly, the tissue sections (lower lobes of right lung), fixed on slides, were deparaffinized on a heating pad and rehydrated with xylene and alcohol concentrations between 100% and 80%. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min. The slides were then placed in a citrate buffer solution (Biogenex, San Ramon, CA) and heated in a microwave for 10 min for antigen retrieval. The tissue sections were then blocked in 10% donkey serum to reduce background. Next the tissue sections were rinsed with phosphate-buffered saline solution and incubated overnight at 4°C with primary antibodies to HIF1α (1:100, mouse monoclonal, Novus Biological), pimonidazole (anti-rabbit, gift from Dr. J. A. Raleigh, UNC, Chapel Hill, NC), NFκB (p65) (1:500, rabbit polyclonal, Abcam Inc., Cambridge, MA), macrophage marker ED1 (1:100, Serotec, Oxford, UK), CA IX (1:2000, rabbit polyclonal, Abcam Inc., Cambridge, MA), 8-OHdG (8-hydroxydeoxyguanine, mouse monoclonal, 1:2000, JaICA, Shizuoka, Japan), VEGF (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA), CD 105 (Dako Cytomation, Carpinteria, CA), and active TGFβ1 (1:200, Santa Cruz Biotechnology). Slides were then washed three times in phosphate-buffered saline solution for 5 min followed by the incubation with the appropriate secondary antibody (1:200, Jackson ImmunoResearch, West Grove, PA) for 30 min at room temperature. Again slides were washed three times in phosphate-buffered saline for 5 min each followed by incubation with ABC-Elite (Vector Laboratories, Burlingame, CA) for 30 min at room temperature and developed using DAB working solution (Laboratory Vision, Fremont, CA). Finally, the slides were counterstained with Harris hematoxylin (Fisher Scientific, Pittsburgh, PA) and mounted with cover slips.
Semi-quantification of Immunohistochemistry
Image analysis was carried out as described previously (5). Briefly, slides were systematically scanned at a lower magnification to define the lung injury at a specific time by evaluating H&E-stained slides along with immunohistochemistry-stained consecutive slides. Eight to 10 representative digital images were acquired from each slide using a 40× objective. Activated macrophage marker ED1, HIF1α, NFκB and 8-OHdG staining were quantified as positively stained cells or nuclei per high-power field. Results were expressed as the percentage of positively stained cells or nuclei to total cells. CD 105 expression was quantified as positively stained endothelial cells per high-power field and expressed as the proliferating capillary index (PCI).
Staining for pimonidazole, CA IX, active TGFβ and VEGF was analyzed in Adobe Photoshop (Version 7.0; Adobe Systems, San Jose, CA). After the positive expression of staining per digitized image was quantified, the total tissue area regardless of expression was quantified. The percentage of positively stained pixels was determined by the ratio of positive staining over the total tissue area per digital image acquired at low magnification. The results represent the average percentage of positive-staining pixels from 8–10 digital images per animal and five animals per time.
Western Blot
Snap-frozen irradiated and control lung tissues were extracted with T-PER Tissue Protein Extraction kit (Pierce, Rockford, IL). Briefly, 0.1 g of tissue was homogenized in 2 ml T-PER reagent containing proteinase inhibitors (Roche Diagnostics, Mannheim, Germany) for 15 min and then centrifuged at 10,000g for 5 min to remove tissue debris. Supernatants were collected and concentrations were determined by the DC protein assay (Bio-Rad Lab, Hercules, CA). For Western blotting, 40 μg of total protein was separated using 4–20% SDS-PAGE gels, transferred onto a polyvinylidene fluoride membrane (PVDF), and probed with specific antibodies against NFκB p50, HIF1α and CA IX. Images were acquired with an ECL chemiluminescence kit (Amersham, Arlington Heights, IL). Western blot data were quantified as described (14).
Statistical Analysis
All data are presented as means ± SEM for each parameter. Times for each parameter were compared to the preirradiation controls by t tests. All P values reported were two-sided, and statistical significance was defined as P < 0.05.
