Abstract
Developmental exposure to estrogenic chemicals induces morphological, functional, and behavioral anomalies associated with reproduction. Humans are exposed to bisphenol-A (BPA), an estrogenic compound that leaches from dental materials and plastic food and beverage containers. The aim of the present study was to determine the effects of perinatal exposure to low, environmentally relevant doses of BPA [25 and 250 ng BPA/kg body weight (bw)·d] on the peripubertal development of the mammary gland. BPA exposure enhanced the mammary glands' sensitivity to estradiol in ovariectomized CD-1 mice. In their intact 30-d-old littermates, the area and numbers of terminal end buds relative to the gland ductal area increased whereas their apoptotic activity decreased. There was a positive correlation between ductal length and the age at first proestrus; that was reduced as the BPA dose increased, suggesting that BPA exposure slows down ductal invasion of the stroma. There was also a significant increase of progesterone receptor-positive ductal epithelial cells that were localized in clusters, suggesting future branching points. Indeed, lateral branching was significantly enhanced at 4 months of age in mice exposed to 25 ng BPA /kg bw·d. In conclusion, perinatal exposure to environmentally relevant BPA doses results in persistent alterations in mammary gland morphogenesis. Of special concern is the increased terminal end bud density at puberty as well as the increased number of terminal ends reported previously in adult animals, as these two structures are the sites at which cancer arises in humans and rodents.
Perturbations in the fetal environment may predispose individuals to disease and/or dysfunction, such as hypertension and coronary heart disease that become apparent in adulthood (1). Epidemiological studies also suggest that the intrauterine milieu may have an influential role in predisposing an individual to carcinogenesis. For example, dizygotic twin births correlate with an increased incidence of breast cancer in female siblings (2); on the other hand, females born to mothers who had eclampsia or preeclampsia, have a decreased breast cancer risk. These outcomes have been attributed to changes in the fetal hormonal milieu, in particular estrogens (3). The mechanisms underlying this observation are presently unknown.
During the last decade, observations made in the estrogen receptor (ER)-α and -β knockout models suggested that the mammary gland develops in an estrogen-independent manner until puberty (4, 5). The corollary of this particular view of development was that perinatal exposure to exogenous estrogens would have no effect on the development of the mammary gland. This concept was reinforced by the fact that the mouse mammary gland does not express a proliferative response to estrogens before the third week of age (6). However, administration of supraphysiological doses of estradiol (E2) (i.e. doses of 35 μg/mouse·d or higher) to mice at postnatal d (PNDs) 1–5 resulted in changes in the mammary gland, such as increased ductal branching, which did not become obvious until the fifth week of life (7). Similarly, prenatal exposure to pharmacological levels of the estrogen diethylstilbestrol (DES) enhanced the sensitivity of the gland to hormones and carcinogens administered during adulthood, thus increasing mammary cancer incidence in rodent models (8, 9). These studies required addressing the notoriously pathological effects of in utero exposure to high doses of DES in humans (10, 11). Presently the concern about effects of estrogen exposure is focused on a far more subtle exposure, represented by environmental estrogens, which may affect mammary gland development and/or enhance the risk of breast cancer later in life.
Over the last 60 yr, humans have been exposed to a plethora of synthetic hormonally active chemicals overtly because of their deliberate use in agriculture and medicine; inadvertently as byproducts of industrial use; or as waste released into rivers, lakes, and the atmosphere. Environmental exposure to these chemicals has coincided with an increase in endocrine-related diseases of the male reproductive system (12) and increases in testicular (13) and breast cancers (14).
Among the endocrine disruptors, bisphenol-A (BPA) is receiving increased attention due to its high potential for human exposure. Used in the manufacture of polycarbonate plastics and epoxy resins, BPA leaches from food containers (15), beverage containers (16), and dental sealants and composites (17) under normal conditions of use (18). BPA is also used in the manufacture of many products in addition to those stated above (19), which would further increase levels of human exposure to this compound. These reports suggest that humans routinely ingest BPA. Indeed, a recently published study, the first involving a reference human population (394 samples analyzed), reported that BPA was found in 95% of the urine samples (20). In a smaller study (36 samples analyzed), Arakawa et al. (21) reported a median daily urinary excretion of BPA of 1.2 μg/d and a maximum daily intake of BPA per body weight to be 0.23 μg/kg·d.
Before these studies, research on the effects of low-dose BPA exposure in animal models have used measurements of the amount of BPA leached from food cans, containers, and dental sealants as guidance for estimating a plausible exposure range of 2–20 μg BPA/kg body weight (bw)·d administered orally (22). BPA has recently been measured in human sera (adult men: 1.49 ± 0.11 ng/ml; adult women: 0.64 ± 0.10 ng/ml) (23), the maternal and fetal plasma, and placental tissue at birth in humans (24, 25). The concentration of BPA in amniotic fluid was approximately 5-fold higher than that measured in maternal plasma. The range of BPA concentrations assessed in the placenta was 1–100 ng/g with a median level of 12 ng/g, whereas in fetal plasma, levels ranged from 0.2 to 9.2 ng/ml. Recognizing that it is not feasible to delineate definitive exposure levels from the existing data, we have chosen to administer 25 and 250 ng BPA/kg bw·d sc by means of osmotic pumps to female mice on d 9 of pregnancy until PND4. Based on the data reported by Arakawa et al. (21), we estimate that this level of BPA exposure should fall within the range of human exposures reported to date.
Intrauterine exposure to BPA has been shown to advance puberty and disrupt estrous cyclicity in mice (26–28). Although the mechanism by which BPA is able to induce developmental abnormalities in estrogen-target tissues is unknown, it is plausible that ERs may mediate BPA-induced effects because this chemical binds both ERα and ERβ (29–31). Interaction between BPA and ERs may take place during fetal development because ERα and ERβ are first detected in the mouse mammary gland primordium at d 12.5 of gestation (32).
The purpose of the present study was to test whether in utero and neonatal exposure to environmentally relevant levels of BPA alters the peripubertal development of the mammary gland.
Materials and Methods
Animals
Sexually mature female CD-1 mice (8 wk of age; Charles River Laboratories, Wilmington, MA) were maintained in temperature-controlled and light-controlled (14-h light, 10-h dark cycle) conditions in the Tufts University School of Medicine animal facility. All experimental procedures were approved by the Tufts University–New England Medical Center Animal Research Committee in accordance with the Guide for Care and Use of Laboratory Animals. Food, cages, and bedding all tested negligible for estrogenicity by the E-SCREEN assay (33); water was supplied from glass bottles only. Female mice were mated with CD-1 males of known reproductive competence, and the morning on which a vaginal plug was observed was designated pregnancy d 1. On d 9 of pregnancy, mice were weighed and implanted with Alzet osmotic pumps (Alza Corp., Mountain View, CA) designed to deliver either dimethyl sulfoxide (vehicle control), 25 or 250 ng BPA/kg bw·d dissolved in dimethyl sulfoxide (Sigma, St. Louis, MO) for 14 d, which encompassed the remainder of the pregnancy through PND4 (n = 6–10/treatment). Offspring born on d 20 of gestation were culled to 10 pups per mother on PND7. The actual dose decreased as pregnancy progressed because the weight of the mother increased over this period. Female offspring were killed by CO2 inhalation on PND20 and 30 and at 4 months of age (one pup/litter, n = 6–10/treatment). For mice killed at first proestrus, vaginal smears were taken daily after vaginal opening until a proestrus smear was detected. For animals killed at 4 months of age, vaginal smears were assessed daily for 2–3 wk to assess the pattern of estrous cyclicity; these mice were killed on the afternoon of proestrus. Proestrus was confirmed by the presence of uterine ballooning.
