Summary
Malignant gliomas are associated with a very high risk of venous thromboembolism (VTE). While many clinical risk factors have previously been described in brain tumor patients, the risk of VTE associated with newer anti-angiogenic therapies such as bevacizumab in these patients remains unclear. When VTE occurs in this patient population, concern regarding the potential for intracranial hemorrhage complicates management decisions regarding anticoagulation, and these patients have a worse prognosis than their VTE-free counterparts. Risk stratification models identifying patients at high risk of developing VTE along with predictive plasma biomarkers may guide the selection of eligible patients for primary prevention with pharmacologic thromboprophylaxis. Recent studies exploring disordered coagulation, such as increased expression of tissue factor (TF), and tumorigenic molecular signaling may help to explain the increased risk of VTE in patients with malignant gliomas.
Keywords: bevacizumab, malignant glioma, thromboembolism, tissue factor
Clinical significance of VTE in malignant gliomas
Risk factors and outcomes
The risk of venous thromboembolism (VTE) in adults with malignant gliomas is high; the estimated incidence varies widely and has been reported to be as high as 72%, but is generally accepted to be in the range of 20–30% over the course of the disease [1–6]. The highest risk is in the first few months postoperatively, but the risk of VTE remains higher than other malignancies throughout the course of disease [7], suggesting that increased VTE risk may be a reflection of alternate tumor biology specific to malignant gliomas. In the immediate postoperative period, for example, glioma patients have a higher incidence of VTE than a comparable cohort of colon cancer patients [8,9]. Surgical resection may cause release of procoagulant microparticles (MPs) into the circulation, and post-operative immobility and paresis may further contribute to thrombosis [8,10]. The increased risk of VTE in glioma patients directly attributable to neurosurgery is difficult to quantify because of the lack of standardized prophylaxis methods across available studies; however, one series showed the hazard ratio (HR) for developing VTE was 1.7 [95% confidence interval (CI) 1.3–2.3] within 61 days after neurosurgery [8].
Established ‘generic’ risk factors for VTE such as prolonged immobility or indwelling central venous catheter devices can be reasonably extrapolated to contribute to the risk of VTE in malignant glioma. Other risk factors have been confirmed specifically in glioma patients including age greater than 75 (HR 1.8; CI 1.4–2.5) [8]. Proven and possible disease-specific risk factors include glioblastoma (GBM) tumor subtype [5], (HR 1.7; CI 1.4–2.1) [8]; subtotal surgical resection compared with total resection (HR 3.58; CI 0.98–13.13) [6]; glioma size greater than 5 cm (HR 2.2; CI 1.0–4.5) [11]; intraluminal thrombosis in the tumor pathologic specimen [odds ratio (OR) 17.8; CI 4–79.3] [12]; A and AB blood type (HR 2.7; CI 1.0–7.0 and HR 9.4; CI 2.7–32, respectively) [11]; and limb paresis [5,10,13]. Therapy-specific risk factors have also been suggested in glioma patients including treatment with thalidomide [6], and administration of chemotherapy [13]. While radiotherapy is an essential treatment modality for malignant gliomas and has been shown to predispose patients to VTE in other cancers, there are no similar data available for brain tumors. Likewise, corticosteroids are a mainstay of management of vasogenic edema in glioma and are associated with increased rates of VTE in other tumors; however, the role of corticosteroids as an independent risk factor for VTE in malignant glioma patients remains undefined [14].
Bevacizumab (Avastin®; Genentech, South San Francisco, CA, USA) is an anti-vascular endothelial growth factor (VEGF) monoclonal antibody that recently received U.S. Food and Drug Administration approval in recurrent GBM [15]. A well-documented side effect of bevacizumab in extra-central nervous system (CNS) malignancies is intratumoral bleeding [16,17]. Bevacizumab has been linked to an increased risk of arterial and venous thromboembolic events in cancer patients as well [16,18,19]. Thus far, however, there are no data to support the contention that bevacizumab increases the risk of arterial or venous thromboembolism, or indeed intracranial hemorrhage, in GBM patients [20]. In general, composite data from multiple tumor types are inconsistent regarding the risk of thromboembolic events on bevacizumab therapy [21,22], but a recent meta-analysis concluded that bevacizumab is associated with an increased risk of VTE [relative risk (RR) 1.33; CI 1.13–1.56; P < 0.001] in non-primary CNS malignancies [23].
In recent years, a great deal has been learned about the epidemiology of VTE in cancer. Thromboembolic events in cancer patients generally portend a worse outcome compared with patients with cancer but without thromboembolic complications [9,24,25]. Furthermore, compared with patients without malignant disease, cancer patients present with larger clot burdens, a greater tendency towards clinical deterioration despite anticoagulation, diminished venographic resolution of the clot despite anticoagulation and a greater propensity for recurrent thromboembolic events after completion of a course of anticoagulation [26]. Cancer diagnosed within 1 year of an episode of VTE correlates with advanced stage and poor prognosis; one study found the 1-year survival of patients diagnosed with cancer and VTE concurrently was 12% compared with 36% in those diagnosed with cancer alone [24]. In addition, hospitalized cancer patients with VTE have a greater in-hospital mortality rate than hospitalized cancer patients without VTE. Finally, the risk of fatal pulmonary embolism (PE) in patients with cancer undergoing surgery is 3-fold greater than that of patients without cancer undergoing similar surgery [21]. Outcomes data for malignant glioma patients are similar; a large neurosurgical cohort showed that patients with VTE had a 30% higher risk of death within 2 years (HR 1.3; CI 1.2–1.4) compared to those without VTE [8].
