Abstract
Phosphatidylinositol-3-phosphate kinase (PI3K) has been reported to exhibit anti-inflammatory roles as a negative modulator of the NF-κB pathway (MyD88- and Mal-dependent) triggered upon Toll-like receptor (TLR)4 activation by lipopolysaccharide (LPS). Here, we investigated the role of PI3K on the TLR4-dependent, MyD88-independent signaling cascade which is activated in macrophages infected by Vesicular Stomatitis Virus (VSV) and leads to interferon production, thus conferring antiviral protection. We show that VSV induces TLR4 (and CD14)-dependent Akt phosphorylation. We observed hypersusceptibility to viral infections after pharmacological inactivation of the PI3K pathway in macrophages, which indicates that normal PI3K functions are critical for type I interferon synthesis and viral resistance. Conversely, we noticed increased resistance in macrophages isolated from genetically modified mice in which the PI3K pathway is constitutively active. Our data, which demonstrate that PI3K-Akt axis is an important component of the TLR4-dependent antiviral mechanism, also indicate that pharmacological modulation of this pathway to regulate the inflammatory response could promote viral susceptibility.
Keywords: TLR, PI3K, VSV, Signal transduction
1. Introduction
It is well established that Toll-like receptors (TLRs) are able to discriminate a variety of pathogens and constitute essential sentinels of the innate immune system (reviewed in Beutler et al., 2006). Among this family, TLR4 has been extensively studied. Binding of its prototypic ligand, lipopolysaccharide (LPS), induces a signaling cascade that involves the adaptors MyD88 (Kawai et al., 1999) and Mal (also named Tirap) (Fitzgerald et al., 2001; Yamamoto et al., 2002) and culminates in the activation of the transcription factor NF-κB and to the production of pro-inflammatory cytokine such as TNF-α or IL-6 (Alexander and Rietschel, 2001; Beutler et al., 2001; Poltorak et al., 2000; Yu et al., 1997). The production of these molecules, which are essential to the early immune response, has to be tightly regulated because any excess may have a dramatic outcome, such as septic shock syndrome in the case of TNF-α (Beutler et al., 1985, reviewed in Liu and Malik, 2006). The phosphoinositide 3-kinase (PI3K) pathway has recently been identified as one of these modulators of the inflammatory response to LPS (Guha and Mackman, 2002; Schabbauer et al., 2004). Indeed, many studies have shown that pharmacological PI3K inactivation can inhibit TLR-mediated signaling (see Fukao and Koyasu, 2003 for review). However, TLR4 is also able to activate a separate pathway, in which the proteins Trif and Tram serve as adapters in a signaling cascade that specifically leads to the production of type I (α/β) interferons (Hoebe et al., 2003; Yamamoto et al., 2003a,b). This pathway is particularly relevant during viral infections, as we recently demonstrated for the Vesicular Stomatitis Virus (Georgel et al., 2007). In this study, we investigated the regulatory functions of the PI3K pathway on the TLR4-dependent interferon production. For this, we realized VSV infections of peritoneal macrophages after pharmacological inactivation of PI3K and quantified the cells immune responses. We also studied the effects of genetic ablation of components of the PI3K cascade on VSV susceptibility ex vivo. Western blots experiments using antibodies directed against phospho-Akt revealed that PI3K activation is VSV-dependent. Furthermore, as demonstrated for LPS, Akt phosphorylation in response to VSV signaling emanates from TLR4/CD14 activation. However, we observed that PI3K exerts a strong positive effect on INF-mediated antiviral resistance. Whereas PI3K impairment was shown to increase TNF-α production in response to LPS, our data unexpectedly, indicate that pharmacological inactivation of the PI3K pathway reduces VSV-dependent IFN production and consequently, renders macrophages more susceptible to VSV infection. Conversely, we noted increased resistance to viral infection in macrophages isolated from mice in which PI3K activity is constitutively activated upon genetic ablation of the Phosphatase and Tensin Homologue gene (PTEN) which negatively regulates this pathway. These data, which demonstrate that PI3K differentially modulates the signaling pathways (MyD88-dependent and -independent) that are activated upon TLR4 activation, show that drugs targeting PI3K could exert both anti- and pro-inflammatory activities.