To assess whether perinatal BPA treatment altered the response to E2, animals (one pup/litter) from each treatment group were ovariectomized at 25 d of age and immediately implanted with one Alzet pump delivering vehicle or 0.5 μg E2/kg bw·d (n = 10/group). Animals were killed 10 d after ovariectomy.
Tissue collection
One and a half hours before kill, the animals were weighed and injected ip with 5-bromo-2′-deoxyuridine (BrdU) (1.5 mg/100 g bw; Roche, Indianapolis, IN). Trunk blood was collected in heparinized tubes and plasma was separated by centrifugation and stored at −80 C until assayed for E2. The fourth and fifth mammary gland pairs were dissected out; the right mammary glands were whole mounted, and the left mammary glands were embedded in paraffin for light microscopy and immunohistochemistry. Mammary glands from an additional series of mice killed at first proestrus were collected under RNase-free conditions and immediately frozen for subsequent RNA isolation and RT-PCR.
Whole mounts and paraffin sections
To prepare the whole mounts, the mammary glands were spread onto glass slides, fixed with 4% paraformaldehyde in 0.1 m PBS overnight, stained with Carmine Alum (Sigma), dehydrated, and whole mounted with Permount. To prepare paraffin sections, mammary glands were fixed with 4% paraformaldehyde in 0.1 m PBS for 18–20 h at room temperature (RT), washed in 0.1 m PBS, dehydrated through a series of alcohol and xylene, and embedded with Paraplast paraffin (Fisher, Pittsburgh, PA) under vacuum. Five-micrometer sections were cut on an RM2155 rotary microtome (Leica, Nusslock, Germany), mounted on Superfrost slides (Fisher Scientific International Inc., Hampton, NH), and stained with hematoxylin and eosin or processed for immunohistochemistry to assess DNA synthesis, apoptosis, or expression of ERα and progesterone receptor (PR).
Immunohistochemistry
BrdU was immunolocalized within paraffin sections of mammary glands from 30-d-old mice using the streptavidin-biotin labeling system as described previously (34). Sections were hydrated through a series of alcohols, microwaved in 10 mm citrate buffer (pH 6) for antigen retrieval, subjected to acid hydrolysis for DNA denaturation, and treated for both endogenous peroxidase and nonspecific binding with methanol/H2O2 and 2% normal goat serum in 0.01 m PBS, respectively. Sections were incubated with monoclonal BrdU antibody IgG (1:400; clone 85–2C8; Novocastra, Newcastle upon Tyne, UK) overnight in a humid chamber at 4 C. Biotinylated goat antimouse IgG (Sigma) was applied and reaction visualized by streptavidin-peroxidase-diaminobenzidine (DAB) (Sigma) to assess cellular incorporation of BrdU. Sections were lightly counterstained with Harris' hematoxylin and mounted with a glass coverslip for light microscopy.
The expression of ERα and PR was evaluated by immunohistochemistry in paraffin sections of mammary tissue obtained from 30-d-old mice, as previously described (35). Using the biotin-streptavidin-peroxidase method, tissue was incubated with primary antibodies for ERα (1:60; mouse 6F-11 clone; Novocastra Laboratories) and PR (1:100; mouse PR-AT 4.14 clone; Affinity BioReagents Inc., Golden, CO) at 4 C for 14–16 h, and DAB was used as the chromogen to visualize reactions. Sections were counterstained with Mayer's hematoxylin. Each immunohistochemical run included positive and negative controls. In the negative control slides, the primary antibody was replaced with nonimmune mouse serum (Sigma). To minimize spurious variation among experiments, each immunostaining procedure was performed in sets containing tissues from an equal number of subjects from all the experimental groups (0, 25, and 250 ng BPA/kg bw·d).
In situ detection of apoptosis
Apoptosis was evaluated in mammary glands from 30-d-old mice. Sections were analyzed for in situ detection of cells with DNA strand breaks using the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) technique (ApopTag; Intergen Co., Purchase, NY) as previously described (36). Briefly, after deparaffinization and rehydration, sections were incubated with proteinase K (5 μg/ml; Intergen) for 10 min at 37 C and then treated with hydrogen peroxide PBS for 10 min at RT to quench endogenous peroxidase activity. Sections were then incubated with a mixture containing digoxigenin deoxynucleotide triphosphate, unlabeled deoxynucleotide triphosphate, and terminal dideoxy transferase enzyme in a humidified chamber at 37 C for 1 h Subsequently slides were rinsed with PBS and incubated with antidigoxigenin-peroxidase for 30 min at RT and substrate-chromogen mixture (DAB; Sigma) for 4 min. Samples were counterstained with Mayer's hematoxylin, dehydrated, and mounted with permanent mounting medium. Negative control slides were run using exactly the same procedures, except distilled water was added instead of terminal dideoxy transferase enzyme. An involuting mouse mammary gland collected 3 d after weaning was processed in an identical manner and used as positive control.
Quantitative analysis of the expression of BrdU, ERα, PR, and apoptosis
The incorporation of BrdU in mammary glands from 30-d-old mice was quantified using a Zeiss microscope (Carl Zeiss Inc., Göttinger, Germany) and a ×100 objective oil immersion lens. A graticule was placed in the eyepiece to quantitate the percentage of BrdU-stained cells within the epithelium per total number of cells in approximately 50 arbitrarily chosen fields per mammary gland.
Quantitative analysis of ERα and PR expression was performed in both epithelial and stromal compartments of the mammary gland. Two different stromal areas were defined: first, a periepithelial stroma (stroma immediately surrounding epithelial structures, composed of fibroblastic-like cells and a dense extracellular matrix) and second, adipose stroma (fatty stroma not adjacent to epithelial structures) (37). Receptors associated with the capsule of the mammary gland were not included in the analysis.
For analysis of cells expressing ERα and PR, 50 representative fields (at least 1000 epithelial cells/section) in each section were analyzed using a Dplan ×100 objective lens. All immunostained epithelial and stromal nuclei in the defined regions, regardless of intensity, were defined as positive. Positive cells were expressed as the percent ratio of total number of epithelial or stromal cells evaluated. In tissue from 30-d-old mice, the percentage of apoptotic cells was quantified in the body cells of the terminal end buds (TEBs) at the peripheral leading edge of the advancing ductal mass, according to the methodology described by Humphreys et al. (38).