RESULTS
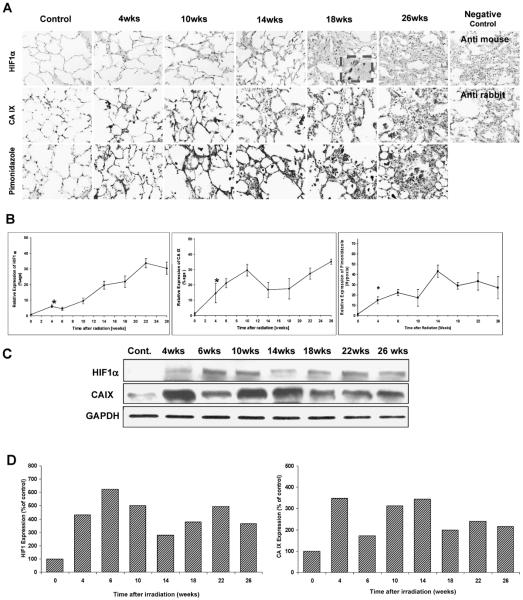
Irradiated lungs exhibited strong HIF1α staining, that was localized mainly to damaged areas and inflammatory cells (Fig. 1A). A significant increase in HIF1α staining was observed 4 weeks after irradiation compared to controls (P < 0.001), and it increased progressively with time after irradiation (Fig. 1B). Western blot analysis basically confirmed the increase in HIF1α expression observed in the immunochemistry assay with small discrepancies in levels at some times (Fig. 1C, D).
FIG. 1.
Panel A: HIF1α, CA IX and pimonidazole. Irradiated lungs exhibited strong HIF1α and CA IX staining localized mainly in damaged areas and inflammatory cells. The extent and intensity of staining increased with time. Original magnification 400×. Panel B: Semi-quantitative analysis of HIF1α, CA IX and pimonidazole: A significant increase in HIF1α-positive staining (P < 0.001) and CA IX (P = 0.05) expression was observed at 4 weeks compared to the control. The levels of HIF1α and CA IX remained significantly elevated after irradiation. Error bars represent ± SEM. Panel C: Western blots of HIF1α and CA IX. Western blot analysis also confirmed the radiation-induced HIF1α and CA IX expression over the observation period. Panel D: Densitometric readings (HIF1α/GAPDH and CA IX/GAPDH) of the Western blot expressed as percentage of control (control set to 100%).
Tissue hypoxia is associated with increased expression of CA IX. A significant increase in CA IX-positive staining was observed at 4 weeks after irradiation compared to the control group (P = 0.05) (Fig. 1A and B). CA IX staining increased further up to 10 weeks after irradiation and decreased slightly thereafter (10 weeks compared to 14 weeks: P < 0.05) but remained significantly increased compared to control animals (P = 0.017). From 18 weeks after irradiation to the end of the observation period, CA IX expression rose again. Western blot analysis basically confirmed the increase in CA IX expression observed in the immunochemistry assay with small discrepancies at some times (Fig. 1C, D).
Staining for the exogenous hypoxia marker pimonidazole revealed little immunoreactivity in control lung tissue (Fig. 1A, B). However, pimonidazole staining at 4 weeks after irradiation showed significantly higher levels of hypoxia in irradiated lungs that remained elevated during the follow-up period.
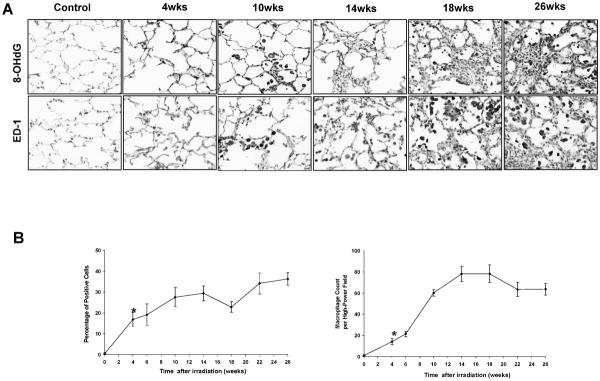
8-OHdG is a marker of oxidative stress. 8-OHdG-positive nuclear staining was significantly higher at 4 weeks after irradiation compared to control levels (P = 0.002) and increased progressively with time (Fig. 2A and B). ED1, a marker of activated macrophages, increased in irradiated lung under the inflammatory response (Fig. 2A). The number of macrophages increased significantly at 4 weeks after irradiation (4 weeks compared to controls: P = 0.001) and remained elevated throughout the follow-up period (Fig. 2B).