2. Materials and methods
2.1. Mice
C3H/HeN and C57BL/6 mice were obtained from Charles River and C3H/HeJ mice from the Jackson Laboratories. Heedless mutant mice have been previously described (Jiang et al., 2005). Floxed Pten, LysM cre transgenic mice were backcrossed for 10 generations against C57BL/6 to ensure homogeneity of the genetic background. Thioglycolate-elicited macrophages were recovered 3 days after i.p. injection of 3 ml bbl thioglycolate medium brewer modified (4%; Becton Dickinson) by peritoneal lavage with 5 ml phosphate buffer saline (PBS). All experiments were carried out in compliance with the rules of the TSRI Animal Use Committee and with the French Government's ethical and animal experiments regulations.
2.2. Reagents
LPS (Escherichia coli serotype O111:B4), wortmannin and LY294002 were purchased from Sigma–Aldrich.
2.3. Viral infection, titration and survival assay
VSV (Indiana Strain) was propagated and amplified by infection of a monolayer of Vero cells. Twenty-fours hour after infection, the supernatant was harvested and clarified by centrifugation. Viral titer was determined by plaque assay on Vero cells. For the VSV cytolytic assay, 100,000 cells were plated and infected at different multiplicities of infection (m.o.i.). Forty hours post-infection, cell survival was quantified by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) staining. OD was measured at 590 nm.
2.4. Reverse transcription and semi-quantitative PCR
Total mRNA were prepared using Trizol reagent (Invitrogen) and quantified by spectrophotometric analysis. Two micrograms were used according to the manufacturer's recommendations (Ambion) in a 20 μl reaction volume for reverse transcription. Two microliters of RT reaction was used for each PCR whose number of cycles was optimized to avoid saturation. Five microliters of reaction were loaded on agarose gels.
Actin transcripts were used as internal normalization controls.
Primers Ifnβ forward: 5′ TCCAAGAAAGGACGAACATTCG
Ifnβ reverse: 5′ TGAGGACATCTCCCACGTCAA
Ifnα4 forward: 5′ CCTGGTAATGATGAGCTACTACTGGT
Ifnα4 reverse: 5′ ATTTCTTCCAGGACTGTCAAGGC
VSV forward: 5′ GAATTCATGAAGTGCTTTTTGTACTTAGC
VSV reverse: 5′ TCTAGAAAGTCGGTTCATCTCTATGTCTG
Irf-7 forward: 5′ CCAGTGACTACAAGGCATCACAGAGTAGTAGC
Irf-7 reverse: 5′ TTGGGATTCTGAGTCAAGGCCACTGAC
Actin forward: 5′ TTCGTTGCCGGTCCACA
Actin reverse: 5′ ACCAGCGCAGCGATATCG
2.5. Statistical analysis
Data were analyzed using ANOVA test with GraphPad software.
2.6. Western blots
Proteins were separated by SDS–PAGE on 10% Tris–glycine gels and transferred to Immobilon-P membrane (Millipore Corp., Billerica, MA). The phosphorylation of AKT and GSK-3β and the expression of PTEN and AKT were determined by overnight incubation at 4 °C with a 1:2000 dilution of primary antibodies (Cell Signaling Technology, Danvers, MA). Actin antibody was from Sigma. This was followed by incubation for 1 h at room temperature with a secondary anti-rabbit IgG-HRP conjugated antibody diluted at 1:5000 (Amersham Biosciences, Piscataway, NJ). Membranes were washed and incubated with Supersignal West Femto substrate (Pierce Biotechnology, Rockford, IL) solution and bands were detected by a Fluor Chem HD2 (Alpha Innotec).
