Abstract
The severity of ischaemic heart disease is markedly enhanced in type 2 diabetes. We recently reported that complement activation exacerbates I/R injury in the type 2 diabetic heart. The purpose of this study was to isolate and examine MBL pathway activation following I/R injury in the diabetic heart. ZLC and ZDF rats underwent 30 minutes of left coronary artery occlusion followed by 120 minutes of reperfusion. Two different groups of ZDF rats were treated with either FUT-175, a broad complement inhibitor, or P2D5, a monoclonal antibody raised against rat MBL-A. ZDF rats treated with FUT175 and P2D5 had significantly decreased myocardial infarct size, C3 deposition and neutrophil accumulation compared with untreated ZDF controls. Taken together, these findings indicate that the MBL pathway plays a key role in the severity of complement-mediated I/R injury in the type 2 diabetic heart.
Keywords: complement, Zucker Diabetic Fatty rat, inflammation, ischaemia-reperfusion injury
Introduction
Cardiovascular disease is the leading cause of death and serious illness in the developed world. Among this population, people with type 2 diabetes have more than twice the risk of severe and fatal heart attacks than non-diabetics. In an effort to understand why ischaemic heart disease is enhanced in type 2 diabetes, roles for oxidative stress,1 AGEs,1 and impairments in NO-mediated pathways2 have been explored.
A consistent relationship has been found between chronic inflammation and the cardiovascular complications of diabetes.1,3 Complement activation plays an important role in the inflammatory response and is known to be involved in I/R injury in the non-diabetic heart.4,5 Recently, we reported a significant role for complement activation in increasing myocardial I/R injury in a rat model of type 2 diabetes.6 Involvement of complement in the enhanced I/R injury in type 2 diabetes was a novel finding; however, the specific complement pathway responsible for the increased I/R injury was not addressed. Understanding which specific pathway of the complement system is activated in the diabetic heart following I/R is necessary for proper treatment and prevention of reperfusion injury in this rapidly growing patient population.
The complement cascade is part of the innate immune system responsible for the initiation of inflammation and elimination of invading foreign cells. There are three independent pathways which can initiate the complement cascade: the classical, alternative, and more recently described lectin pathway. Complement activation contributes to tissue injury in non-human primate myocardial infarction,7 as well as cardiac injury in animal models of ischaemia.6 Indeed, post-mortem examination of human hearts has identified deposition of the MAC C5b-9 on damaged muscle fibres.8
It is now well understood that neutrophil trapping and sequestration in the coronary microcirculation plays a significant role in myocardial I/R injury.9 However, the effects of neutrophil accumulation are not solely confined to mechanical plugging of small coronary capillaries, contributing to the so-called ‘no-reflow’ phenomenon. Under reduced flow rates, such as during ischaemia and reperfusion, neutrophils initially sequester in the heart by adhering to the venular endothelium.10 Production of the complement cleavage product C5a primarily acts as the principal chemotactic factor for circulating neutrophils, thereby increasing neutrophil adhesion to the endothelium by mobilising internal stores of neutrophil complement receptor-1, CD11b/CD18, and CD11c.11 C5a is also capable of stimulating neutrophils to produce and release reactive oxygen species, proteolytic enzymes, and lipoxygenase products (leukotrienes) that influence platelet and endothelial function, inducing vasoconstriction and platelet aggregation.12 The finding that C5a indirectly induces the production of chemokines, cytokines, and other pro-inflammatory mediators13,14 provides yet another link between complement activation, inflammation, and impaired cardiovascular function in the type 2 diabetic heart.
Jordan et al. found that blockade of the lectin pathway is cardioprotective following I/R by reducing neutrophil infiltration and attenuating pro-inflammatory gene expression.4 Additionally, echocardiographic analysis of cardiac function in MBL-null mice following myocardial I/R indicated improved LV function, as measured by ejection fraction, compared with both wild-type and C1q-null mice.15 Together, these data point to a significant contribution of MBL to cellular injury and LV function in post-ischaemic myocardial tissues. Hansen and colleagues reported that patients with a history of cardiovascular disease had significantly elevated MBL levels, suggesting that MBL may be involved in the pathogenesis of micro- and macrovascular complications in type 1 diabetes.16 To our knowledge, no studies have examined the role of the MBL pathway in I/R in the type 2 diabetic heart. Thus, the present study was performed to test the specific role of MBL pathway activation in myocardial I/R injury in a rat model of type 2 diabetes.