Management of VTE in malignant gliomas
Historically, physicians have often favored inferior vena cava (IVC) filters over anticoagulation in patients with malignant glioma and VTE because of the perceived high risk of bleeding with anticoagulation [27–29]. However, some authorities suggest that the theoretical risk of bleeding is overestimated, and that anticoagulation can be used safely and effectively in many instances [2,7,30,31]. The risk of intratumoral hemorrhage on therapeutic anticoagulation is estimated to be 2% [2]. Further, IVC filters carry inherent risks including a higher risk of recurrent VTE, IVC or filter thrombosis and postphlebitic syndrome [1,2,32,33]. Furthermore, while IVC filters are associated with complications in < 10% of patients without malignancy, in glioma patients complication rates have been reported to be as high as 62% [7,34,35]. Therefore, caution is advised in relying solely upon IVC filters for VTE treatment in cancer patients in whom long-term survival is expected [26].
Absolute contraindications to anticoagulation for VTE are relatively limited. Thrombolytic agents for life-threatening PE are absolutely contraindicated in patients with intracranial malignancy. Some authorities recommend a non-contrast head computed tomography (CT) to rule out active intracranial bleeding prior to initiating anticoagulation in patients with brain tumors and concurrent VTE [1,2,7].
In general, anticoagulation is recommended for at least 3 months after the diagnosis of a first episode of VTE in patients with brain tumors in the absence of any other contraindications. This may be followed by a more prolonged period of less intense anticoagulation with the goal of minimizing the risk of recurrence, depending on a clinical assessment of risks and benefits. Notably, while reported cohort sizes remain small, recent studies have suggested that current anticoagulation for VTE is not necessarily a contraindication to starting bevacizumab despite the theoretically increased bleeding risk associated with this combination of therapies [36].
Thromboprophylaxis
Data from the neurosurgical literature suggest that peri-operative triple thrombosis prophylaxis with graduated compression stockings, pneumatic compression and low-molecular weight heparin (LMWH) or subcutaneous heparin maximizes VTE prevention with a low risk of bleeding [1,37,38]. Given the high risk of VTE in patients with malignant gliomas, outpatient thromboprophylaxis has been proposed. The 2008 ACCP guidelines recommend against primary pharmacologic prevention of VTE in general cancer patients [39]. However, recent studies have shown that anticoagulation administered in usual prophylactic doses slightly increases the bleeding risk and effectively reduces the risk of thromboembolic events [6,40–43]. To date, only one study has examined this issue in malignant glioma patients; the PRODIGE study was a randomized controlled trial designed to determine the efficacy and safety of dalteparin 5000 anti-Xa units or placebo for 6 months for the prevention of VTE in newly diagnosed patients with malignant glioma. This trial was terminated early secondary to expiration of study medication. Preliminary data suggested a trend towards a reduction in VTE incidence in the LMWH group (11% and 17%, LMWH group v. placebo, P = 0.3), but with a disconcerting concomitant trend toward an increased risk of major intracranial bleeding (5.1% and 1.2%, LMWH v. placebo, P = 0.2) [44]. Therefore, given the seemingly narrow therapeutic window associated with LMWHs, the role of primary prophylactic anticoagulation in this patient population currently remains unclear, and further data are needed to address this important issue.
Validated prediction models to risk-stratify non-CNS cancer patients according to their specific VTE risk have been published, and could be used to select outpatients with cancer for thromboprophylaxis [41,45,46]. However, few patients receiving potentially thrombogenic anti-angiogenic agents such as thalidomide, lenalidomide and bevacizumab were included. While malignant glioma patients were not included in these analyses as a result of limited patient numbers [45,46], one recent study did include glioma patients and showed that elevated d-dimer and prothrombin fragment 1 + 2 (F 1 + 2) identified patients prone to developing VTE and stratified them into low- and high-risk groups [47]. A prediction model combining clinical markers and measurable biomarkers such as circulating d-dimer, F 1 + 2, VEGF or plasminogen activator inhibitor-1 (PAI-1) levels, as well as tumoral expression of tissue factor (TF) [48–50], could be developed and validated to risk stratify for VTE in malignant glioma patients.
Anticoagulation with LMWH has been proposed to enhance survival through inhibition of angiogenesis and a consequent antineoplastic effect of heparin itself in cancer patients. A recent meta-analysis showed that anticoagulants, specifically LMWHs, both reduced overall mortality in cancer patients without VTE as well as increased bleeding risk [51]. However, these studies all excluded patients with malignant gliomas. A single study examined dalteparin 5000 U subcutaneously (SC) daily with and after traditional radiotherapy in newly diagnosed GBM patients. No significant improvement in survival was noted between treatment and control groups; however, this study was underpowered to resolve this question [52]. Inhibition of coagulation could attenuate cancer progression by decreasing thrombin generation and fibrin formation. Thrombin acts as a potent growth factor and pro-angiogenic factor for cancer cells. Fibrin matrices support migration of tumor cells and provide a scaffold for formation of new blood vessels. Fibrin coats tumors cells and protects them from immune attack, conferring resistance to chemotherapy and mediating attachment to vascular walls, thus enhancing metastasis. LMWH also blocks the adhesion molecules P- and L-selectin, which likely contributes to its anti-angiogenic and anti-metastatic effects in cancer patients [53–56]. The effectiveness of anticoagulation as an antineoplastic strategy remains theoretical, and recent ASCO guidelines conclude that the data are insufficient to support this approach [21].