FIG. 2.
Panel A: Oxidative stress (8-OHdG) and inflammation (ED-1). Oxidative stress is associated with increased expression of 8-OHdG in irradiated lung tissue, which was increased with time after irradiation. There was an increase in the numbers of both total and activated macrophages detected in the right lung with time after irradiation. Original magnification 400×. Panel B: Semi-quantitative analysis of 8-OHdG and ED-1. 8-OHdG-positive staining was found at significantly elevated levels 4 weeks after irradiation (P = 0.002) and increased with time. The number of macrophages increased significantly at 4 weeks after irradiation (P = 0.001) and remained elevated throughout the follow-up period. Error bars represent ± SEM.
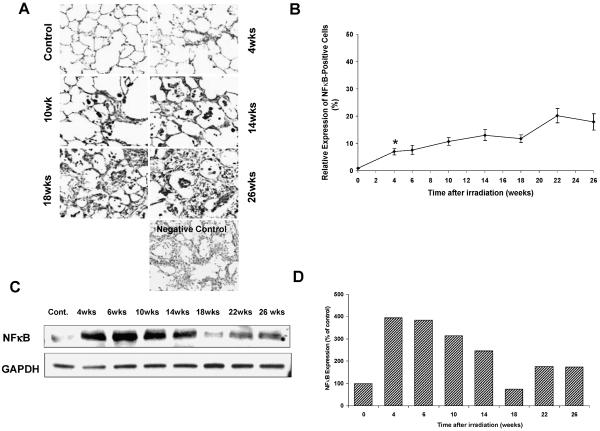
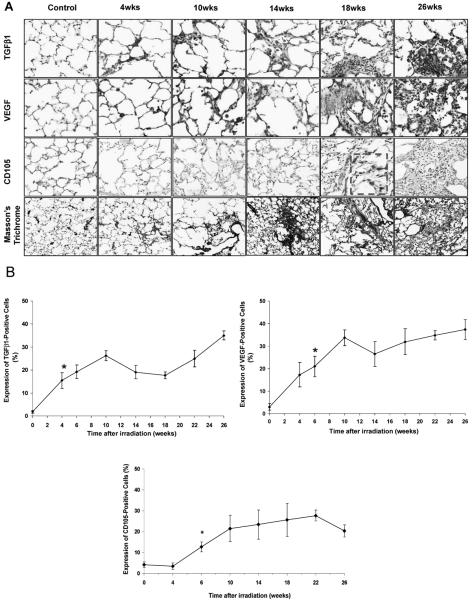
NFκB expression also increased at 4 weeks after irradiation compared to the controls (P < 0.001) (Fig. 3A and B). Western blot analysis for NFκB also revealed a positive expression, but NFκB did not increase with time after irradiation (Fig. 3C, D). As expected, the expression of TGFβ1 activity increased at 4 weeks after irradiation (4 weeks compared to control: P = 0.006) and increased continuously over time (Fig. 4A, B). TGFβ1-positive staining was found mainly in macrophages and damaged focal areas in the lung.
FIG. 3.
Panel A: NFκB expression increased starting at 4 weeks after fractionated irradiation of the right lung. Original magnification 400×. Panel B: Semi-quantitative analysis of NFκB. NFκB expression also increased 4 weeks after irradiation compared to the controls (P < 0.001) (panels A and B). It increased further with time. Error bars represent ± SEM. Panel C: Western blot showing NFκB expression. Western blot analysis for NFκB also showed the positive expression but did not increase with time after irradiation. Panel D: Densitometric readings (NFκB/GAPDH) of the Western blot expressed as percentage of control (control set to 100%).
FIG. 4.
Panel A: TGFβ1, VEGF and CD 105 expression and collagen deposition (Masson's trichrome). TGFβ1 and VEGF had similar expression patterns, with strongly stained signals mainly in the macrophages and damaged foci. Starting from 10 to 14 weeks, collagen deposition was prominent and increased with time. Panel B: Semi-quantitative analysis of TGFβ1, CD 105 and VEGF. The expression of active TGFβ1 increased 4 weeks after irradiation (P = 0.006) and continued to increase over time. VEGF levels also showed a significant increase 6 weeks after irradiation compared to the unirradiated controls (P = 0.007). VEGF levels remain elevated throughout the 26-week observation period. Expression of CD 105 after irradiation increased significantly at 6 weeks and was associated with the formation of apparently nonfunctional blood vessels. Error bars represent ± SEM.