Research design and methods
Animals
All procedures were reviewed and approved by the Institute for Laboratory Animal Research Guide for Care and Use of Laboratory Animals. Male ZDF fa/fa rats and their aged matched lean litter mates (ZLC fa/-) were obtained from Charles River GMI Labs at 10 weeks of age. Housing was under controlled conditions of light (12 h light–dark) and temperature (22–24°C). Rats were fed Purina 5008, a 6% fat rodent diet, ad libitum. This animal model of non-insulin dependent diabetes mellitus develops hyperglycaemia and insulin resistance at approximately 7 weeks of age and glucose concentration reaches 500 mmol/L by 10 to 11 weeks of age.17
Complement inhibition
FUT-175 (Futhan, nafamostat mesylate; BIOMOL International) is a serine protease inhibitor with potent inhibitory activity against the C1r and C1s subunits of the classical pathway of the complement cascade, as well as factors B and D of the alternative pathway.18,19 To determine if FUT-175 inhibits MBL-dependent C3 deposition ex vivo, rat sera samples were collected and subjected to a MBL-dependent complement-activation FLISA as previously described and modified herein to evaluate C3b deposition for rat sera.20 Briefly, 2% rat serum (diluted with veronal-buffered saline with Ca++ and Mg++ (VBS++)) was added to BSA–GlcNAc-coated 384-well microplates and incubated at 37°C for 30 min. The plates were washed and incubated with goat anti-rat C3 antibody (MP Biomedicals), followed by washing and detection with donkey anti-goat IRDye® 800 antibody (1:3,000; Rockland). After washing, C3 deposition was quantified with an Odyssey system, as we have described.20 Background integrated intensity (e.g. wells containing only VBS++) was subtracted from all wells. The data are presented with background integrated intensity from control wells (e.g. VBS++ only). FUT-175 inhibits MASP-2 activity in a concentration-dependent manner as measured using a C4b and C3b deposition FLISA (data not shown). FUT-175 prevents complement activation in vitro and in vivo.21,22 In this study, FUT-175 was dissolved in sterile saline and was administered 5 min prior to reperfusion (1 mg/kg body weight) via intravenous bolus.
Anti-rat MBL-A antibody
Anti-rat (r) MBL-A mAb, P2D5, was raised and purified as previously described.4 An MBL-dependent complement-activation ELISA was used to determine antibody specificity. Briefly, GlcNAc was conjugated to BSA (Sigma) and plated onto microtiter plates. Rat serum was incubated with: (1) vehicle consisting of veronal-buffered saline with calcium and magnesium (VEH); (2) d-mannose, a known MBL inhibitor; or (3) various concentrations of mAb P2D5. These serum samples were added to the GlcNAc-BSA plates and incubated for 30 min at 37°C. The plates were washed, incubated with HRP-conjugated goat anti-rat C3 antibody (1:2,000; Cappel), and C3 deposition was quantified using a microplate reader. Background optical density (VEH only) was subtracted from experimental wells. In this study, a group of animals were dosed with 10 mg/kg (body weight) P2D5 intravenously 5 min prior to ischaemia.
Myocardial I/R protocol
The regional myocardial I/R protocol was described in detail earlier.6 ZLC and ZDF rats were anaesthetised with sodium pentobarbital (50 mg/kg IP) and polyethylene catheters (PE-10) were inserted and secured into the right femoral artery and vein for blood sampling, blood pressure monitoring, and drug administration. Rats were divided into four groups: (1) ZLC+PBS bolus infusion; (2) ZDF+PBS bolus infusion; (3) ZDF+FUT-175 bolus infusion (1 mg/kg); or (4) ZDF+P2D5 bolus infusion (10 mg/kg). Following 30 min of ischaemia, the ligature was unclamped and the ischaemic myocardium was reperfused for 120 min.
Myocardial infarct size
The protocol for determining infarct size was described in detail previously.6 Infarct size is expressed as the percentage of the AAR (AI/AAR×100).
Histology
Following I/R, ZLC and ZDF hearts were excised and cut into 4 mm coronal sections. Sections were frozen, sectioned, and mounted on slides as previously described.6 Analysis of C3 immunostaining was performed blinded, in duplicate, and data expressed as the percentage of C3-positive area to total LV area (%C3/LV).
A separate set of slides was stained for neutrophil sequestration within regions of C3 deposition using Naphthol AS-D Choloroacetate Esterase (Sigma) as previously described.6,23 Data are expressed as the number of neutrophils per 5 fields at 40× magnification.