3. Results
3.1. Pharmacological inactivation of PI3K renders macrophages susceptible to VSV infection
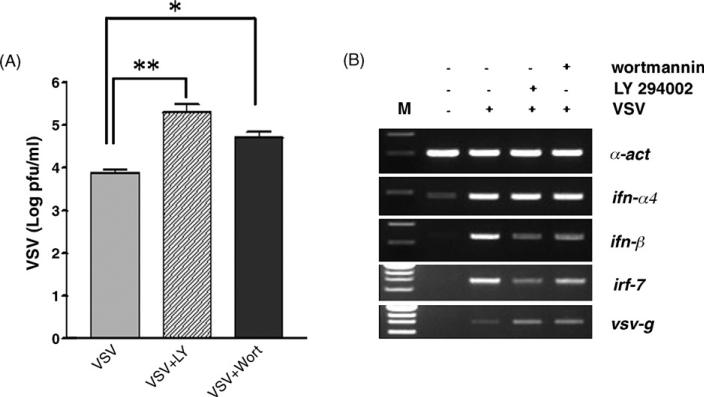
Phosphoinositide-3-kinase (PI3K) is known regulator of the LPS- and TLR4-dependent TNF-α production. To study the potential involvement of this pathway on the interferon production in response to Vesicular Stomatitis Virus (VSV) engagement of TLR4, we first studied the effect of LY294002 and wortmannin on thioglycolate-elicited peritoneal macrophages infected with VSV. As shown in Fig. 1A, pharmacological inactivation of PI3K by these compounds renders macrophages more susceptible to an ex vivo viral infection. Under control conditions, more than 50% of the macrophages survive when infected at a multiplicity of infection (m.o.i.) of 1, whereas treated cells exhibit only 30% survival (P < 0.001). To confirm that this effect of LY294002 and wortmannin on the increased cytotoxicity of VSV is caused by PI3K inactivation, we analyzed Akt phosphorylation by Western blotting. Akt is a target of PI3K and is activated by phosphorylation on ser 473 and thr 308 upon LPS stimulation. This modification is abolished in the presence of LY294002. Using these conditions as controls (Fig. 1B, top panel), we were also able to detect phosphorylated Akt in response to VSV, whereas this activated form of Akt is absent when macrophages are infected by VSV in the presence of LY294002 (Fig. 1B, bottom panel). These data clearly indicate that PI3K activation after VSV infection is important for efficient antiviral response.
Fig. 1.
Pharmacological inactivation of VSV-dependent PI3K activation reduces cell survival. (A) Survival of control peritoneal macrophages isolated from C57BL/6 mice or treated with 2 μM LY294002 or 500 nM wortmannin 40 h after VSV infection (m.o.i. 1 and 10). Statistical significance of the difference between VSV-infected treated and control cells was assessed by one-way ANOVA (P < 0.001). (B) Upper panel: Western blot analysis of Akt phosphorylation in control conditions (with 100 ng/ml LPS and with LPS+ 2 μM LY294002). The lower panel shows VSV-induced Akt phosphorylation and its inhibition by LY294002. In both panels, the blots were probed with an antibody specific for the phosphorylated form of Akt at serine (ser) 473 before being washed and reprobed with an antibody that allows detection of all forms of Akt for loading control.
To complete our analysis of PI3K pharmacological impairment, we also showed increased viral replication when macrophages are treated with LY294002 or wortmannin (Fig. 2A). Using RNA isolated from control and treated cells with and without VSV infection, we performed semi-quantitative RT-PCR analysis of genes involved in interferon-mediated antiviral immunity. As shown in Fig. 2B, interferon-β expression is decreased when macrophages are incubated in the presence of PI3K inhibitors, which is consistent with uncontrolled viral replication that we confirmed in this assay using the VSV glycoprotein G (vsv-g) as target gene. This is also accompanied by a reduction in the VSV-dependent activation of irf-7, a transcription factor critical for interferon-β expression.
Fig. 2.
PI3K participates in the early interferon response to VSV infection. (A) Quantification of viral titer measured by plaque assay in the supernatant of macrophages 24 h after infection with VSV (m.o.i. 10). Cells were left untreated or cultured in the presence of 2 μM LY294002 (VSV + LY) or 500 nM wortmannin (VSV + wort). (B) RNA from macrophages (infected or not by VSV, with and without LY294002 or wortmannin in the same conditions as in panel (A)) was isolated 20 h post-infection. After reverse transcription, semi-quantitative PCR reactions were performed using primers specific for ifn-α4, ifn-β, irf-7 and vsv-g genes. a-actine (α-act) serves as internal control. M, size marker.