Morphometric analysis of whole mounts
Digital images of whole mounted mammary glands were captured with a SPOT-real time digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI) attached to a dissecting microscope (Carl Zeiss). Quantitative analyses of mammary glands were performed using the Optimus 6.5 program (Media Cybernetics, Silver Spring, MD). In 30-d-old mice, the length of the ductal tree along its longitudinal axis, the total area of the mammary gland occupied by the epithelial ducts, and the total number and size of all TEBs were analyzed. The ductal area was determined by measuring the region defined by the outermost edges of the epithelial tree relative to the area of the entire fat pad (or the total area of the mammary gland epithelium was determined on digital images of mammary glands (for d 20: objective, ×1, magnified, ×400; for d 30: objective, ×0.8, magnified, ×400) by using the threshholding tool within Optimus, taking advantage of the greater staining intensity of the mammary gland epithelium relative to the remainder of the gland). The total number and size of TEBs were measured on digital images of the entire mammary gland (×0.8 objective, magnified ×400); the size of the TEBs was determined by placing each structure into a size category, ranging from smallest to largest, based on its area and perimeter length.
Morphometric analysis of ductal side branching was performed on whole mounted mammary glands of 4-month-old mice. Digital images were captured using a ×4 objective. Side-branching evaluation was carried out using Image Pro-Plus 4.1.0.1 system (Media Cybernetics) by counting the total number of branch points along the ductal network. Results were expressed as the number of branch points per 500 μm of duct length.
RNA isolation
Total RNA was isolated from whole mammary glands using TRIzol (Invitrogen Inc., Carlsbad, CA) according to the manufacturer's instructions and stored at −80 C until analyzed. RNA concentration was determined using the ratio of UV absorbance read at 260/280 nm.
Analysis of Wnt4 mRNA expression using RT-PCR
To remove genomic DNA, RNA isolated from mammary glands of 30-d-old mice was treated with DNase (Invitrogen) before performing reverse transcription with random primers and Superscript II RNase H− reverse transcriptase (Invitrogen). All of the above procedures were carried out according to the manufacturer's instructions. An aliquot of cDNA (2.9 μl) from control and treated mammary glands was added to each PCR tube along with 10 μl master mix (Quantitect SYBR Green PCR kit, QIAGEN Inc., Valencia, CA) containing SYBR green as the detection dye and 6-carboxy-X-rhodamine as the normalizing dye, and 1 U/tube uracil-DNA-glycosylase, heat labile (Roche Diagnostics Corp., Indianapolis, IN) to prevent DNA contamination from previous PCRs, according to the instructions of the manufacturer. Forward and reverse primers for Wnt4, GATGTGCAAACGGAACCTTGA, and GTCAC-CACCTTCCCAAAGACAG (PCR product = 144 bp), respectively, were also added to the tubes. The internal control, ribosomal protein L19, was run in separate tubes using the primers: forward, ATCGCCAATGC-CAACTCC and reverse, TCATCCTTCTCATCCAGGTCA (PCR product = 205 bp). Real-time PCR was performed in the MX4000 thermocycler (Stratagene, La Jolla, CA) under the following conditions: 50 C for 2 min and 95 C for 15 min, followed by 45 cycles of 95 C for 15 sec, 63 C for 30 sec, and 72 C for 30 sec.
The SYBR green fluorescence data for each sample were analyzed on the MX4000 software (Stratagene); after normalization with the 6-carboxy-X-rhodamine signal followed by baseline correction, an amplification curve was obtained to calculate the threshold cycle for each sample. Absolute standards (200 × 103 to 390 copies/tube) for both Wnt4 and L19, prepared from purified cDNA identical with real-time PCR products, were included on each plate to ensure equal efficiency of amplification between standards and PCR products generated in sample wells. The efficiency of the PCR was more than 95% for both Wnt4 and L19 as calculated from the standard curves with R2 = 0.9861 and R2 = 0.9714, respectively. The expression of Wnt4 was then determined by interpolating the threshold cycle values of each sample from the Wnt4 standard curve and was normalized with the expression of L19 of that sample, which was calculated in the same way. An average of values obtained from three runs was used as the final result to compare Wnt4 expression between control and treated groups. Contamination by genomic DNA was assessed in each sample by running the reaction in the absence of reverse transcriptase. The purity and specificity of the PCR products was confirmed by the SYBR Green dissociation curve set up at the end of each PCR run.
RIA for plasma E2
Steroids were extracted from 200 μl of plasma using 6 ml diethyl ether (>99% pure; Sigma). Percent recovery of extraction was calculated by the addition of a fixed amount of tracer. Plasma E2 levels were determined by RIA (DSL-39100; Diagnostic Systems Laboratories Inc., Webster, TX) following the manufacturer's instructions.
Statistics
The statistical program SPSS (SPSS, Inc., Chicago, IL) was used. Statistical analysis was performed by one-way ANOVA and significance between groups was determined by Dunn's post hoc test. In specific cases in which the data were not distributed normally, a Kruskal-Wallis test was employed and differences between the control and BPA treatment groups were assessed by Mann-Whitney U tests (39). Results were considered significant at P < 0.05.
Results
Number, size, and area of TEBs
The period around puberty, i.e. between 20 and 30 d of age, is characterized by the reinitiation of ductal growth in the mammary glands. Estrogens play a major role in this process (40). Bulbous TEBs (area > 0.03 mm2) form at the tips of the ducts. These structures invade the stroma and mediate the longitudinal growth of the subtending ducts. When the ductal tree reaches the edge of the fat pad, the TEBs mature into a resting structure, the terminal ends, and ductal growth ceases. The number of bulbous TEBs was similar in the mammary glands of all groups at 20 d of age (Table 1). At 30 d of age, there was a significant increase in the number of TEBs relative to the area occupied by the ductal tree in the animals exposed to 250 ng BPA/kg bw·d, compared with that in the vehicle-treated controls (P = 0.008), whereas the increase in the 25 ng BPA/kg bw·d approached significance (P = 0.054) when compared with control (Fig. 1A and Table 1). Similarly, when these data were expressed as TEB area relative to ductal tree area, a significant increase was observed at 250 ng BPA/kg bw·d with respect to the vehicle-treated control (P < 0.05) (Fig. 1B and Table 1). The absolute number of TEBs and the total area occupied by TEBs were increased in the mammary glands of BPA-treated animals when compared with the control group (Table 1); however, this increase was not statistically significant. The increased number and area of TEBs relative to the ductal area in the BPA-exposed animals suggested that ductal growth might be impaired.
TABLE 1.