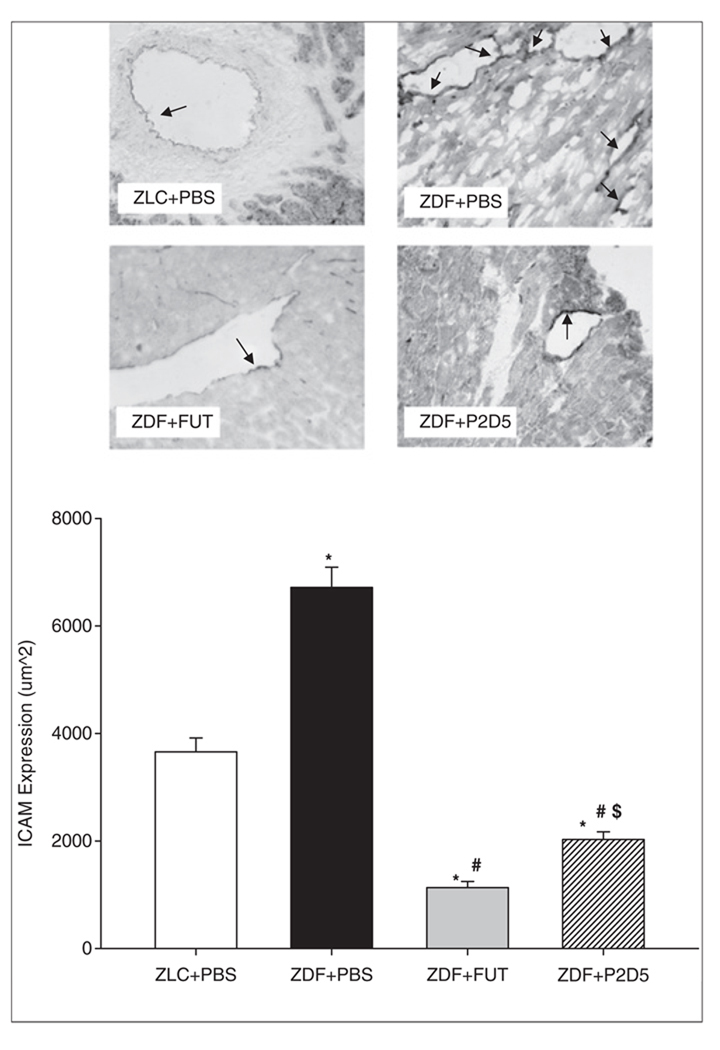
ICAM-1 (CD54) expression was examined in frozen myocardial tissue sections as a measure of endothelial cell activation following I/R. ICAM-1 expression was analysed as previously described.6 Data are presented as the area of ICAM staining (µm2). The camera acquisition settings remained the same for all groups and 25 non-overlapping semi-random fields were examined for each heart section.
Statistical analysis
All values are expressed as means ± standard error (SEM). Comparisons between non-diabetic and diabetic groups were made using a two-tailed independent t-test. Differences were considered significant at p≤0.05. SigmaStat 3.0 software (Jandel Scientific) was used for statistical analysis.
Results
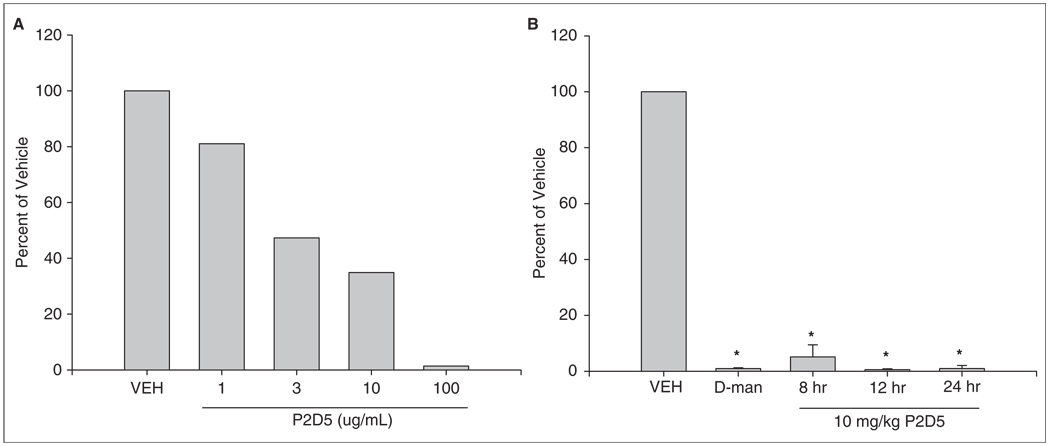
Recognition of rMBL-A by P2D5 mAb
Complement activation and C3 deposition via the lectin pathway were assessed in vitro by C3 ELISA. P2D5 exhibits a concentration-dependent inhibition of the lectin pathway in vitro in the GlcNAc-BSA C3 deposition ELISA (Figure 1A), similar to that observed for a similar previously published mAb against rMBL-A.4 Furthermore, P2D5 was as effective at inhibiting MBL-mediated C3 deposition as D-mannose, a known inhibitor of lectin pathway activation.4 In vivo, rats were dosed with 10 mg/kg (body weight) and this dose was found to inhibit MBL-A mediated C3b deposition ex vivo in the GlcNAc-BSA assay for at least 8–24 hours (Figure 1B). These data demonstrate that the mAb P2D5 recognises and binds MBL-A, inhibiting the lectin pathway.
Figure 1.