3.2. TLR4-dependent activation of PI3K during viral infection
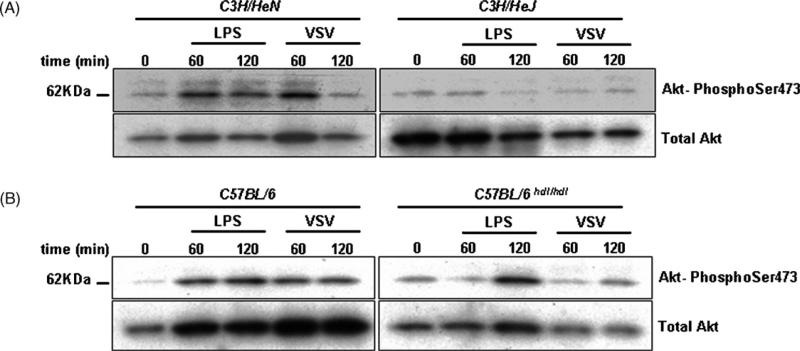
To identify the upstream components responsible for PI3K activation by VSV, we used macrophages isolated from TLR4- and CD14-deficient mice which are highly susceptible to VSV infection (Georgel et al., 2007). We first showed (Fig. 3A) that the TLR4 defect (Lpsd, Poltorak et al., 1998) present in C3H/HeJ mice prevents LPS- and VSV-induced Akt phosphorylation. As controls, we used C3H/HeN mice which show normal response to LPS and VSV. Next, we studied the VSV response in Heedless mutant macrophages which carry an ENU-induced point mutation in the Cd14 gene (Jiang et al., 2005). As observed for LPS, VSV infection is unable to activate PI3K when CD14 is not functional, whereas control macrophages isolated from C57BL/6 animals show a normal response (Fig. 3B). These data show that PI3K activation responds to a signal that emanates from the TLR4/CD14 complex upon VSV infection.
Fig. 3.
VSV activates TLR4- and CD14-dependent Akt phosphorylation. (A) Macrophages isolated from control (C3H/HeN) or tlr4-deficient (C3H/HeJ) mice were left untreated or activated by LPS (100 ng/ml) or VSV (m.o.i. 10) for 60–120 min. After proteins extraction and polyacrylamide gel electrophoresis, blots were probed with a specific antibody directed against the phosphorylated form of Akt at serine (ser) 473. After extensive washing, the blots were reprobed with an antibody specific for all forms of Akt (total Akt). (B) The same conditions as in panel (A) were applied to macrophages isolated from control (C57BL/6) and homozygous cd14 mutant (heedless C57BL/6hdl/hdl) mice.
3.3. Overactivation of PI3K in genetically modified animals increases resistance to VSV infection
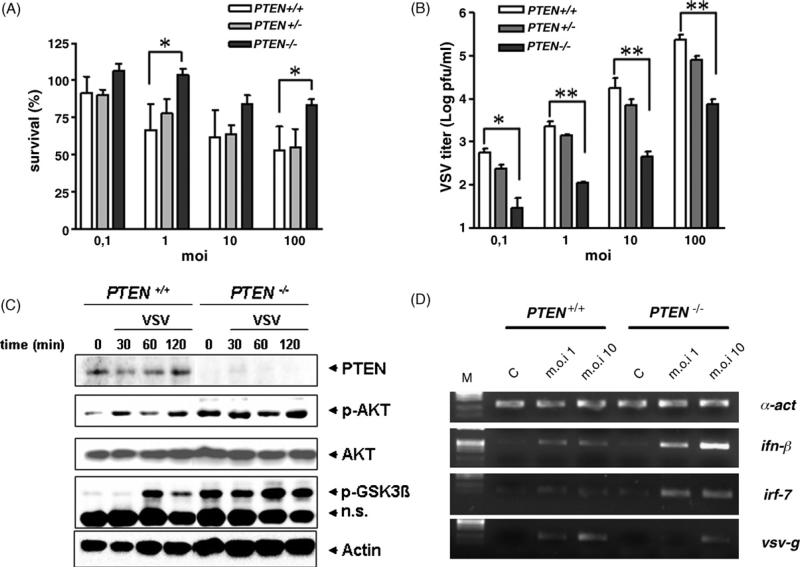
To obtain in vivo confirmation of the role of PI3K in viral resistance, we used macrophages isolated from mice in which the pten gene has been deleted by a cre-lox mechanism (Suzuki et al., 2001). A macrophage-specific PTEN knockout was achieved by overexpression of a LysM promoter regulated cre recombinase in mice carrying both alleles of a floxed pten gene (Luyendyk et al., JI in revision). As a consequence of this genetic alteration, PI3K is constitutively active (Cao et al., 2004). As illustrated in Fig. 4A, macrophages isolated from homozygous mutants animals (PTEN –/–) show increased resistance to VSV infection as compared to wild-type (+/+) mice (*P < 0.05). No gene dosage effect could be detected, as macrophages from heterozygous (+/–) mice do not exhibit statistically significant differences when compared to control cells. We also observed that mutant cells produce fewer viruses 24 h after the infection (Fig. 4B). Again, our semi-quantitative gene expression assay revealed that higher inf-β and irf-7 expression in infected pten –/– macrophages (Fig. 4C) correlates with increased resistance and low virus production in these cells. Finally, we performed Western blot experiments to monitor PI3K activity in wild-type and pten KO cells upon VSV infection. As shown in Fig. 4D, we confirmed that absence of PTEN induces constitutive phosphorylation of Akt. As noted above, we also detected VSV-induced phospho-AKT in control cells. Downstream components of PI3K pathway, such as Glycogen Synthase Kinase-3 beta (GSK-3β) are also affected in this experiment. Using an antibody specific for the activated phosphoprotein, we show here that GSK-3β phosphorylation is constitutively detected in pten mutant cells and occurs 30–60 min upon VSV infection.
Fig. 4.
Genetic overactivation of the PI3K pathway in pten-KO mice renders macrophages more resistant to VSV infection. (A) Quantification of cell survival 40 h after VSV infection (m.o.i. of 0.1–100) of peritoneal macrophages isolated from control (PTEN +/+, n = 6), heterozygotes (PTEN +/–, n = 2), and homozygote mutant (PTEN –/–, n = 6) mice. Statistical analysis was assessed by one-way ANOVA (*P < 0.05). (B) Viral titers in the supernatant of macrophages isolated control (+/+), heterozygotes (+/–) and homozygotes PTEN mutants (–/–) 24 h after VSV infection (m.o.i. 10). *P < 0.05; **P < 0.01. (C) Western blot analysis of untreated macrophages or 30, 60 and 120 min after VSV infection (m.o.i. 10). Cells were isolated from control (PTEN +/+) or mutant (PTEN –/–) mice. Blots were successively probed with antibodies specific for PTEN, phosphorylated Akt, Akt, phosphorylated GSK-3β and actin. n.s. refers to a non-specific band detected with the anti-p-GSK-3β antibody and which serves as loading control in addition to actin. (D) Gene expression analysis by semi-quantitative RT-PCR. cDNA from control (C), or VSV-infected (m.o.i. 1 and 10) macrophages isolated from wild-type (PTEN +/+) or mutant (PTEN –/–) mice 20 h post-infection were PCR amplified with specific primers. α-Actin serves as loading control. M, size marker.