VEGF was elevated after irradiation as well, showing a significant increase at 6 weeks compared to the controls (P = 0.007) (Fig. 4A, B). VEGF expression increased further up to 10 weeks after irradiation, and its levels remained elevated during the entire observation period. The proliferating capillaries index (PCI) showed a significant increase 6 weeks after irradiation compared to the controls (P = 0.003) (Fig. 4A, B). The PCI increased further up to 22 weeks after irradiation and remained elevated during the follow-up period.
The histological images demonstrated the typical radiation-induced lesions in the lung in respect to time after irradiation. The H&E-stained lung sections of irradiated animals showed the deterioration of lung tissue with time (data not shown). Masson's trichrome staining indicated collagen deposition starting from 10 to 14 weeks that increased with time and eventually obliterated the alveoli at the later times (Fig. 4A).
DISCUSSION
The results of the current study indicated HIF1α activation in rat lung after fractionated irradiation. This up-regulation was observed consistently during the complete study period of 26 weeks after irradiation. Increased oxidative stress coincided with tissue hypoxia in immunohistochemistry analysis. Inflammation and expression of TGFβ1, VEGF, CD 105 and NFκB also occurred at elevated levels after radiation exposure throughout the follow-up period.
The current results suggest that sustained chronic oxidative stress, a mediator through which radiation stabilizes HIF1α, may be associated with increased HIF1α expression in irradiated lung (11, 15, 16). When Mn porphyrin-based catalytic antioxidant treatment was delivered during and after fractionated radiotherapy of the lung, significant decreases were observed in oxidative stress as well as HIF1α and its regulated proteins (17). Such effects may be the consequence of the antioxidant's ability to remove reactive species and/or the direct interaction with signaling proteins (18–21). In agreement with those studies, the current data also support the conclusion that radiation not only induces HIF1α activity but also produces reactive species that might serve as regulators of HIF1α activity in lung injury.
Recent work suggested that an imbalance between oxygen demand and supply can occur early in irradiated and diseased normal tissue before any histological evidence of injury is detected (5, 22). Our previous studies demonstrated a significant decrease in lung perfusion with the occurrence of tissue hypoxia (CA IX, an endogenous marker of hypoxia) that intensified further over time after irradiation (5). In the present study, we used pimonidazole and CA IX to evaluate tissue hypoxia in irradiated lung at different times after fractionated irradiation. The levels of pimonidazole and CA IX expression were significantly up-regulated throughout the observation period. Collectively, our studies suggest an important role of tissue hypoxia in radiation-induced lung injury (5, 13).
Different mechanisms have been used to explain how radiation exposure leads to the development of normal tissue hypoxia. They may share a common landmark: HIF1α induction through radiation-induced nonhypoxic or hypoxic HIF1α pathways. In nonhypoxic activation of HIF1α signaling, free radicals directly or indirectly regulate the stabilization, translocation and activation of HIF1 via cytokines in an ROS-dependent mechanism (11, 23). In addition to setting off this oxidative pathway and cytokine activity, acute ischemia (low perfusion) also affects the translational machinery in the irradiated tissue, contributing further to the radiation-induced activation of the HIF1 pathway (5, 9). In a recent study, we hypothesized that one of the mechanisms involved in the development of radiation-induced lung hypoxia is the direct damage to the vasculature that resulted in reduced tissue perfusion (5). The observation in neuron-specific knockout mice indicated that HIF1 deficiency could protect mice from cerebral ischemic injury (24). Thus it appears that oxidative stress and hypoxia play important roles in controlling cell signaling pathways and in regulating the activity of important transcription factors involved in radiation-induced lung injury (5, 9).