Recognition of rMBL-A by P2D5 mAb. BSA-GlcNAc was coated onto microtiter plates and exposed to rat serum co-incubated with either vehicle (VEH) or MBL inhibitors, including D-mannose (D-man) and mAb P2D5. (A) Dose-dependent decrease in C3 deposition in response to mAb P2D5. (B) Time course of inhibition by mAb P2D5. C3 deposition was measured by ELISA and expressed as a percentage of vehicle. *p<0.05 vs. vehicle
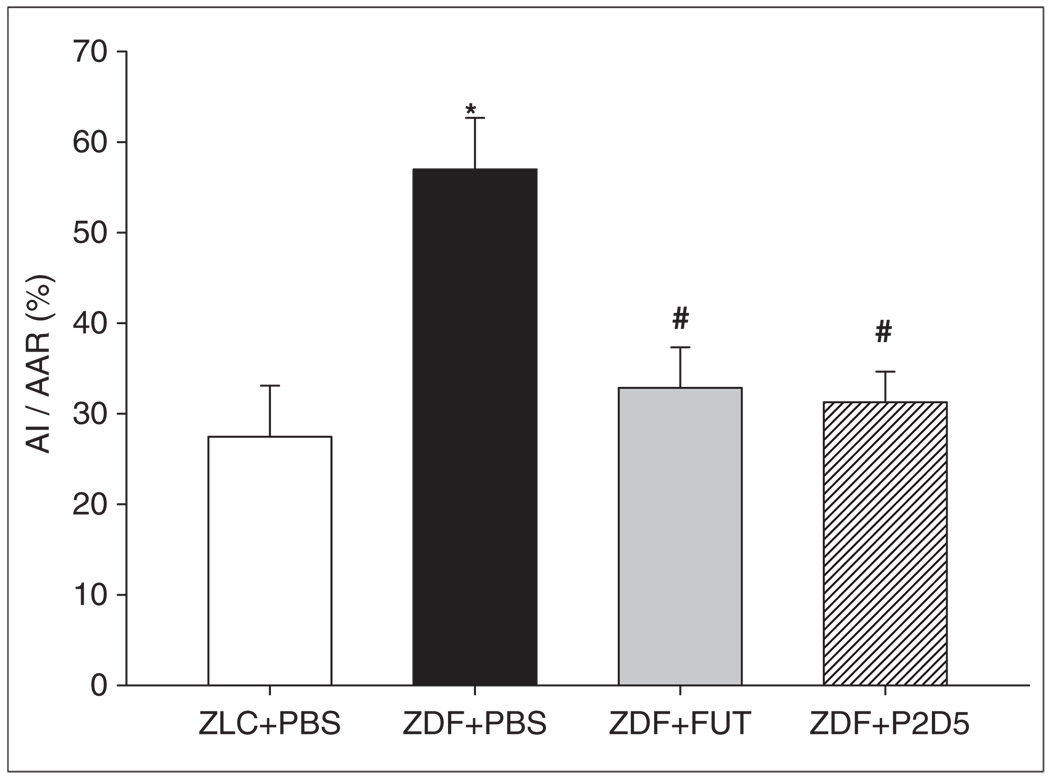
Left ventricular infarct size
Myocardial injury following 30 min ischaemia and 120 min reperfusion was assessed by examining the size of the infarct as a percentage of the AAR (%AI/AAR). The AAR did not differ among PBS-, FUT- or P2D5-treated rats (ZLC+PBS: 52.8±3.7 %AAR/LV; ZDF+PBS: 46.3±4.3 %AAR/LV; ZDF+FUT: 41.4±7.6 %AAR/LV; ZDF+P2D5: 44.3±5.4 %AAR/LV, respectively), indicating a comparable degree of ischaemic insult among all groups. However, infarct size normalised to AAR was significantly greater in the untreated ZDF rat hearts compared with the untreated ZLC and ZDF treated with FUT-175 or P2D5 (Figure 2; ZLC+PBS: 27.5±5.6 %AI/AAR; ZDF+PBS: 57.0±5.7 %AI/AAR; ZDF+FUT: 32.8±4.5 %AI/AAR; ZDF+P2D5: 31.3±3.4 %AI/AAR; p<0.05). Thus, ZDF rats treated with the complement inhibitors FUT-175 and P2D5 had similar and significant decreases in myocardial injury compared with PBS-treated controls.
Figure 2.
P2D5-treatment attenuates myocardial infarct size. Anti-complement treatment in diabetic (ZDF) rats (n=6 in both FUT and P2D5 groups) resulted in significantly decreased infarct size compared with untreated ZDF rats (n=9). *p<0.05 ZLC+PBS vs. ZDF+PBS; #p<0.05 ZDF+FUT and ZDF+P2D5 vs. ZDF+PBS.