4. Discussion
Upon ligand binding, Toll-like receptors activate a large panel of signaling pathways. In addition to the classical MyD88-dependent and -independent cascades which are activated by LPS (reviewed in Kawai and Akira, 2006), an intricate interplay of factors downstream of the TRL4/CD14 receptor complex has been described. These include G proteins, MAP kinases and many additional regulatory proteins which identify sub-pathways essential for an efficient positive and negative regulation of potentially harmful molecules (such as TNF-α) in an elaborate and orchestrated cross-talk (Oda and Kitano, 2006). PI3K are a family of lipid kinases that produce phosphoinosides which are second messengers essential for intracellular signaling in several important cellular processes, such as survival, migration and cytoskeleton rearrangement. In combination with other factors, they have been described as negative regulators of the TLR4-activated pathway (Fukao and Koyasu, 2003). Indeed, pharmacological inactivation of PI3K enhances NF-κB activation leading to excessive and prolonged inflammatory response with dramatic outcome to the host (Schabbauer et al., 2004). These data are, however, challenged by studies which demonstrate that PI3K activation induces cytokine secretion downstream of TLR4 and CD14 (Ojaniemi et al., 2003; O'Toole and Peppelenbosch, 2006). This conflicting situation is likely the result of the relative non-specificity of the inhibitors regarding the different classes of PI3K as well as the heterogeneity of the cells and TLR inducers which have been studied. In addition, most investigations were focused on the potential involvement of PI3K in the modulation of the MyD88-independent pathway which, after LPS binding to TLR4, activates NF-κB and induces the transcription of inflammatory cytokines such as TNF-α. Few controversial data are available on the relationships between PI3K and the interferon-mediated antiviral pathway elicited by TLR4. Whereas impaired IRF-3 dimerization was demonstrated when influenza virus-infected culture cells were treated with PI3K inhibitors (Ehrhardt et al., 2006), others reported increased IFN-β synthesis when human dendritic cells were activated with TLR3 or TLR4 ligands and PI3K inhibitors (Aksoy et al., 2005). In this study, we combined both pharmacological and genetic analysis to investigate the role of PI3K in innate antiviral immunity. Using inhibitors of this pathway, we show that the integrity of the PI3K-Akt axis, which is activated downstream of the TLR4/CD14 complex, contributes to an efficient IFN production which is necessary for full resistance to an ex vivo viral infection. We extended our observation of the role of PI3K to mouse models in which this pathway has been genetically engineered. First, we showed that in cells lacking the phosphatase PTEN (which antagonizes the action of PI3K), antiviral reactions are more effective than in control cells. We observed decreased viral amplification and augmented inf-β gene transcription upon VSV infection as a result of this artificial overactivation of the PI3K pathway achieved by genetic means. We also studied the VSV susceptibility in p85α (the regulatory subunit of PI3K) knockout animals (Brachmann et al., 2005). Genetic ablation of this gene is normally lethal but we were able to identify few animals which survived several weeks after birth (G. Schabbauer, personal observation). These mice were then used as donors of bone marrow cells which were transferred in irradiated recipients. Macrophages isolated from such reconstituted animals show a normal resistance to VSV infection (data not shown), which we interpret as a consequence of potential redundancy between α and β PI3K regulatory subunits. Further investigations with mice carrying a targeted disruption of the catalytic p110 component in cells of myeloid origin should clarify this discrepancy. However, increased resistance to VSV infection was also observed when cells were harvested from transgenic mice overexpressing a mutated (constitutively active) form of the Akt kinase in cells of myeloid origin (data not shown) (Pengal et al., 2006). Altogether, these genetic data confirm the positive effect of PI3K activation on viral resistance in macrophages and extend our results obtained with pharmacological impairment of this pathway. The observed effects of pharmacological inactivation of genetic exacerbation of the PI3K pathway may appear marginal in our ex vivo model of VSV infection. Indeed, we observed modest (5–10-fold) differences in viral replication, 2–4-fold modifications in ifn-β and irf-7 target gene expression (fluorescence quantification of PCR bands were realized after agarose gels electrophoresis and in some experiments, quantitative PCR were performed, data not shown) and slight, but statistically significant, discrepancies were observed for cell survival. However, this might reflect the myriad of signaling molecules which are activated upon TLR activation and converge on target genes such as ifn-β. In addition, full resistance to viral infection relies on the synergistic activation of multiple pathways (TLR-dependent and -independent, Otsuka et al., 2007) following pathogen's recognition. Future studies in vivo with mice carrying genetic alteration of components in the PI3K pathway will allow direct evaluation of the role of this cascade in innate antiviral immunity.