Morphometric analyses of mammary gland structures and plasma estradiol levels in animals exposed perinatally to vehicle or BPA
| Parameter | 0 ng BPA/kg bw·d | 25 ng BPA/kg bw·d | 250 ng BPA/kg bw·d |
|---|---|---|---|
| No. of TEBs at PND 20 | 2.0 ± 1.1 | 3.0 ± 1.38 | 2.1 ± 0.7 |
| No. of TEBs at PND30 | 12.0 ± 1.75 | 15.67 ± 1.52 | 15.83 ± 1.16 |
| No. of TEBs/ductal area at PND 30 | 0.184 ± 0.029 | 0.271 ± 0.029a | 0.290 ± 0.016b |
| Total area (mm2) TEBs at PND30 | 0.580 ± 0.091 | 0.715 ± 0.076 | 0.802 ± 0.073 |
| Area TEBs/ductal area at PND30 | 0.0091 ± 0.0017 | 0.012 ± 0.0014 | 0.153 ± 0.0012c |
| Ductal area (mm2) at PND30 | 67.55 ± 6.60 | 65.22 ± 7 | 59.47 ± 5.08 |
| Ductal tree length (mm) at PND30 | 5.79 ± 0.64 | 6.11 ± 0.62 | 5.73 ± 0.33 |
| % ER-positive epithelial cells at PND 30 | 52.03 ± 1.62 | 53.4 ± 1.56 | 58.17 ± 2.83 |
| E2 levels at 1st proestrus pg/ml | 18.11 ± 0.99 | 18.42 ± 0.812 | 15.92 ± 0.875 |
Data are represented as the mean ± sem.
P = 0.054.
P = 0.007.
P = 0.005 when compared with the respective vehicle-treated control.
Fig. 1.

Effect of perinatal exposure to BPA (25 and 250 ng/kg bw·d) on the number of terminal end buds (A) and the terminal end bud area (B) relative to the total ductal area in the fourth mammary gland at 30 d of age (bars indicate mean ± sem). The asterisk indicates statistical significance relative to the control (A, P = 0.008; B, P = 0.005).
Analysis of epithelial ducts in whole mounts in 30-d-old animals
To assess whether ductal growth was affected at 30 d of age, two parameters were measured: the length of the ductal tree from the center of the lymph node to the leading edge and the total area covered by the ductal tree. There were no significant differences among the experimental groups regarding the area covered by the epithelial ducts of the mammary gland; however, a trend toward reduction of the ductal area was observed in the BPA-treated animals (Table 1). A measure of the length of the ductal tree from the center of the lymph node to the leading edge also did not show a significant difference between the control and treated groups.
The length of the ductal tree was also measured at the first proestrus. There was a positive correlation between ductal length from the center of the lymph node to the leading edge and the age at first proestrus (Fig. 2). The slope was steepest in the controls (m = 0.8822) and became reduced as the BPA dose was increased (25 ng BPA/kg bw·d: m = 0.501; 250 ng BPA/kg bw·d: m = 0.1249), suggesting that BPA exposure slows down ductal invasion of the stroma, a phenomenon that is regulated by estrogens. Indeed, there was a statistically significant difference between the slopes of the control and 250 ng BPA/kg bw·d-exposed groups (P = 0.005). Moreover, the length of the ductal tree was significantly reduced in the subset of animals exposed to 250 ng BPA/kg bw·d, which had their first proestrus at 34 d of age or later (4.43 ± 0.71 mm), when compared with the controls (10.05 ± 1.63; P = 0.027). Two possible underlying causes are that perinatal exposure to BPA alters either the plasma E2 levels or the sensitivity of the mammary gland to these hormones. Measuring the plasma E2 levels did not provide a clear-cut answer. Whereas there were no significant differences among the treatments regarding the plasma level of E2 at proestrus (Table 1), E2 levels were slightly, but not significantly, lower in the 250 ng BPA/kg bw·d group than the vehicle or 25 ng BPA/kg bw·d.
Fig. 2.

Length of the ductal tree from the center of the lymph node to the leading edge measured in mammary glands of control (■, solid line) and BPA [25 ng (△, dashed line) and 250 ng (◆, dotted line) per kg bw·d] treated mice killed at the first proestrus.
Perinatal BPA treatment alters the sensitivity of the mammary gland to E2
To assess whether the increase in the number of TEBs/ductal area observed at 30 d of age in animals exposed perinatally to BPA was due to altered sensitivity to E2, a set of animals exposed perinatally to vehicle or to 250 ng BPA/kg bw·d was ovariectomized at 25 d of age and treated for 10 d with pumps delivering 0 or 0.5 μg E2/kg bw·d. Perinatal exposure to 250 ng BPA/kg bw·d significantly increased the response to 0.5 μg E2/kg bw·d regarding the following parameters: total number of TEBs, total TEB area, mean TEB size, TEB number/ductal area, and TEB area/ductal area (Table 2 and Fig. 3). These findings suggest that perinatal exposure to BPA enhances the sensitivity of the mammary gland to estrogens.
TABLE 2.
Perinatal exposure to 250 ng/kg·d alters the sensitivity to estradiol in the mammary glands of ovariectomized animals
| A.0BPA/0E2 (mean ± sem) |
B.0BPA/0.5E2 (mean ± sem) |
P (A and B) |
C.250BPA/0E2 (mean ± sem) |
D.250BPA/0.5E2 (mean ± sem) |
P (C and D) |
P (B and D) |
|
|---|---|---|---|---|---|---|---|
| No. of TEBs | 1.4 ± 0.58119 | 5.5 ± 1.16667 | 0.004 | 2.09091 ± 0.77991 | 10 ± 0.64979 | 0.000 | 0.007 |
| TEB area | 0.048 ± 0.0201 | 0.219 ± 0.05374 | 0.007 | 0.07818 ± 0.02885 | 0.428 ± 0.0396 | 0.000 | 0.005 |
| Ductal extension | 2.6155 ± 0.62599 | 4.41091 ± 0.74227 | ns | 2.80273 ± 0.7631 | 4.686364 ± 0.5776 | 0.016 | ns |
| Ductal area | 79.081 ± 8.65917 | 126.135 ± 9.20329 | 0.002 | 82.2555 ± 6.96275 | 118.5518 ± 8.21476 | 0.005 | ns |
| TEB size | 0.0137 ± 0.00559 | 0.03384 ± 0.0043 | 0.023 | 0.02021 ± 0.00599 | 0.04228 ± 0.00164 | 0.000 | ns |
| No. of TEBs/area | 0.0146 ± 0.00622 | 0.04195 ± 0.00896 | 0.029 | 0.02289 ± 0.00599 | 0.084893 ± 0.00621 | 0.000 | 0.003 |
| TEB area/area | 0.0005 ± 0.00021 | 0.00167 ± 0.00041 | 0.019 | 0.00086 ± 0.0003 | 0.003576 ± 0.00029 | 0.000 | 0.004 |
Animals exposed perinatally to 250 ng BPA/kg bw·d or to vehicle were ovariectomized at 25 d of age and implanted with osmotic pumps delivering 0 or 0.5 μg estradiol/kg bw·d from 25–35 d of age. A, Perinatal treatment with vehicle and implanted with pumps delivering vehicle at 25–35 d old; B, perinatal treatment with vehicle and implanted with pumps delivering 0.5 μg estradiol/kg bw·d at 25–35 d old; C, perinatal treatment with 250 ng BPA/kg bw·d and implanted with pumps delivering vehicle at 25–35 d old; D, perinatal treatment with 250 ng BPA/kg bw·d and implanted with pumps delivering 0.5 μg E2/kg bw·d at 25–35 d old. P (A and B) denotes the P value of the comparison between groups A and B; P (C and D) denotes the P value of the comparison between groups C and D; and P (B and D) denotes the P value of the comparison between groups B and D. ns, Lack of statistically significant differences. No. of TEBs denotes number of TEBs at the leading edge of the 4th mammary gland; ductal area is expressed in mm2. Ductal extension denotes the distance from the center of the lymph node to the leading edge in mm; TEB size is expressed in mm2. TEBs/area denotes the number of TEBs relative to the ductal area; it is expressed as number/mm2, and TEB area/area denotes the area (mm2) occupied by the TEBs relative to the total area (mm2) of the ductal tree. There were no statistically significant differences between the mice treated perinatally with 0 and 250 ng BPA/kg bw·d after ovariectomy for any of the parameters measured (groups A and C).