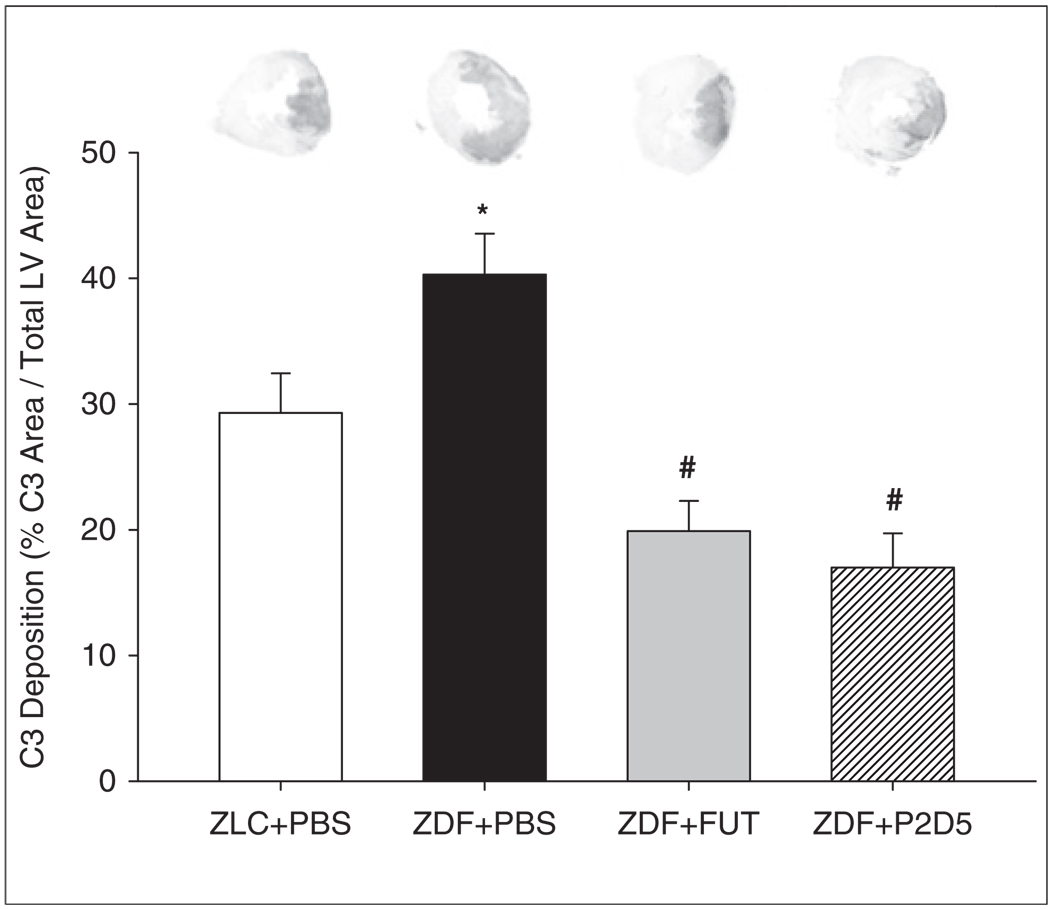
Myocardial complement deposition
Complement activation and deposition plays a significant role in the pathophysiology of reperfusion injury.4,6 To analyse complement activity, we immunohistologically stained LV cardiac tissue sections for complement component C3 (Figure 3). C3 deposition was localised to the AAR region of the LV and was significantly greater in ZDF+PBS-treated rat hearts compared with ZLC+PBS-treated hearts (Figure 2; ZLC+PBS: 29.3±3.1 %C3/LV and ZDF+PBS: 40.3±3.3 %C3/LV, p<0.05). Both FUT-175 and P2D5 treatment resulted in significantly decreased C3 staining in ZDF hearts (ZDF+FUT: 19.9±2.4 %C3/LV; ZDF+P2D5: 17.0±2.7 %C3/LV; p<0.05). No C3 staining was observed in non-ischaemic heart tissue or in slides stained only with DAB chromogen, indicating that these findings were not due to basal complement deposition or endogenous peroxidase activity.
Figure 3.
P2D5-treatment decreases myocardial C3 deposition. Treatment with anti-MBL (P2D5) in ZDF rats (n=6) resulted in similar decreases in C3 deposition as seen in ZLC+PBS (n=9) and ZDF+FUT (n=6) groups. There was significantly greater C3 deposition in the LV of untreated ZDF+PBS animals (n=9). *p<0.05 ZLC+PBS vs. ZDF+PBS; #p<0.05 ZDF+FUT and ZDF+P2D5 vs. ZDF+PBS.