The levels of TGFβ1 continued to increase throughout the study (Fig. 4), which was the same expression pattern observed for tissue hypoxia (Fig. 1). This confirmed our previous findings that hypoxia is found in reactive tissue areas characterized by TGFβ1 expression and collagen deposition (5, 13, 17). Moreover, TGFβ1 and hypoxia have been shown to act synergistically with regard to various types of collagen production (25, 26). Different genes that play important roles in fibrogenic response are direct HIF1α targets, such as TIMP-1, PAI-1 and CTGF (27–29). Thus increased HIF1α expression is most likely to play an important role in the pathogenesis of irradiated tissue through direct transcriptional regulation of specific profibrotic genes and TGFβ1 signaling.
As expected, the number of activated macrophages also increased significantly during the follow-up period of 26 weeks after fractionated irradiation. Similar inflammatory response in lung was seen in our rat model of single doses of 28 Gy (5). It was thought that tissue hypoxia might influence the pathogenesis of radiation-induced lung disease through the regulation of inflammatory responses (5, 13, 17, 30), affecting inflammatory cell recruitment (31) and function (32). Thus HIF1α signaling in inflammatory cells may also play a significant role in irradiated lung.
Previous studies indicated that radiation is also associated with NFκB activity, which in turn induces a number of pro-inflammatory, apoptotic and oncogenic genes that collectively function to foster cellular adaptation to stress (33–36). Here we also demonstrated for the first time that levels of NFκB, a redox-sensitive transcription factor, were significantly elevated after fractionated irradiation, during the period when oxidative stress and hypoxia were already present in irradiated lung.
The current results are not conclusive in describing the time-related events involved in fractionated radiation-induced lung injury. Follow-up studies are in progress to explore the HIF1α signaling, along with tissue hypoxia, oxidative stress and cytokines, prior to 4 weeks. Although Western blot analyses basically confirmed the findings in immunochemistry assay, there was a small discrepancy in the expression of HIF1α, CA IX and NFκB at some times. For example, the immunohistochemistry analysis showed an increase in expression over time, whereas Western blot analysis only confirmed the increases in these proteins at each time after irradiation but did not show a time-dependent change. This difference can be explained by the differences in experimental methodology and data analysis. For instance, the immunohistochemistry assay used the whole lower lobe of the irradiated right lung, which revealed a focal and nonrandom distribution of obvious changes in lung architecture and radiation-induced damage. These focally damaged areas correlated with strong expression of tested markers. For analysis of expression, representative areas with the most severe damage were assessed. Thus the time-dependent changes shown in the immunohistochemistry assay reflect the progressive damage in focal areas. For Western blot analysis, a random and relatively small piece of the lower lobe from the irradiated right lung and of the nonirradiated right lung of control animals was excised. Thus severely damaged areas might have been missed during tissue sampling. The ratio of damage tissue to normal tissue in the sample would cause different outcomes when molecular markers were tested with this protocol. Therefore, it is important to optimize the tissue sampling, and the exact same area should be used for both the immunohistochemistry and Western blot assays.
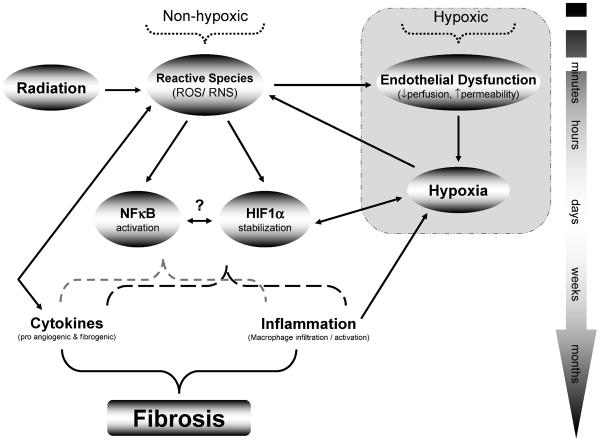
In summary, the current data show a time-related similarity in the levels of oxidative stress, tissue hypoxia, angiogenesis and inflammation. Compared with our previous findings, the current observation showed similarity in the temporal expressions but not in the extent of these markers (5). The data provide further insight into the role of HIF1α in the pathogenesis of radiation-induced lung injury. Most importantly, HIF1α expression was found early in the pathogenesis of the injury, at a time when no clear histological evidence of injury was present. This suggests that HIF1α activation can represent an early event and a potential pro-fibrotic stimulus. Radiation-induced lung damage is not confined to a single event but involves complex signaling between different pathways, enhancing and maintaining the processes that lead to lung damage (Fig. 5). However, whether radiation-induced HIF1α expression in lung tissue is a consequence of tissue hypoxia or a result of oxidative stress or both remains to be determined.