Fig. 3.

Effect of perinatal exposure to 250 ng BPA/kg bw·d on the response to 0.5 μg E2/kg bw·d administered from 25 to 35 d of age in animals ovariectomized at 25 d of age. A, Comparison of the number of TEBs per ductal area. B, Comparison of the area occupied by TEBs relative to the ductal area of the fourth mammary gland. The gray bars represent E2 treatment; black bars represent controls (bars indicate mean ± sem).
Apoptotic and proliferative activity in the mammary gland
At puberty, as the TEBs become bulbous, they show both high proliferative and high apoptotic activity. Death of the body cells is essential for the formation of the lumen on the proximal side of the TEBs (38) and to the growth of the subtending duct. A large number of apoptotic cells were identified by the TUNEL method in TEBs of control mice at 30 d of age as shown in Fig. 4A. In utero exposure to BPA resulted in a significant decline in the number of apoptotic cells in TEBs of both treated groups (25 ng BPA/kg bw·d, P < 0.001; 250 ng BPA/kg bw·d, P < 0.05) relative to the controls (Fig. 4B). This decreased apoptotic activity suggests impaired ductal growth and may explain the increased number of TEBs/ductal area.
Fig. 4.
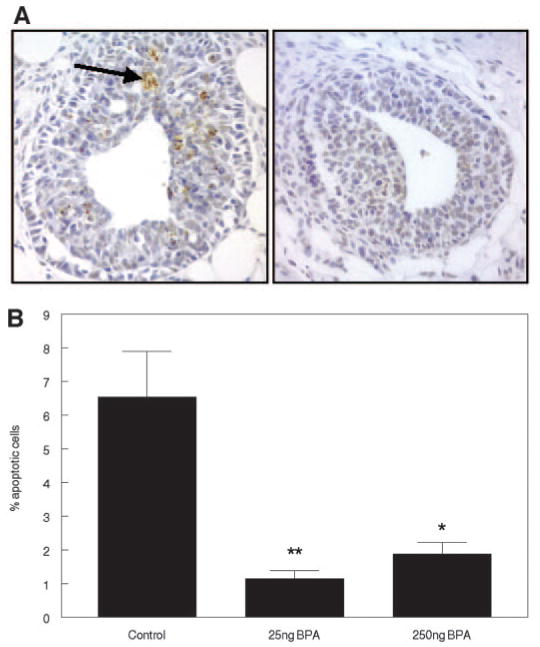
Detection of apoptotic cells by the TUNEL method in mammary glands of mice treated perinatally with BPA or vehicle. A, Photomicrographs showing cells stained positive for apoptosis in terminal end buds of 30-d-old mice treated perinatally with vehicle (left panel) and BPA (right panel). The arrow indicates a cluster of apoptotic bodies. B, Graph summarizing percentage of apoptotic cells in TEBs of control and treated mice (bars indicate mean ± sem). *, P < 0.05; **, P < 0.01.
In contrast to the strong inhibitory effect on apoptosis, BPA exposure did not affect BrdU incorporation, a marker of proliferative activity, in the epithelial compartment. On the other hand, the percentage of stromal cells that incorporated BrdU decreased by 50% (P < 0.05) in the 250 ng/kg BPA group at 30 d of age (Table 3). Because the development of ductal structures involves remodeling of the stroma, these results suggest that perinatal exposure to BPA also alters stroma-epithelium interactions.
TABLE 3.
Percent of BrdU-positive cells in the epithelial and stromal compartment of the 4th mammary gland of 30-d-old animals
| BPA dose | 0 ng/kg bw·d | 25 ng/kg bw·d | 250 ng/kg bw·d |
|---|---|---|---|
| Epithelium | 8.18 ± 2.51 | 3.31 ± 1.02 | 5.51 ± 1.15 |
| Stroma | 1.78 ± 0.29 | 1.27 ± 0.18 | 0.87 ± 0.10a |
Data are represented as the mean ± sem.
A significant difference with the control (P < 0.05).
Expression of ERα and PR in the mammary glands
Because ER and PR are clearly involved in the pathways regulating ductal development (41, 42), their expression was assessed at 30 d of age. Expression of ERα was observed in both the epithelial and stromal compartments of the mammary glands, whereas expression of PR was observed only in the epithelium. Treatment with BPA had no effect on the expression of ERα in the stroma or the epithelial ducts of mammary glands at 1 month of age (Table 1). The number of cells expressing PR in the epithelial compartment of both treated groups was found to be significantly higher (25 ng BPA/kg bw·d, P < 0.001; 250 ng BPA/kg bw·d, P < 0.05) than in controls (Fig. 5A). In addition, a characteristic cluster of PR-positive cells was frequently seen in the ductal epithelium of BPA-treated mice (Fig. 5B). These clusters are believed to be indications of future branching points (43).
Fig. 5.

A, Histogram representing the percentage of cells stained positive for PR in the epithelial compartment of mammary glands of 30-d-old mice treated perinatally with vehicle, 25 and 250 ng BPA/kg bw·d (**, P < 0.01; *, P < 0.05) (bars indicate mean ± sem). B, Photomicrograph showing clusters (as indicated by the arrow) of PR-expressing cells in the mammary gland ducts of BPA-treated mice.
Expression of Wnt4 in the mammary glands
The PR expression results described above suggested that BPA might increase lateral branching. Wnt4 expression is an important mediator of lateral branching downstream of PR (44). Compared with the basal levels of Wnt4 seen in controls, the mean expression of Wnt4 was increased in the 250 ng BPA/kg bw·d group, whereas the levels in the 25 ng BPA/kg bw·d group remained similar to the control levels (Fig. 6). However, this increasing trend of Wnt4 levels did not reach statistical significance at the single time point chosen for this study.