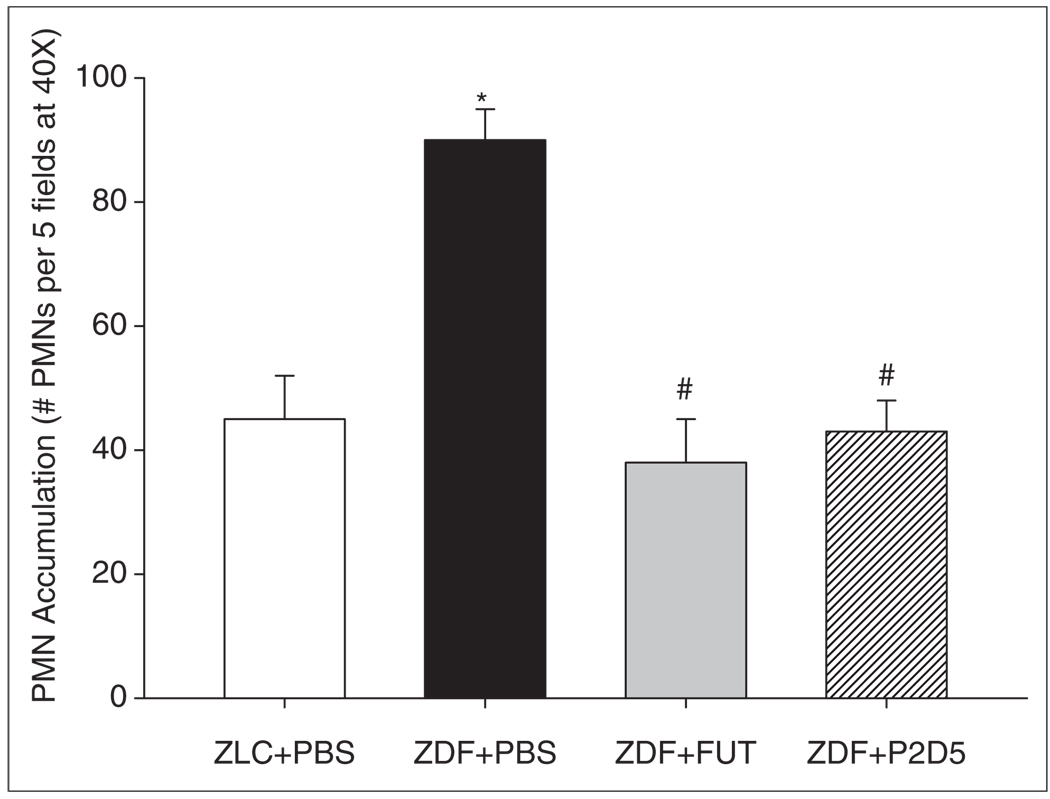
Neutrophil accumulation
Neutrophil sequestration and infiltration plays a significant role in reperfusion injury and endothelial dysfunction.9,24 Complement contributes to neutrophil accumulation in the post-ischaemic heart. Vakeva et al. found that tissue myeloperoxidase activity, a measure of neutrophil accumulation, is decreased following complement inhibition.25 We examined the relationship between complement inhibition and neutrophil accumulation in hearts from untreated rats and those treated with FUT-175 or P2D5. We observed significantly greater neutrophil accumulation in the LV of the untreated ZLC and ZDF rats compared with the FUT-175 and P2D5-treated rats (Figure 4; ZLC+PBS: 45±7; ZDF+PBS: 90±5; ZDF+FUT: 38±7; and ZDF+P2D5: 43±5 PMNs/five fields, p<0.001). Anti-MBL treatment with P2D5 decreased the accumulation of neutrophils to the same degree as the broad-based inhibitor, FUT-175, suggesting that the MBL pathway is largely responsible for neutrophil accumulation in post-ischaemic tissue.
Figure 4.
P2D5-treatment decreases myocardial neutrophil accumulation. LV neutrophil (PMN) accumulation was significantly increased in the untreated ZDF animals compared with non-diabetic and anti-complement treated animals. Treatment with anti-MBL (P2D5) effectively ameliorated the effect of diabetes on PMN accumulation since there is no difference between non-diabetic (ZLC) and ZDF+P2D5 LV PMN counts. *p<0.05 ZLC+PBS vs. ZDF+PBS; #p<0.05 ZDF+FUT and ZDF+P2D5 vs. ZDF+PBS.
Vascular ICAM-1 (CD54) expression
A primary event in the immune response to injury is the recruitment of circulating neutrophils to the inflammatory site. Adhesion to the vascular endothelium is the prerequisite initial step for neutrophil sequestration and extravasation to the site of injury. ICAM-1 (CD54) expression is a marker of endothelial cell activation. Neutrophil CD11b/CD18 ligand binds to ICAM-1, enabling firm adherence of neutrophils to the vascular endothelium. Using immunohistochemical techniques, we observed significantly decreased ICAM-1 expression in the LV of P2D5-treated ZDF rat hearts compared with untreated ZDF hearts (Figure 5, ZLC+PBS: 3659.0±256.7 µm2; ZDF+PBS: 6716.5±378.1 µm2; ZDF+FUT: 1132.1±115.0 µm2; ZDF+P2D5: 2025.9±143.9 µm2; p<0.001). These results suggest that activation of the lectin pathway is a key step in endothelial cell activation following ischaemia. Furthermore, these data indicate that MBL-mediated endothelial cell activation can be attenuated by P2D5.
Figure 5.
P2D5-treatment decreases myocardial ICAM-1 expression. Treatment with the broad complement inhibitor FUT-175 (ZDF+FUT, grey bar) resulted in significantly decreased areas of vascular ICAM-1 expression compared to both untreated ZLC (ZLC+PBS, open bar) and untreated ZDF (ZDF+PBS, black bar) rats. Anti-MBL treatment (ZDF+P2D5, hashed bar) was effective at decreasing ICAM expression compared to ZLC+PBS and ZDF+PBS, however, there was more ICAM expression in P2D5-treated ZDF rats than in FUT-175 treated ZDF rats. *p<0.001 ZLC+PBS vs. ZDF+PBS; #p<0.001 ZDF+FUT and ZDF+P2D5 vs. ZDF+PBS; $p<0.001 ZDF+FUT vs. ZDF+P2D5.