Pathophysiology of VTE in malignant gliomas
GBM histology
A complex cascade of genetic and cellular events leads to the development of GBM. Genetic events that characterize the transition from lower-grade to malignant gliomas include loss of phosphatase and tensin homolog (PTEN) and VEGF overexpression, two molecular events that have been shown to further up-regulate TF expression [57]. PTEN loss – a genetic signature of glioblastoma rarely noted in lower grade astrocytomas – leads to Akt activation and tumor progression [58]. In GBM, intra-luminal thrombosis is seen in nearly all resected specimens, and the degree of thrombosis correlates with both advanced histologic grade and the subsequent development of VTE [1,12]. Glioblastomas are distinguished from lower-grade gliomas by either necrosis or microvascular hyperplasia, which almost always co-exist. Cells surrounding areas of necrosis are known as ‘pseudopalisading cells’, and they are thought to represent a wave of tumor cells migrating away from a central hypoxic zone created after intravascular thrombosis. These cells secrete pro-angiogenic factors that promote microvascular hyperplasia and tumor expansion. TF expression levels vary within glioblastoma tissues in a pattern consistent with hypoxic regulation with the highest level of expression in hypoxic cells surrounding areas of necrosis. The development of necrosis could be initiated or propagated by vaso-occlusion after intravascular thrombosis within the neoplasm. Both the Ras/MEK/ERK and PI3K/Akt/mTOR pathways are capable of modulating TF expression, particularly under hypoxic conditions; hypoxia has also been shown to induce TF expression via induction of the transcription factor early growth response protein 1 (Egr-1) [59–61]. Vaso-occlusion after intravascular thrombosis could lead directly to the development of hypoxia; the resultant necrosis could be responsible for the induction of the microvascular hyperplasia that defines GBM histology. Factors that may contribute to intra-luminal thrombosis include abnormal blood flow within distorted vasculature, increased interstitial pressure and dysregulation of the balance between pro- and anti-coagulant factors [57,58]. Overexpression of epidermal growth factor receptor (EGFR) in human glioma cells causes increased TF expression, and stimulation of EGFR leads to dose-dependent up-regulation of TF independently of hypoxia [62]. Glioma cells often express a truncated, oncogenic form of EGFR known as EGFRvIII; expression of this form stimulates formation of MPs containing EGFRvIII, leading to increased TF expression, enhanced VEGF production and angiogenesis [63,64]. Oncogenic mechanisms are responsible for the up-regulation of TF expression by GBM cells; this TF-rich, prothromobotic environment could lead to local thrombosis, necrosis, hypoxia-induced angiogenesis and peripheral tumor growth in gliomas [62]. In fact, a potent exogenous TF inhibitor, Ixolaris, has recently been shown to block the in vivo growth of human glioblastoma cells in a xenograft model. Ixolaris may function by both attenuating the procoagulant state, as well as preventing angiogenesis, thus interrupting both tumor growth and metastasis, representing a novel therapeutic target for the treatment of malignant glioma patients [65].
Cellular mechanisms of thrombogenesis
The mechanistic relationship between cancer and thrombogenesis has been extensively studied but remains incompletely understood [66,67]. Tumor-mediated extrinsic vascular compression and invasion can obstruct venous return resulting in clot formation, endothelial injury and activation of the coagulation cascade. Tumor cells may promote thrombin generation by eliciting intravascular TF expression on host monocytes and endothelial cells [26,68]. TF expression is induced by inflammatory cytokines such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α); these factors also downregulate endothelial expression of the anticoagulant protein thrombomodulin leading to a net prothrombotic condition in the vasculature. PAI-1, a member of the serine protease inhibitor (SERPIN) family that inhibits fibrinolysis, is also transcriptionally up-regulated thereby contributing to the pro-thrombotic state [58,69,70].
TF is a target of two of the most common genetic alterations in tumorigenesis, namely inactivation of p53 and mutation of k-ras [71]. TF is a member of the interferon receptor family and it has been described to participate in both regulation of tumor cell growth and stimulation of tumor angiogenesis [70]; inhibition of TF reduces tumor growth in several mouse models [72]. TF may enhance metastatic potential by influencing cellular adhesion and migratory functions [73], and supporting the survival of micrometastases [74]. Tumoral TF expression correlates with advanced clinical stage, histologic grade and poor prognosis in several solid tumor types including gastric, prostate and ovarian malignancies. TF expression has also been shown to correlate with enhanced angiogenesis in several malignancies including hepatocellular, colorectal and prostate cancers, and VTE is 4-fold more common among patients with high TF-expressing carcinomas [16,18,48,49,68,71].
Correlation between enhanced TF expression and advanced histologic grade has also been shown in gliomas [70]. In glioma patients, the combination of TF up-regulation and PAI-1 release can lead to a low-grade disseminated intravascular coagulation (DIC) state [2,68,75]. Elevated PAI-1 levels are seen more prominently in glioblastomas than low-grade gliomas, and its secretion has been correlated with tumor aggressiveness and infiltration of surrounding healthy brain tissue [76]. Glioma patients manifest higher plasma levels of thrombosis-associated biomarkers including D-dimer, lipoprotein (a), homocysteine, VEGF, tissue plasminogen activator (tPA) and PAI-1 relative to patients without glioma, and it has been suggested that these changes contribute to the increased risk of VTE [77,78].