FIG. 5.
Possible mechanisms of development of normal tissue hypoxia after irradiation. In nonhypoxic activation of the HIF1α pathway, oxidative stress has been implicated in irradiated tissue (11). Recent studies showed that HIF1α can be stabilized by exposure to radiation, and triggering signals were identified to be reactive species (11, 15). In addition to setting off this oxidative pathway, tissue ischemia/hypoxia also contributes to the radiation-induced activation of the HIF1α pathway. Moreover, recent work suggested that disturbance between oxygen demand and supply can even occur early in irradiated normal tissue before visible scarring is detected (5). HIF1α signaling could promote the development of tissue damage by exerting a direct influence on profibrotic gene expression, inflammation, NFκB and cytokine activity.
ACKNOWLEDGMENTS
We would like to thank Liguang Chen, M.D., Ph.D. and Susan Poulton for providing technical assistance and suggestions. Jing Mi and Yu Zhang contributed equally to this work. This study was supported by the National Institutes of Health Grant number R01 CA 098452 (Zeljko Vujaskovic).
REFERENCES
- 1.Marks LB, Yu X, Vujaskovic Z, Small W, Jr., Folz R, Anscher MS. Radiation-induced lung injury. Semin. Radiat. Oncol. 2003;13:333–345. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 2.Anscher MS, Chen L, Rabbani Z, Kang S, Larrier N, Huang H, Samulski TV, Dewhirst MW, Brizel DM, Vujaskovic Z. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2005;62:255–259. doi: 10.1016/j.ijrobp.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Riley PA. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Oncol. Biol. Phys. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 4.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS, Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:1056–1066. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 5.Fleckenstein K, Zgonjanin L, Chen L, Rabbani Z, Jackson IL, Thrasher B, Kirkpatrick J, Foster WM, Vujaskovic Z. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, Vujaskovic Z. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int. J. Radiat. Oncol. Biol. Phys. 2007;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 8.Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000;60:321–327. [PubMed] [Google Scholar]
- 9.Rabbani ZN, Salahuddin FK, Yarmolenko P, Batinic-Haberle I, Thrasher BA, Gauter-Fleckenstein B, Dewhirst MW, Anscher MS, Vujaskovic Z. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic. Res. 2007;41:1273–1282. doi: 10.1080/10715760701689550. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 11.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 12.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 13.Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS, Dewhirst MW, Haroon ZA. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int. J. Radiat. Oncol. Biol. Phys. 2001;50:851–855. doi: 10.1016/s0360-3016(01)01593-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Lee TH, Davidson C, Lazarus C, Wetsel WC, Ellinwood EH. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology. 2007;32:377–387. doi: 10.1038/sj.npp.1301101. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1alpha stability through S-nitrosylation. Mol. Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, Dewhirst MW. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Rabbani ZN, Salahuddin FK, Yarmolenko P, Batinic-Haberle I, Thrasher B, Gauter-Fleckenstein B, Anscher MS, Dewhirst MW, Vujaskovic Z. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic. Res. 2007;41:1273–1282. doi: 10.1080/10715760701689550. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, St. Clair D, St. Clair W, Chaiswing L, Oberley TD, Batinic-Haberle I, Epstein CJ. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 19.Beckman JS. –OONO: rebounding from nitric oxide. Circ. Res. 2001;89:295–297. [PubMed] [Google Scholar]
- 20.Sheng H, Enghild JJ, Bowler R, Patel M, Batinic-Haberle I, Calvi CL, Day BJ, Pearlstein RD, Crapo JD, Warner DS. Effects of metalloporphyrin catalytic antioxidants in experimental brain ischemia. Free Radic. Biol. Med. 2002;33:947–961. doi: 10.1016/s0891-5849(02)00979-6. [DOI] [PubMed] [Google Scholar]