Discussion
In 1979, the Framingham study reported that impaired glucose tolerance is a major risk factor for cardiovascular disease. The most common cardiovascular complication of type 2 diabetes is ischaemic heart disease. In the heart, both the incidence and severity of ischaemic events are significantly increased in individuals with diabetes. In fact, ischaemic cardiovascular disease is now the single major cause of death among patients with diabetes, with four out of five deaths due to either acute myocardial infarction or stroke.26,27 The severe atherosclerosis observed in diabetic vessels places these individuals at a significantly increased risk of thrombo-embolism and ischaemic vascular disease.
While it is generally accepted that the complement system plays a significant role in the manifestation of myocardial reperfusion injury, the lectin pathway is the most recently described contributor to tissue injury following oxidative stress5 and myocardial I/R.4 Activation of the lectin pathway is antibody-independent, beginning with the binding of MBL to carbohydrate structures present on the surface of bacteria and other parasites.
In a non-diabetic rat model, Jordan et al. reported that blockade of the lectin pathway using monoclonal antibodies against rat MBL-A is cardioprotective following I/R by reducing neutrophil infiltration and attenuating pro-inflammatory gene expression.4 In the current study, we found that inhibition of lectin pathway activation via mAb P2D5 significantly decreases infarct size, C3 deposition, neutrophil accumulation and ICAM expression in the diabetic heart. In fact, we found that inhibition of MBL alone provided similar protection as FUT-175, a broad complement inhibitor, indicating that the MBL pathway is primarily responsible for the observed increase in complement-mediated I/R injury in the diabetic heart.
The role of complement in diabetes has not been extensively examined. Hansen and colleagues16 found that patients with a history of cardiovascular disease had significantly elevated MBL levels, suggesting that MBL may be involved in the pathogenesis of micro- and macrovascular complications in type 1 diabetes. Similarly, another study reported that circulating MBL levels are increased in type 1 diabetic patients with nephropathy; however, normoalbuminuric diabetic patients had increased levels of serum MBL compared with non-diabetic controls, and circulating MBL levels correlated with increasing levels of urinary albumin excretion within the normal range.28 Jerath et al. (2005) demonstrated a systemic increase in C3a, Bb, and C5b-9 prior to and during the treatment of diabetic ketoacidosis in adolescents with type 1 diabetes.29
Patients with type 2 diabetes are hyperinsulinaemic for a significant period during the disease, as the pancreas attempts to handle the high glucose load. Interestingly, adipocytes increase C3 production in response to insulin,30,31 and serum C3 levels correlate strongly with fasting insulin levels.32 Hansen et al.33 found that MBL levels are suppressed by insulin, and Engstrom et al. reported a significant association between complement component C3 and diabetes, concluding that the risk of developing type 2 diabetes is related to plasma levels of complement C3.34 McMillan reported that C3 and C4 components were positively correlated with fasting plasma glucose in patients with type 2 diabetes.35 The simultaneous development of complement elevation and glucose intolerance strongly suggests a metabolic basis for increased complement expression. Interestingly, the increased C3/C4 appeared unrelated to the presence or severity of diabetic microvascular complications. In middle-aged men, plasma C3 levels were significantly correlated with diabetes even after adjustments for HOMA-IR, BMI, insulin, and inflammatory markers.34 From these studies, we can hypothesise that C3 deposition may be present in the diabetic heart and/or vasculature prior to the appearance of overt cardiovascular complications.
In agreement with our current findings, recent work by Busche et al. found a significant role for MBL in hyperglycaemia-associated hypertrophic cardiac remodelling and myocardial I/R injury in a mouse model of streptozocin-induced type 1 diabetes.36 Although the end-stage vascular complications are similar between type 1 and type 2 diabetes, conclusions drawn in an STZ model of type 1 diabetes do not necessarily translate to a type 2 diabetic model. Moreover, even the type 2 diabetic mouse model does not demonstrate the same blood changes – in either coagulation or inflammation – as diabetic people and the ZDF rat model.37 In addition, Paul et al. recently characterised significant differences between the ZDF and STZ models of diabetes with respect to platelet aggregation, finding that the STZ model does not support what is observed clinically – namely, hypercoagulability.38 These reports alone warrant the study of neutrophil-mediated myocardial I/R injury in the ZDF model of type 2 diabetes.
Myocardial infarct size
The lectin pathway of complement activation is the most recently described contributor to tissue injury following oxidative stress, suggesting an association between MBL and vascular events.5 Fiane et al. reported that MBL-deficient patients undergoing thoracic abdominal aortic aneurism repair had less pro-inflammatory markers (IL-1β, TNF-α, and IL-8) following surgery.39 Ischaemic injury in a variety of vascular beds initiates lectin-complement pathway activation, and pretreatment with anti-MBL antibody reduced post-ischaemic myocardial reperfusion injury in a rat model of I/R.4
Ischaemia and reperfusion are associated with an acute local and systemic inflammatory response that is recognised by reactive oxidant production, complement activation, leukocyte–endothelial cell adhesion, leukocyte diapedesis, platelet–leukocyte aggregation, increased vascular permeability, and decreased endothelium-dependent relaxation.40 A number of pro-inflammatory and lytic mechanisms have been identified by which complement exacerbates reperfusion injury, ultimately through formation of the lytic MAC, C5b-9.