Fig. 6.

Expression of Wnt4 mRNA in mammary glands of control and BPA-treated mice (25 and 250 ng BPA/kg bw·d) at 30 d of age estimated by quantitative RT-PCR. Wnt4 mRNA levels were measured relative to L-19 expression (internal control). Bars indicate mean ± sem.
Analysis of ductal branching
As suggested by the clustering of PR positive cells at 30 d of age, an analysis of mammary gland whole mounts at 4 months of age revealed a significant increase in the number of lateral branches in mammary glands of mice treated with 25 ng BPA/kg bw·d (P < 0.05), whereas an increase observed in the 250 ng BPA/kg bw·d group was not statistically significant (P = 0.09) (Fig. 7, A and B). Interestingly, in the latter group, the data points form two clusters, one control-like and the other similar to the 25 ng BPA/kg bw·d dose. This plot also reveals that there is less variance among the controls than among the individual animals in the treated group (P = 0.007) (Fig. 7A).
Fig. 7.

A, Number of side branches per 500 μm of ductal length in mammary glands of 4-month-old mice treated perinatally with vehicle, 25 and 250 ng BPA/kg bw·d (*, P < 0.05) (n = 6, 6, 10 for controls, 25 and 250 ng BPA/kg bw·d, respectively). Error bars indicate sem. B, Photomicrographs of whole mounts of mammary glands of animals treated with vehicle (left panel) and 25 ng BPA/kg bw·d (right panel). The bar represents 1 mm.
Discussion
Prior data obtained from siblings of the animals used in the present study revealed that prenatal exposure to environmentally relevant levels of BPA resulted in alterations of the reproductive tract and mammary gland that were manifested long after exposure ended. These alterations encompassed functional changes such as alteration of estrous cyclicity observed at 3 months of age (27); morphological changes in the ovary, uterus, and vagina, also observed at 3 months of age (27); and cellular changes, such as an increase in the ER- and PR-positive cells observed in the lining of the endometrium at 3 months of age (45). In the mammary gland, an increase in the total area of the ductal tree and increases in the number of terminal ducts, terminal ends, and alveolar buds were observed at 6 months of age (34).
The objective of the present study was to assess whether perinatal BPA exposure also affected the development of the mammary gland at puberty, a phenomenon initiated by the rise in estrogen plasma levels. During this period, the ductal tree undergoes growth by invasion of the stroma, accrual of new cells, and lumen formation; these processes are mediated by a highly dynamic structure, the TEB. We observed a positive correlation between ductal length from the center of the lymph node to the leading edge and the age at first proestrus; the slope of this curve was significantly reduced as the BPA dose increased. At 30 d of age, the number of TEBs relative to the ductal area increased significantly in animals exposed perinatally to BPA. Whereas the distal aspect of the TEB is the invasive organ, the solid primordium of body cells near the neck is involved in the formation of the subtending duct and its lumen, a process that is manifested by a high apoptotic rate (38); the ductal lumen is formed as a consequence of this apoptotic activity. A large and significant decrease in the number of apoptotic cells was observed within the TEBs in animals of both BPA-treated groups. Hence, decreased cell death may be the factor underlying the impaired ductal elongation and the increased number and area of TEBs per ductal area in the BPA-treated animals. Cell proliferation, instead, seemed to be unaffected because no significant changes in the BrdU labeling index of epithelial cells were observed. However, a significant decrease of the labeling index of stromal cells was found in animals exposed to 250 ng BPA/kg bw·d. Because mammary gland morphogenesis is mediated by complex stromal-epithelial interactions, this result suggests that BPA also affects the stromal compartment and may disrupt important stromal-epithelial interactions.
The morphological changes found in 30-d-old animals exposed perinatally to BPA could be attributed, at least in part, to an increased sensitivity to estrogens. Indeed, the magnitude of the response to E2 was significantly enhanced in their siblings that were ovariectomized and exposed to E2 for 10 d. In particular, a significant increase in the number and area of TEBs relative to the ductal area was observed in these ovariectomized E2-treated animals as well as in their intact siblings that were exposed perinatally to BPA. Although it seems counterintuitive that E2 would decrease ductal growth, we observed that the response of the normal mammary gland to E2 is biphasic, with low doses producing a larger effect than higher doses (Vandenberg L., personal communication). This nonmonotonic behavior was also observed regarding the percent area of the mammary gland occupied by the ductal tree (46). Alternatively, BPA may affect ductal growth by acting on other yet-unknown end points in addition to increasing the sensitivity to estrogens. Indeed, recent evidence indicates a complex interaction among E2, IGF-I, and progesterone on ductal morphogenesis (47).
Progesterone is the main mediator of lateral branching and alveolar growth, a fact clearly illustrated by the PR null mutant mice (48). Perinatal BPA exposure resulted in a significant increase in the number of epithelial cells expressing PR. This finding may result from the observed increased estrogen sensitivity of the mammary gland in BPA-exposed animals. In these animals, the ductal PR-positive cells often formed clusters. Lateral branching points and alveoli originate in these clusters (49). In the normal mammary gland, the transition from uniform PR expression to PR clustering takes place between 8 and 12 wk of age (49). Wnt 4 expression is a crucial event downstream from PR signaling in the lateral branching process (44). Perinatal BPA exposure resulted in increased wnt4 mRNA levels at puberty, the only time point assayed; however, this increase did not reach significance. Nevertheless, morphometric analysis of the mammary glands at 4 months of age clearly revealed an increase in the number of lateral branches in the animals exposed to BPA.
These observations suggest that perinatal exposure to BPA significantly enhances the response to estrogens and increases the expression of PR. Furthermore, it suggests that the increased expression of PR may be the mechanism underlying the enhanced side branching observed at 4 months of age and the significant increase in the percentage of alveolar buds observed at 6 months of age (34).
The mechanisms by which BPA affects the morphology of the mammary gland long after the period of exposure are largely unknown. One pathway may involve the direct action of BPA on the fetal mammary gland by altering the expression of genes that regulate mesenchymal-epithelial interactions and ductal morphogenesis (50). Misexpression of these genes has been associated with mammary gland dysgenesis and carcinogenesis (51). E2 regulates certain homeobox genes (51–53); thus, it is plausible that BPA may also have this effect.