TF expressing microparticles
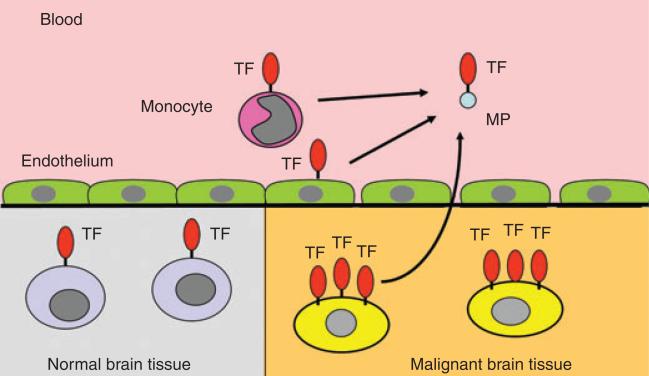
Normal glial tissue has a very high constitutive expression of TF [68,79,80]. As already described, TF has been shown to function in tumor initiation, tumor growth, angiogenesis and metastasis in glioma cells [63], and a direct correlation between TF levels and tumor grade has been shown in gliomas [70]. The constitutive expression of TF in tumor cells may be augmented by TF expression in host monocytes and vascular cells. TF circulates on small membrane fragments, or MPs, that are shed during cellular activation or apoptosis and may serve to disseminate membrane-bound TF throughout the circulation (Fig. 1). Recent studies have shown that MPs expressing TF (TF-MPs) may be detected in plasma from a variety of cancer patients, including those with lung, pancreatic, breast and colon cancers; further, the level of TF-MPs may correlate with the risk of thrombosis in these patients [81–84].
Fig. 1.
Normal brain tissue constitutively expresses tissue factor (TF), and expression is increased on malignant brain tissue cells. In this way, TF may play a role in tumor growth. TF circulates on microparticles (TF-MPs) that are shed during cellular activation or apoptosis. Circulating TF-MPs may be derived from both malignant brain tissue, as well as from upregulated, host cell-derived sources.
TF-MPs account for most of the TF released from cancer cells including glioblastoma cells [85], and tumor cell-derived MPs show strong procoagulant activity in vitro and in vivo [86]. Together these data suggest a mechanism for the prothrombotic state observed in glioma patients. However, this hypothesis has yet to be supported by data from prospective clinical studies in glioma patients.
Cancer treatment-related procoagulant state
Certain chemotherapeutic agents such as cisplatin have been shown to induce the release of procoagulant TF-MPs from cultured endothelial cells [87]. Bevacizumab, a monoclonal VEGF antibody, highlights the interesting interplay of VEGF and the coagulation cascade in the development and treatment of malignant gliomas. The pathophysiologic mechanisms responsible for the dysregulation of coagulation observed with bevacizumab remain elusive. VEGF regulates vascular proliferation and permeability, and functions as an anti-apoptotic factor. It has been postulated that when bevacizumab inactivates VEGF, the renewal capacity of endothelial cells is diminished resulting in bleeding, whereas the clotting risk is simultaneously increased by enhanced exposure of sub-endothelial collagen and TF [88]. Immune complexes of bevacizumab and VEGF also induce platelet aggregation and degranulation via activation of the platelet FCγRIIa receptor [89]. It has previously been demonstrated that VEGF is capable of transcriptionally activating TF expression in endothelial cells [90,91], and conversely, TF can induce VEGF expression [92,93]. Therefore, high TF expression in some tumor cells may up-regulate VEGF expression, thereby accounting for the observation that high pre-treatment VEGF expression predicts poor clinical outcomes in small cell lung cancer, hepatocellular carcinoma and melanoma patients [94–96]. Consistent with this hypothesis, high-grade glioma specimens show normalization of vascular morphology with the development of thin-walled, evenly distributed vessels after treatment with bevacizumab [16,97].
Conclusions
The risk of VTE in malignant glioma patients is extraordinarily high, and extends beyond the post-operative period. The procoagulant molecule TF appears to play an important role in the pathobiology of GBM including tumor growth, angiogenesis, metastasis and possibly also in thrombogenesis related to it. Thromboembolic events portend a worse outcome in malignant glioma patients relative to those without thromboembolic complications. VTE thromboprophylaxis is utilized with caution in glioma patients because of the concern for intracranial hemorrhage, and additional definition of the risks and benefits of thrombosis prophylaxis is urgently needed. The new anti-angiogenic agent bevacizumab used in malignant glioma is associated with a modestly increased risk of thromboembolism in other forms of cancer; while this risk may also extend to glioblastoma, more data are needed to better delineate this relationship. Additional data are necessary to elucidate the pathophysiology and magnitude of excessive VTE risk, and to evaluate the risks and benefits of primary VTE prevention in malignant glioma patients.