- 21.Smith KR, Uyeminami DL, Kodavanti UP, Crapo JD, Chang LY, Pinkerton KE. Inhibition of tobacco smoke-induced lung inflammation by a catalytic antioxidant. Free Radic. Biol. Med. 2002;33:1106–1114. doi: 10.1016/s0891-5849(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto M, Tanaka T, Yamamoto T, Noiri E, Miyata T, Inagi R, Fujita T, Nangaku M. Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J. Am. Soc. Nephrol. 2004;15:1574–1581. doi: 10.1097/01.asn.0000128047.13396.48. [DOI] [PubMed] [Google Scholar]
- 23.Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002;14:879–897. doi: 10.1016/s0898-6568(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 24.Helton R, Cui J, Scheel JR, Ellison JA, Ames C, Gibson C, Blouw B, Ouyang L, Dragatsis I, Barlow C. Brain-specific knock-out of hypoxia-inducible factor-1α reduces rather than increases hypoxic-ischemic damage. J. Neurosci. 2005;25:4099–4107. doi: 10.1523/JNEUROSCI.4555-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falanga V, Zhou L, Yufit T. Low oxygen tension stimulates collagen synthesis and COL1A1 transcription through the action of TGF-b1. J. Cell. Physiol. 2002;191:42–50. doi: 10.1002/jcp.10065. [DOI] [PubMed] [Google Scholar]
- 26.Saed GM, Zhang W, Chegini N, Holmdahl L, Diamond MP. Alteration of type I and III collagen expression in human peritoneal mesothelial cells in response to hypoxia and transforming growth factor-b1. Wound Rep. Regen. 1999;7:504–510. doi: 10.1046/j.1524-475x.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 27.Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 28.Kietzmann T, Roth U, Jungermann K. Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxiainducible factor-1 in rat hepatocytes. Blood. 1999;94:4177–4185. [PubMed] [Google Scholar]
- 29.Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am. J. Physiol. Renal Physiol. 2004;287 doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 30.Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, Chen L, Golson ML, Samulski TS, Dewhirst MW, Vujaskovic Z. Overexpression of extracellular superoxide dismutase reduces acute radiation induced lung toxicity. BMC Cancer. 2005;5:59. doi: 10.1186/1471-2407-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong T, Eltzschig HK, Karhausen J, Colgan SP, Shelley CS. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of integrin gene expression. Proc. Natl. Acad. Sci. USA. 2004;101:10440–10445. doi: 10.1073/pnas.0401339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Johnson RS. HIF-1a is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohan N, Meltz ML. Induction of nuclear factor kappa B after low-dose ionizing radiation involves a reactive oxygen intermediate signaling pathway. Radiat. Res. 1994;140:97–104. [PubMed] [Google Scholar]
- 34.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 35.Prasad AV, Mohan N, Chandrasekar B, Meltz ML. Activation of nuclear factor kappa B in human lymphoblastoid cells by low-dose ionizing radiation. Radiat. Res. 1994;138:367–372. [PubMed] [Google Scholar]
- 36.Sun Z, Andersson R. NF-kappaB activation and inhibition: a review. Shock. 2002;18:99–106. doi: 10.1097/00024382-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Haddad JJ, Safieh-Garabedian B, Saade NE, Kanaan SA, Land SC. Chemioxyexcitation (delta pO2/ROS)-dependent release of IL-1 beta, IL-6 and TNF-alpha: evidence of cytokines as oxygen-sensitive mediators in the alveolar epithelium. Cytokine. 2001;13:138–147. doi: 10.1006/cyto.2000.0789. [DOI] [PubMed] [Google Scholar]
- 38.Haddad JJ, Safieh-Garabedian B, Saade NE, Land SC. Thiol regulation of pro-inflammatory cytokines reveals a novel immunopharmacological potential of glutathione in the alveolar epithelium. J. Pharmacol. Exp. Ther. 2001;296:996–1005. [PubMed] [Google Scholar]
- 39.Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am. J. Physiol. 1999;277:L1067–1088. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- 40.Rahman I, Skwarska E, Henry M, Davis M, O'Connor CM, FitzGerald MX, Greening A, MacNee W. Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis. Free Radic. Biol. Med. 1999;27:60–68. doi: 10.1016/s0891-5849(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 41.Rahman I, Swarska E, Henry M, Stolk J, MacNee W. Is there any relationship between plasma antioxidant capacity and lung function in smokers and in patients with chronic obstructive pulmonary disease? Thorax. 2000;55:189–193. doi: 10.1136/thorax.55.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]