Prenatal exposure to DES down-regulates the expression of Wnt7a in the uterus (54) and AbdB Hoxa in the müllerian duct (55) and induces a posterior shift in Hox gene expression and homeotic anterior transformations of the reproductive tract (56), and this correlates with structural abnormalities of these organs. The observation that the response to E2 was enhanced in ovariectomized animals exposed perinatally to BPA is compatible with this scenario. An additional pathway by which BPA may indirectly affect mammary gland development is by disrupting the hypothalamic-pituitary-ovarian axis. This would alter the secretory patterns of pituitary and ovarian hormones, which are important in postnatal mammary gland morphogenesis. The observations that perinatal exposure of rats to low-dose BPA reduces serum LH levels after ovariectomy in adulthood (57), disrupts estrous cyclicity, and results in morphological changes in the ovary (27) support the potential for alterations in the hypothalamus-pituitary-ovarian axis of BPA-exposed offspring.
What are the implications of these findings regarding human health? Surrogate animal models provide an understanding of human diseases. Although the relationship between the two is not always direct, surrogate models are most useful when used to develop hypotheses linking exposures and health outcomes. They also increase our understanding of the mechanisms underlying these pathologies. For instance, the mouse model has proven to be a generally outstanding model of human DES exposure, thus providing a means to understand the mechanisms underlying the DES syndrome. This excellent performance strengthens the human relevance of the current findings in mice. Within this context, it is useful to speculate how the findings described here may also apply to humans. On the one hand, exposure to estrogens is a main risk factor for the development of breast cancer in humans and also increases the development of mammary cancer induced by chemical carcinogens in rodent models (58). Thus, the increased sensitivity to E2 suggests that prenatal exposure to BPA may increase the likelihood of neoplastic development. On the other hand, TEBs are the structures in which mammary cancer originates in both rodents and humans (59, 60). The increase in the number of TEBs/ductal area is also consistent with an increased risk of breast cancer. Another well-established risk factor for breast cancer is increased mammographic density. Mammographic density is attributed to an increased epithelial compartment and increased nonadipose stroma. The mammary glands of BPA-exposed animals contained significantly more ducts due to lateral branching at 4 months of age. Previous data obtained from sisters of animals in this study revealed that at 6 months of age, the number of all the epithelial structures were significantly increased (34), including terminal ducts, i.e. the structures in which neoplasia originates in adult animals (59). These correlations suggest that perinatal exposure to BPA in particular, and to estrogens in general, may increase susceptibility to breast cancer. This hypothesis is being further tested in our laboratories.
Acknowledgments
The authors are most grateful to Laura Vandenberg, Cheryl Schaeberle, and Jenny Lenkowski for technical assistance in histology and morphometric analyses. We are most appreciative of Dr. David Damassa's advice on the statistical analysis.
This work was supported by National Institutes of Health-ES Grant 08314 and National University of Litoral CAI+D program. M.M.-d.-T. was a recipient of a Senior Fulbright Scholar Award.
Abbreviations
- BPA
Bisphenol-A
- BrdU
5-bromo-2′-deoxyuridine
- bw
body weight
- DAB
diaminobenzidine
- DES
diethylstilbestrol
- E2
estradiol
- ER
estrogen receptor
- PND
postnatal day
- PR
progesterone receptor
- RT
room temperature
- TEB
terminal end bud
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling
References
- 1.Sallout B, Walker M. The fetal origin of adult diseases. J Obstet Gynaecol. 2003;23:555–560. doi: 10.1080/0144361031000156483. [DOI] [PubMed] [Google Scholar]
- 2.Braun MM, Ahlbom A, Floderus B, Brinton LA, Hoover RN. Effect of twinship on incidence of cancer of the testis, breast, and other sites (Sweden) Cancer Causes Control. 1995;6:519–524. doi: 10.1007/BF00054160. [DOI] [PubMed] [Google Scholar]
- 3.Potischman N, Troisi R. In utero and early life exposures in relation to risk of breast cancer. Cancer Causes Control. 1999;10:561–573. doi: 10.1023/a:1008955110868. [DOI] [PubMed] [Google Scholar]
- 4.Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, Korach KS. Induction of mammary gland development in estrogen receptor-α knockout mice. Endocrinology. 2000;141:2982–2994. doi: 10.1210/endo.141.8.7609. [DOI] [PubMed] [Google Scholar]
- 5.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 6.Fendrick JL, Raafat AM, Haslam SZ. Mammary gland growth and development from the postnatal period to postmenopause: ovarian steroid receptor ontogeny and regulation in the mouse. J Mammary Gland Biol Neoplasia. 1998;3:7–21. doi: 10.1023/a:1018766000275. [DOI] [PubMed] [Google Scholar]
- 7.Warner MR. Effect of various doses of estrogen to BALB/cCrgl neonatal female mice on mammary growth and branching at 5 weeks of age. Cell Tissue Kinet. 1976;9:429–438. doi: 10.1111/j.1365-2184.1976.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 8.Mori T, Bern HA, Mills KT, Young PN. Long-term effects of neonatal steroid exposure on mammary gland development and tumorigenesis in mice. J Natl Cancer Inst. 1976;57:1057–1061. doi: 10.1093/jnci/57.5.1057. [DOI] [PubMed] [Google Scholar]
- 9.Rothschild TC, Boylan ES, Calhoon RE, Vonderhaar BK. Transplacental effects of diethylstilbestrol on mammary development and tumorigenesis in female ACI rats. Cancer Res. 1987;47:4508–4516. [PubMed] [Google Scholar]
- 10.Herbst AL, Bern HA. Developmental effects of diethylstilbestrol (DES) in pregnancy. New York: Thieme-Stratton; 1988. [Google Scholar]
- 11.Mittendorf R. Teratogen update: carcinogenesis and teratogenesis associated with exposure to diethylstilbestrol (DES) in utero. Teratology. 1995;51:435–445. doi: 10.1002/tera.1420510609. [DOI] [PubMed] [Google Scholar]
- 12.Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm count and disorders of the male reproductive tract? Lancet. 1993;341:1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- 13.Skakkebaek NE, Meyts ER, Jorgensen N, Carlsen E, Petersen PM, Giwercman A, Andersen A, Jensen TK, Andersson A, Muller J. Germ cell cancer and disorders of spermatogenesis: and environmental connection? APMIS. 1998;106:3–12. doi: 10.1111/j.1699-0463.1998.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 14.Davis DL, Bradlow HL, Wolff M, Woodruff T, Hoel DG, Anton-Culver H. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ Health Perspect. 1993;101:372–377. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brotons JA, Olea-Serrano MF, Villalobos M, Olea N. Xenoestrogens released from lacquer coating in food cans. Environ Health Perspect. 1994;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biles JE, McNeal TP, Begley TH, Hollifield HC. Determination of Bisphenol-A in reusable polycarbonate food-contact plastics and migration to food simulating liquids. J Agric Food Chem. 1997;45:3541–3544. [Google Scholar]