Acknowledgement
N. S. Key and N. Mackman were supported by RO1 HL-095096.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Knovich MA, Lesser GJ. The management of thromboembolic disease in patients with central nervous system malignancies. Current Treat Options Oncol. 2004;5:511–7. doi: 10.1007/s11864-004-0039-x. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Schiff D, Kesari S, Drappatz J, Gigas DC, Doherty L. Medical management of patients with brain tumors. J Neurooncol. 2006;80:313–32. doi: 10.1007/s11060-006-9193-2. [DOI] [PubMed] [Google Scholar]
- 3.Tabori U, Beni-Adani L, Dvir R, Burstein Y, Feldman Z, Pessach I, Rechavi G, Constantini S, Toren A. Risk of venous thromboembolism in pediatric patients with brain tumors. Pediatr Blood Cancer. 2004;43:633–6. doi: 10.1002/pbc.20149. [DOI] [PubMed] [Google Scholar]
- 4.Marras CL, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma. Cancer. 2000;89:640–6. doi: 10.1002/1097-0142(20000801)89:3<640::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Brandes AA, Scelzi E, Salmistraro G, Ermani M, Carollo C, Berti F, Zampieri P, Baiocchi C, Fiorentino MV. Incidence and risk of thromboembolism during treatment of high-grade gliomas: a prospective study. Eur J Cancer. 1997;33:1592–6. doi: 10.1016/s0959-8049(97)00167-6. [DOI] [PubMed] [Google Scholar]
- 6.Simanek R, Vormittag R, Hassler M, Roessler K, Schwarz M, Zielinski C, Pabinger I, Marosi C. Venous thromboembolism and survival in patients with high-grade glioma. Neuro Oncol. 2007;9:89–95. doi: 10.1215/15228517-2006-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerber DE, Grossman SA, Streiff MB. Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol. 2006;24:1310–8. doi: 10.1200/JCO.2005.04.6656. [DOI] [PubMed] [Google Scholar]
- 8.Semrad TJ, O'Donnell R, Wun T, Chew H, Harvey D, Zhou H, White RH. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg. 2007;106:601–8. doi: 10.3171/jns.2007.106.4.601. [DOI] [PubMed] [Google Scholar]
- 9.Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, Zhou H, White RH. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24:1112–8. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- 10.Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol. 1983;13:334–6. doi: 10.1002/ana.410130320. [DOI] [PubMed] [Google Scholar]
- 11.Streiff MB, Segal J, Grossman SA, Kickler TS, Weir EG. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer. 2004;100:1717–23. doi: 10.1002/cncr.20150. [DOI] [PubMed] [Google Scholar]
- 12.Rodas RA, Fenstermaker RA, McKeever PE, Blaivas M, Dickinson LD, Papadopoulos SM, Hoff JT, Hopkins LN, Duffy-Fronckowiak M, Greenberg HS. Correlation of intraluminal thrombosis in brain tumor vessels with postoperative thrombotic complications: a preliminary report. J Neurosurg. 1998;89:200–5. doi: 10.3171/jns.1998.89.2.0200. [DOI] [PubMed] [Google Scholar]
- 13.Dhami MS, Bona RD, Calogero JA, Hellman RM. Venous thromboembolism and high grade gliomas. Thromb Haemost. 1993;70:393–6. [PubMed] [Google Scholar]
- 14.Walsh DC, Kakkar AK. Thromboembolism in brain tumors. Curr Opin Pulm Med. 2001;7:326–31. doi: 10.1097/00063198-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–5. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elice F, Jacoub J, Rickles FR, Falanga A, Rodeghiero F. Hemostatic complications of angiogenesis inhibitors in cancer patients. Am J Hematol. 2008;83:862–70. doi: 10.1002/ajh.21277. [DOI] [PubMed] [Google Scholar]
- 17.Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69(Suppl 3):25–33. doi: 10.1159/000088481. [DOI] [PubMed] [Google Scholar]
- 18.Khorana AA, Rao MV. Approaches to risk-stratifying cancer patients for venous thromboembolism. Thromb Res. 2007;120(Suppl 2):41–50. doi: 10.1016/S0049-3848(07)70129-9. [DOI] [PubMed] [Google Scholar]
- 19.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 20.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Alfred Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 21.Lyman GH, Khorana AA, Falanga A, Clarke-Pearson D, Flowers C, Jahanzeb M, Kakkar A, Kuderer NM, Levine MN, Liebman H, Mendelson D, Raskob G, Somerfield MR, Thodiyil P, Trent D, Francis CW, American Society of Clinical Oncology American society of clinical oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 22.Shah MA, Ilson D, Kelsen DP. Thromboembolic events in gastric cancer: high incidence in patients receiving irinotecan- and bevacizumab-based therapy. J Clin Oncol. 2005;23:2574–6. doi: 10.1200/JCO.2005.81.908. [DOI] [PubMed] [Google Scholar]
- 23.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with angiogenesis inhibitor Bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–85. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 24.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JH. Prognosis of cancers associated with venous thromboembolism. N Eng J Med. 2000;343:1846–50. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 25.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 26.Dietcher SR. Cancer and thrombosis: mechanisms and treatment. J Thromb Thrombolysis. 2003;16:21–31. doi: 10.1023/B:THRO.0000014589.17314.24. [DOI] [PubMed] [Google Scholar]