The involvement of phosphoinosides in TLR4-mediated signal transduction has recently been re-evaluated (Kagan and Medzhitov, 2006). By analogy with the function of Tirap as a “sorting adaptor” which recruits MyD88 (the “signaling adaptor”) to a subset of TLRs through binding to phosphatidylinositol 4,5-bisphosphate (PIP2), a similar role for Tram has been suggested. We therefore speculate that VSV binding on the cell surface, through a receptor that still remains unknown, activates PI3K-dependent phosphoinosides production. This constitutes an important initial event in the signaling pathway activated by TLR4–CD14 and relayed by Tram to produce antiviral effector molecules. In this model, it is highly relevant to note that influenza virus has been able to subvert the PI3K pathway for its own benefit. As recently demonstrated (Hale et al., 2006), binding of NS1 protein to p85β activates PI3K and improves viral replication.
Acknowledgments
We thank Cécile Macquin for technical assistance. This study was initiated in the laboratory of Prof. Bruce Beutler who is gratefully acknowledged for his constant support. This work was financially supported by la Ligue contre le cancer and Fondation pour la Recherche Médicale (PG) and the Austrian Science Fund (FWF) project P19850-B12 (GS).
References
- Aksoy E, Vanden BW, Detienne S, Amraoui Z, Fitzgerald KA, Haegeman G, Goldman M, Willems F. Inhibition of phosphoinositide 3-kinase enhances TRIF-dependent NF-kappa B activation and IFN-beta synthesis downstream of Toll-like receptor 3 and 4. Eur. J. Immunol. 2005;35:2200–2209. doi: 10.1002/eji.200425801. [DOI] [PubMed] [Google Scholar]
- Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- Beutler B, Du X, Poltorak A. Identification of Toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: genetic and evolutionary studies. J. Endotoxin Res. 2001;7:277–280. [PubMed] [Google Scholar]
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol. Cell Biol. 2005;25:1596–1607. doi: 10.1128/MCB.25.5.1596-1607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Wei G, Fang H, Guo J, Weinstein M, Marsh CB, Ostrowski MC, Tridandapani S. The inositol 3-phosphatase PTEN negatively regulates Fc gamma receptor signaling, but supports Toll-like receptor 4 signaling in murine peritoneal macrophages. J. Immunol. 2004;172:4851–4857. doi: 10.4049/jimmunol.172.8.4851. [DOI] [PubMed] [Google Scholar]
- Ehrhardt C, Marjuki H, Wolff T, Nurnberg B, Planz O, Pleschka S, Ludwig S. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell Microbiol. 2006;8:1336–1348. doi: 10.1111/j.1462-5822.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O'Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, Bahram S, Oldstone MB, Beutler B. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Hale BG, Jackson D, Chen YH, Lamb RA, Randall RE. Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14194–14199. doi: 10.1073/pnas.0606109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Oda K, Kitano H. A comprehensive map of the toll-like receptor signaling network. Mol. Syst. Biol. 2006;2:2006. doi: 10.1038/msb4100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur. J. Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- O'Toole T, Peppelenbosch MP. Phosphatidyl inositol-3-phosphate kinase mediates CD14 dependent signaling. Mol. Immunol. 2007;44(9):2362–2369. doi: 10.1016/j.molimm.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Jing Q, Georgel P, New L, Chen J, Mols J, Kang YJ, Jiang Z, Du X, Cook R, Das SC, Pattnaik AK, Beutler B, Han J. Hyper-susceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity. 2007;27:123–134. doi: 10.1016/j.immuni.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Pengal RA, Ganesan LP, Wei G, Fang H, Ostrowski MC, Tridandapani S. Lipopolysaccharide-induced production of interleukin-10 is promoted by the serine/threonine kinase Akt. Mol. Immunol. 2006;43:1557–1564. doi: 10.1016/j.molimm.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van HC, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B. Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2163–2167. doi: 10.1073/pnas.040565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler. Thromb. Vasc. Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, Tsubata T, Ohashi PS, Koyasu S, Penninger JM, Nakano T, Mak TW. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003a;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 2003b;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- Yu B, Hailman E, Wright SD. Lipopolysaccharide binding protein and soluble CD14 catalyze exchange of phospholipids. J. Clin. Invest. 1997;99:315–324. doi: 10.1172/JCI119160. [DOI] [PMC free article] [PubMed] [Google Scholar]