- 17.Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, Pedraza V, Soto AM, Sonnenschein C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markey CM, Rubin BS, Soto AM, Sonnenschein C. Endocrine disruptors from Wingspread to environmental developmental biology. J Steroid Biochem Mol Biol. 2003;83:235–244. doi: 10.1016/s0960-0760(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 19.Markey CM, Michaelson CL, Sonnenschein C, Soto AM. Alkylphenols and bisphenol A as environmental estrogens. In: Metzler M, editor. The handbook of environmental chemistry Vol 3 Part L, Endocrine disruptors, part I. Berlin Heidelberg: Springer Verlag; 2001. pp. 129–153. [Google Scholar]
- 20.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham JL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arakawa C, Fujimaki K, Yoshinaga J, Imai H, Serizawa S, Shiraishi H. Daily urinary excretion of bisphenol A. Environ Health Prevent Med. 2004;9:22–26. doi: 10.1265/ehpm.9.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997;105:70–76. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeuchi T, Tsutsumi O. Serum bisphenol A concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun. 2002;291:76–78. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- 24.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 26.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 27.Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev. 2003;5:1–9. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 28.Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, Tsubura A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod Toxicol. 2004;18:803–811. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Soto AM, Fernandez MF, Luizzi MF, Oles Karasko AS, Sonnenschein C. Developing a marker of exposure to xenoestrogen mixtures in human serum. Environ Health Perspect. 1997;105:647–654. doi: 10.1289/ehp.97105s3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der Saag PT, van der Burg B, Gustafsson J. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan AV, Starhis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 32.Lemmen JG, Broekhof JLM, Kuiper GGJM, Gustafsson JA, Van Der Saag PT, van der Burg B. Expression of estrogen receptor α and β during mouse embryogenesis. Mech Dev. 1999;81:163–167. doi: 10.1016/s0925-4773(98)00223-8. [DOI] [PubMed] [Google Scholar]
- 33.Soto AM, Lin TM, Justicia H, Silvia RM, Sonnenschein C. An “in culture” bioassay to assess the estrogenicity of xenobiotics. In: Colborn T, Clement C, editors. Chemically induced alterations in sexual development: the wildlife/human connection. Princeton, NJ: Princeton Scientific Publishing; 1992. pp. 295–309. [Google Scholar]
- 34.Markey CM, Luque EH, Munoz de Toro MM, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65:1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 35.Munoz de Toro MM, Maffini MV, Kass L, Luque EH. Proliferative activity and steroid hormone receptor status in male breast carcinoma. J Steroid Biochem Mol Biol. 1998;67:333–339. doi: 10.1016/s0960-0760(98)00124-1. [DOI] [PubMed] [Google Scholar]
- 36.Ramos JG, Varayoud J, Bosquiazzo VL, Luque EH, Munoz de Toro MM. Cellular turnover in the rat uterine cervix and its relationship to estrogen and progesterone receptor dynamics. Biol Reprod. 2002;67:735–742. doi: 10.1095/biolreprod.101.002402. [DOI] [PubMed] [Google Scholar]
- 37.Shyamala G, Barcellos-Hoff MH, Toft DO, Yang X. In situ localization of progesterone receptors in normal mouse mammary glands: absence of receptors in the connective tissue and adipose stroma and a heterogeneous distribution in the epithelium. J Steroid Biochem Mol Biol. 1997;63:251–259. doi: 10.1016/s0960-0760(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 38.Humphreys RC, Krajewska M, Krnacik S, Jæger R, Weiher H, Krajewski S, Reed JC, Rosen JM. Apoptosis in the terminal end bud of the murine mammary gland: a mechanism of ductal morphogenesis. Development. 1996;122:4013–4022. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- 39.Seigel S. Nonparametric statistics for the behavioral sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- 40.Topper YJ, Freeman CS. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980;60:1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- 41.Mueller SO, Clark JA, Myers PH, Korach KS. Mammary gland development in adult mice requires epithelial and stromal estrogen receptor α. Endocrinology. 2002;143:2357–2365. doi: 10.1210/endo.143.6.8836. [DOI] [PubMed] [Google Scholar]
- 42.Shi HY, Lydon JP, Zhang M. Hormonal defect in maspin heterozygous mice reveals a role in progesterone in pubertal ductal development. Mol Endocrinol. 2004;18:2196–2207. doi: 10.1210/me.2004-0052. [DOI] [PubMed] [Google Scholar]
- 43.Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, Rosen JM. C/EBPb, but not C/EBPa, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 1998;12:1917–1928. doi: 10.1101/gad.12.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- 45.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 46.Škarda J. Detection of estrogenicity by bioassay on the mouse mammary gland. Physiol Res. 2001;50:275–282. [PubMed] [Google Scholar]
- 47.Ruan W, Monaco ME, Kleinberg DL. Progesterone stimulates mammary gland ductal morphogenesis by synergizing with and enhancing insulin-like growth factor-I action. Endocrinology. 2005;146:1170–1178. doi: 10.1210/en.2004-1360. [DOI] [PubMed] [Google Scholar]
- 48.Lydon JP, DeMayo FJ, Conneely OM, O'Malley BW. Reproductive phenotypes of the progesterone receptor null mutant mouse. J Steroid Biochem. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 49.Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. C/EBPβ(CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol. 2000;14:359–368. doi: 10.1210/mend.14.3.0434. [DOI] [PubMed] [Google Scholar]
- 50.Chen F, Capecchi MR. Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc Natl Acad Sci USA. 1999;96:541–546. doi: 10.1073/pnas.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, Woo I, Roberts-Clark D, Francis-West PH, Liu Y, Maxson R, Hill RE, Dale TC. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122:2729–2737. doi: 10.1242/dev.122.9.2729. [DOI] [PubMed] [Google Scholar]
- 52.Daniel CW, Smith GH. The mammary gland: a model for development. J Mammary Gland Biol Neoplasia. 1999;4:3–8. doi: 10.1023/a:1018796301609. [DOI] [PubMed] [Google Scholar]
- 53.Weber-Hall SJ, Phippard DJ, Niemeyer CC, Dale TC. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation. 1994;57:205–214. doi: 10.1046/j.1432-0436.1994.5730205.x. [DOI] [PubMed] [Google Scholar]
- 54.Sassoon DA. Wnt genes and endocrine disruption of the female reproductive tract: a genetic approach. Mol Cell Endocrinol. 2001;158:1–5. doi: 10.1016/s0303-7207(99)00170-7. [DOI] [PubMed] [Google Scholar]
- 55.Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES) Dev Biol. 1998;197:141–154. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]
- 56.Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing müllerian system. FASEB J. 2000;14:1101–1108. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- 57.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol-A affects body weight, patterns of estrous cyclicity and plasma LH levels. Environ Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller EF. Reflections on gender and science. New Haven, CT: Yale University Press; 1985. [Google Scholar]
- 59.Russo J, Russo IH. DNA labeling index and structure of the rat mammary gland as determinants of its susceptibility to carcinogenesis. J Natl Cancer Inst. 1978;61:1451–1459. [PubMed] [Google Scholar]
- 60.Wellings SR, Jensen HM. On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst. 1973;50:1111–1118. doi: 10.1093/jnci/50.5.1111. [DOI] [PubMed] [Google Scholar]