- 27.Ghanim AJ, Daskalakis C, Eschelman DJ, Kraft WK. A five-year, retrospective, comparison review of survival in neurosurgical patients diagnosed with venous thromboembolism and treated with either inferior vena cava filters or anticoagulants. J Thromb Thrombolysis. 2007;24:247–54. doi: 10.1007/s11239-007-0025-9. [DOI] [PubMed] [Google Scholar]
- 28.Dickinson LD, Miller LD, Patel CP, Gupta SK. Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery. 1998;43:1074–81. doi: 10.1097/00006123-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz RE, Marrero AM, Conlon KC, Burt M. Inferior vena cava filters in cancer patients: indications and outcomes. J Clin Oncol. 1996;14:652–7. doi: 10.1200/JCO.1996.14.2.652. [DOI] [PubMed] [Google Scholar]
- 30.Lin J, Proctor MC, Varma M, Greenfield LJ, Upchurch GR, Jr, Henke PK. Factors associated with recurrent venous thromboembolism in patients with malignant disease. J Vasc Surg. 2003;23:976–83. doi: 10.1067/mva.2003.191. [DOI] [PubMed] [Google Scholar]
- 31.Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer. 1994;73:493–8. doi: 10.1002/1097-0142(19940115)73:2<493::aid-cncr2820730240>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 32.Spyropoulos AC, Brotman DJ, Amin AN, Dietelzweig SB, Jaffer AK, McKean SC. Prevention of venous thromboembolism in the cancer surgery patient. Cleve Clin J Med. 2008;3(Suppl 3):17–26. doi: 10.3949/ccjm.75.suppl_3.s17. [DOI] [PubMed] [Google Scholar]
- 33.Schunn C, Schunn GB, Hobbs G, Vona-Davis LC, Waheed U. Inferior vena cava filter placement in late-stage cancer. Vasc Endovascular Surg. 2006;40:287–94. doi: 10.1177/1538574406291821. [DOI] [PubMed] [Google Scholar]
- 34.Cavaliere R, Schiff D. Fatal pulmonary embolism despite an inferior vena cava filter in gliobastoma multiforme. Neurocrit Care. 2005;3:249–50. doi: 10.1385/NCC:3:3:249. [DOI] [PubMed] [Google Scholar]
- 35.Levin JM, Schiff D, Loeffler JS, Fine HA, Black PM, Wen PY. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology. 1993;43:1111–4. doi: 10.1212/wnl.43.6.1111. [DOI] [PubMed] [Google Scholar]
- 36.Nghiemphu PL, Green RM, Pope WB, Lai A, Cloughesy TF. Safety of anticoagulation use and bevacizumab in patients with glioma. Neuro Oncol. 2008;10:355–60. doi: 10.1215/15228517-2008-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agnelli G, Piovella F, Buoncristiani P, Severi P, Pini M, D'Angelo A, Beltrametti C, Damiani M, Andrioli GC, Pugliese R, Iorio A, Brambilla G. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Eng J Med. 1998;339:80–5. doi: 10.1056/NEJM199807093390204. [DOI] [PubMed] [Google Scholar]
- 38.Goldhaber SZ, Dunn K, Gerhard-Herman M, Park JK, Black PM. Low rate of venous thromboembolism after craniotomy for brain tumor using multimodality prophylaxis. Chest. 2002;122:1933–7. doi: 10.1378/chest.122.6.1933. [DOI] [PubMed] [Google Scholar]
- 39.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW, American College of Chest Physicians Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(Suppl 6):381–453. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 40.Kakkar AK, Haas S, Wolf H, Encke A. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost. 2005;94:867–71. doi: 10.1160/TH04-03-0189. [DOI] [PubMed] [Google Scholar]
- 41.Cohen AT, Alikhan R, Arcelus JI, Begmann JF, Haas S, Merli GJ, Spyropoulos AC, Tapson VF, Turpie AG. Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients. Thromb Haemost. 2005;94:750–9. [PubMed] [Google Scholar]
- 42.Levine M, Hirsh J, Gent M, Arnold A, Warr D, Falanga A, Samosh M, Bramwell V, Pritchard KI, Stewart D, Goodwin P. Double-blind randomised trial of very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994;343:886–9. doi: 10.1016/s0140-6736(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 43.Agnelli G, Verso M. Thromboprophylaxis during chemotherapy after advanced cancer. Thromb Res. 2007;120(Suppl 2):128–32. doi: 10.1016/S0049-3848(07)70141-X. [DOI] [PubMed] [Google Scholar]
- 44.Perry JR, Rogers L, Laperriere N, Julian J, Geerts W, Agnelli G, Malkin M, Sawaya R, Baker R, Levine M. PRODIGE: a phase III randomized placebo-controlled trial of thromboprophylaxis using dalteparin low molecular weight heparin (LMWH) in patients with newly diagnosed malignant glioma. J Clin Oncol. 2007;25(Suppl):2011. doi: 10.1111/j.1538-7836.2010.03973.x. ASCO Annual Meeting Proceedings. [DOI] [PubMed] [Google Scholar]
- 45.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zakai NA, Wright J, Cushman M. Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score. J Thromb Haemost. 2004;2:2156–61. doi: 10.1111/j.1538-7836.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 47.Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the vienna cancer and thrombosis study. J Clin Oncol. 2009;27:4124–9. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 48.Nakasaki T, Wada H, Shigemori C, Miki C, Gabazza EC, Nobori T, Nakamura S, Shiku H. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol. 2002;69:247–54. doi: 10.1002/ajh.10061. [DOI] [PubMed] [Google Scholar]
- 49.Poon RT, Lau CP, Ho JW, Yu WC, Fan ST, Wong J. Tissue factor expression correlates with tumor angiogenesis and invasiveness of human hepatocellular carcinoma. Clin Cancer Res. 2003;9:5339–45. [PubMed] [Google Scholar]
- 50.Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, Hostetter G, Harvey J, Taubman MB. Tissue factor expression, angiogenesis and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–5. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 51.Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment. Cancer. 2007;110:1149–60. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 52.Robins HI, O'Neill A, Gilbert M, Olsen M, Sapiente R, Berkey B, Mehta M. Effect of dalteparin and radiation on survival and thromboembolic events in glioblastoma multiforme: a phase II ECOG trial. Cancer Chemother Pharmacol. 2008;62:227–33. doi: 10.1007/s00280-007-0596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frank RD, Schabbauer G, Holscher T, Sato Y, Tencati M, Pawlinski R, Mackman N. The synthetic pentasaccharide fondaparinux reduces coagulation, inflammation and neutrophil accumulation in kidney ischemia–reperfusion injury. J Thromb Haemost. 2005;3:531–40. doi: 10.1111/j.1538-7836.2005.01188.x. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Brown JR, Varki A, Esko JD. Heparin's anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J Clin Invest. 2002;110:127–36. doi: 10.1172/JCI14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic agents. Nat Rev Cancer. 2005;5:526–42. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 56.Borsig L, Wong R, Feramisco R, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98:3352–7. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tehrani M, Friedman TM, Olson JJ, Brat DJ. Intravascular thrombosis in central nervous system malignancies: a potential role in astrocytoma progression to glioblastoma. Brain Pathol. 2008;18:164–71. doi: 10.1111/j.1750-3639.2007.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rong Y, Post DE, Pieper RO, Durden DL, van Meir EG, Brat DJ. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res. 2005;65:1406–13. doi: 10.1158/0008-5472.CAN-04-3376. [DOI] [PubMed] [Google Scholar]
- 59.Guha M, O'Connell MA, Pawlinski R, Hollis A, McGovern P, Yan SF, Stern D, Mackman N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood. 2001;98:1429–39. doi: 10.1182/blood.v98.5.1429. [DOI] [PubMed] [Google Scholar]
- 60.Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky DJ, Stern DM. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355–61. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- 61.Pawlinski R, Pedersen B, Kehrle B, Aird WC, Frank RD, Guha M, Mackman N. Regulation of tissue factor and inflammatory mediators by Egr-1 in a mouse endotoxemia model. Blood. 2003;101:3940–7. doi: 10.1182/blood-2002-07-2303. [DOI] [PubMed] [Google Scholar]
- 62.Rong Y, Belozerov VE, Tucker-Burden C, Chen G, Durden DL, Olson JJ, vanMeir EG, Mackman N, Brat DJ. Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/Activator Protein-1 transcriptional activity. Cancer Res. 2009;69:2540–3549. doi: 10.1158/0008-5472.CAN-08-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milsom CC, Yu JL, Mackman N, Micallef J, Anderson GM, Guha A, Rak JW. Tissue factor regulation by epidermal growth factor receptor and epithelial-to-mesenchymal transitions: effect on tumor initation and angiogenesis. Cancer Res. 2008;68:100068–76. doi: 10.1158/0008-5472.CAN-08-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumor cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 65.Carneiro-Lobo TC, Konig S, Machado DE, Nasciutti LE, Forni MF, Francischetti IMB, Sogayar MC, Monteiro RQ. Ixolaris, a tissue factor inhibitor, blocks primary tumor growth and angiogenesis in a glioblastoma model. J Thromb Haemost. 2009;7:1855–64. doi: 10.1111/j.1538-7836.2009.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–9. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furie B, Furie BC. Mechanisms of thrombus formation. N Eng J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 68.Thoron L, Arbit E. Hemostatic changes in patients with brain tumors. J Neurooncol. 1994;22:87–100. doi: 10.1007/BF01052885. [DOI] [PubMed] [Google Scholar]
- 69.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–10. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 70.Hamada K, Kuratsu J, Saitoh Y, Takeshima H, Nishi T, Ushio Y. Expression of tissue factor correlates with grade of malignancy in human glioma. Cancer. 1996;77:1877–83. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1877::AID-CNCR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 71.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–41. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 72.McEachron T, Mackman N. Tumors, ticks and tissue factor. J Thromb Haemost. 2009;7:1852–4. doi: 10.1111/j.1538-7836.2009.03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruf W, Fischer EG, Huang HY, Miyagi Y, Ott I, Riewald M, Mueller BM. Diverse functions of protease receptor tissue factor in inflammation and metastasis. Immunol Res. 2000;21:289–92. doi: 10.1385/IR:36:1:289. [DOI] [PubMed] [Google Scholar]
- 74.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Hu Z, Barney KA, Degen JL. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-depedent and -independent mechanisms. Blood. 2007;110:133–41. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerber DE, Segal JB, Salhotra A, Olivi A, Grossman SA, Streiff MB. Venous thromboembolism occurs infrequently in meningioma patients receiving combined modality prophylaxis. Cancer. 2007;109:300–5. doi: 10.1002/cncr.22405. [DOI] [PubMed] [Google Scholar]
- 76.Rao JS, Rayford A, Morantz RA, Festoff BW, Sawaya R. Increased levels of plasminogen activator inhibitor-1 (PAI-1) in human brain tumors. J Neurooncol. 1993;17:215–21. doi: 10.1007/BF01049977. [DOI] [PubMed] [Google Scholar]
- 77.Iberti TJ, Miller M, Abalos A, Fischer EP, Post KD, Benjamin E, Oropello JM, Wiltshire-Clement M, Rand JH. Abnormal coagulation profile in brain tumor patients during surgery. Neurosurgery. 1994;34:389–94. doi: 10.1227/00006123-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 78.Sciacca FL, Ciusani E, Silvani A, Corsini E, Frigerio S, Pogliani S, Parati E, Croci D, Boiardi A, Salmaggi A. Genetic and plasma markers of venous thromboembolism in patients with high grade glioma. Clin Cancer Res. 2004;10:1312–7. doi: 10.1158/1078-0432.ccr-03-0198. [DOI] [PubMed] [Google Scholar]
- 79.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues: implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–97. [PMC free article] [PubMed] [Google Scholar]
- 80.Eddleston M, De la Torre JC, Oldstone MB, Loskutoff DJ, Edgington TS, Mackman N. Astrocytes are the primary source of tissue factor in the murine central nervous system. J Clin Invest. 1993;92:349–58. doi: 10.1172/JCI116573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tesselaar MT, Romijn FP, van der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2006;5:520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 82.Tilley RE, Holscher T, Belani R, Nieva J, Mackman N. Tissue factor activity is increased in a combined platelet and microparticle sample from cancer patients. Thromb Res. 2008;122:604–9. doi: 10.1016/j.thromres.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tesselaar MT, Osanto S. Risk of venous thromboembolism in lung cancer. Curr Opin Pulm Med. 2007;13:362–7. doi: 10.1097/MCP.0b013e328209413c. [DOI] [PubMed] [Google Scholar]
- 84.Hron G, Kollars M, Weber H, Sagaster V, Quehenberger P, Eichinger S, Kyrle PA, Weltermann A. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97:119–23. [PubMed] [Google Scholar]
- 85.Yu JL, Rak JW. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J Thromb Haemost. 2004;2:2065–7. doi: 10.1111/j.1538-7836.2004.00972.x. [DOI] [PubMed] [Google Scholar]
- 86.Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost. 2008;6:1517–24. doi: 10.1111/j.1538-7836.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- 87.Lechner D, Kollars M, Gleiss A, Kyrle PA, Weltermann A. Chemo-therapy-induced thrombin generation via procoagulant endothelial microparticles is independent of tissue factor activity. J Thromb Haemost. 2007;5:2445–52. doi: 10.1111/j.1538-7836.2007.02788.x. [DOI] [PubMed] [Google Scholar]
- 88.Kilickap S, Abali H, Celik I. Bevacizumab, bleeding, thrombosis and warfarin. J Clin Oncol. 2003;21:3543. doi: 10.1200/JCO.2003.99.046. [DOI] [PubMed] [Google Scholar]
- 89.Meyer T, Robles-Carrillo L, Robson T, Langer F, Desai H, Davila M, Amaya M, Francis JL, Amirkhosravi A. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost. 2009;7:171–81. doi: 10.1111/j.1538-7836.2008.03212.x. [DOI] [PubMed] [Google Scholar]
- 90.Mechtcheriakova D, Schabbauer G, Lucerna M, Clauss M, De Martin R, Binder BR, Hofer E. Specificity, diversity, and convergence in VEGF and TNF-a signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells. FASEB J. 2001;15:230–42. doi: 10.1096/fj.00-0247com. [DOI] [PubMed] [Google Scholar]
- 91.Armesilla AL, Lorenzo E, Gomez del Arco P, Martinez-Martinez S, Alfranca A, Redondo JM. Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression. Mol Cell Biol. 1999;19:2032–43. doi: 10.1128/mcb.19.3.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Belting M, Ahamed J, Ruf W. Signaling of the tissue factor pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005;25:1545–50. doi: 10.1161/01.ATV.0000171155.05809.bf. [DOI] [PubMed] [Google Scholar]
- 93.Chen J, Kasper M, Heck T, Nakagawa K, Humpert PM, Bai L, Wu G, Zhang Y, Luther T, Andrassy M, Schiekofer S, Hamann A, Morcos M, Chen B, Stern DM, Nawroth PP, Bierhaus A. Tissue factor as a link between wounding and tissue repair. Diabetes. 2005;54:2143–54. doi: 10.2337/diabetes.54.7.2143. [DOI] [PubMed] [Google Scholar]
- 94.Ustuner Z, Saip P, Yasasever V, Vural B, Yazar A, Bal C, Ozturk B, Ozbek U, Topuz E. Prognostic and predictive value of vascular endothelial growth factor and its soluble receptors, VEGFR-1 and VEGFR-2 levels in the sera of small cell lung cancer patients. Med Oncol. 2008;25:394–9. doi: 10.1007/s12032-008-9052-4. [DOI] [PubMed] [Google Scholar]
- 95.Poon RT, Lau C, Pang R, Ng KK, Yuen J, Fan ST. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: importance of tumor biomarker in ablative therapies. Ann Surg Oncol. 2007;14:1835–45. doi: 10.1245/s10434-007-9366-z. [DOI] [PubMed] [Google Scholar]
- 96.Ascierto PA, Leonardi E, Ottaiano A, Napolitano M, Scala S, Castello G. Prognostic value of serum VEGF in melanoma patients: a pilot study. Anticancer Res. 2004;24:4255–8. [PubMed] [Google Scholar]
- 97.Fischer I, Cunliffe CH, Bollo RJ, Raza S, Monoky D, Chiriboga L, Parker EC, Golfinos JG, Kelly PJ, Knopp EA, Gruber ML, Zagzag D, Narayana A. High-grade glioma before and after treatment with radiation and Avastin: initial observations. Neuro Oncol. 2008;10:700–8. doi: 10.1215/15228517-2008-042. [DOI] [PMC free article] [PubMed] [Google Scholar]