Studies by de Vries et al.41 and Homeister et al.22 performed in the isolated heart and in neutrophil-depleted animal models found that complement activation is a significant regulator of I/R injury even in the absence of neutrophils. MAC formation on post-ischaemic endothelial cells may result in increased rates of cellular activation, apoptosis, and necrosis.42 MAC-mediated endothelial cell activation may result in adhesion molecule expression and reactive oxygen species production, thereby enhancing leukocyte accumulation. In support of a role for MAC-mediated myocardial injury following I/R, Ito et al. reported that C6-deficient rabbits had significantly decreased infarct size and increased area of reflow, suggesting that the terminal complement components contributes to the ‘no-reflow’ phenomenon.43 In this study, we found that the MBL-pathway of complement activation plays a significant role in infarct expansion in a type 2 diabetic rat model. We conclude that a majority of diabetic myocardial I/R injury is associated with lectin complement pathway activation.
C3 deposition, neutrophil accumulation and ICAM-1 expression
In the presence of an intact complement system, C1q begins to concentrate in ischaemic tissues soon after coronary occlusion,44 and subsequent production of C5a induces the accumulation of neutrophils (PMNs) in the ischaemic tissue.45 The complement cleavage fragment C5a is produced downstream from C3 following complement activation by all three pathways. C5a is a potent anaphylatoxin and chemoattractant for circulating PMNs. The C5a receptor (C5aR, CD88) is expressed mainly on myeloid cells, particularly PMNs, as well as the salivary gland, lung, liver, and cardiomyocyte.46,47 C5a is also capable of activating non-myeloid cells, especially endothelial cells, by up-regulating the secretion of cytokines, chemokines, and acute phase proteins, and increasing the expression of adhesion molecules. Atsuumi et al. found consistent results with the induction of PMN chemotaxis into the ischaemic myocardium by activation of the complement cascade.48 PMN infiltration and accumulation in post-ischaemic tissues is a significant contributor to myocardial,49,50 cerebral,51,52 and renal53 reperfusion injury. Thus, activation of C5 is particularly important in the inflammatory response, and studies using inhibitors of C5 and its receptor found significant cardioprotective benefits following I/R.25,41,47
Previously, we reported a significant increase in PMN accumulation in the ZDF heart following I/R.6 In the present study, we found that blockade of the lectin complement pathway with P2D5 significant attenuated the influx of PMNs to post-ischaemic tissues (Figure 3). Additionally, MBL inhibition decreased the number of PMNs to the same degree as FUT-175, an effective inhibitor of all three complement activation pathways. There was no significant difference in LV PMN accumulation between the untreated non-diabetic ZLC and the treated diabetic ZDF rats, indicating that much of the PMN accumulation can be attributed to complement activation products, specifically from the MBL pathway. These data suggest that use of MBL inhibitors such as P2D5 may be of use in other pathological conditions characterised by excessive PMN accumulation.
Vascular endothelial cell dysfunction is enhanced in diabetes and may be partially explained by alterations in microvascular and blood cell adhesion properties. Activated endothelial cells express several types of leukocyte adhesion molecules, which cause blood cells rolling along the vascular surface to adhere at the site of activation. Adhesion molecules such as ICAM-1 and VCAM-1 are known to participate in leukocyte–endothelial binding and play important roles in leukocyte adhesion and extravasation. Indeed, Booth et al. reported that acute hyperglycaemia results in augmented microvascular adhesion properties which are manifested as increased VCAM-1 and endothelial P-selectin expression.54
PMN firm adhesion to the vascular endothelium occurs via binding of CD11b to its ligand, ICAM-1. The low-grade chronic inflammation that is present in type 2 diabetes may result in increased basal expression and/or transcription of ICAM-1, and there is significantly increased ICAM-1 expression in the type 2 diabetic heart compared with the non-diabetic heart following I/R.6 In this study we found that P2D5 treatment significantly attenuates vascular ICAM-1 expression, even more than FUT-175 treatment, suggesting that there may be unknown anti-inflammatory mechanisms underlying lectin pathway inhibition. Additionally, the P2D5-mediated decrease in ICAM-1 expression is likely responsible for the attenuated PMN infiltration in the P2D5-treated animals.
In this study we found a significant role for the lectin pathway in the pathophysiology of myocardial ischaemia and reperfusion injury in the setting of type 2 diabetes. Specifically, MBL inhibition alone decreased infarct size, C3 deposition, neutrophil accumulation and ICAM expression to a similar degree as the broad complement inhibitor FUT-175. Type 2 diabetes constitutes roughly 90–95% of the diabetic population worldwide,55 therefore from a clinical perspective, the use of a mAb raised against MBL-A demonstrates the efficacy of acute pharmacological anti-complement treatment for myocardial I/R. Thus, future studies aimed at further elucidating the mechanisms underlying the excessive complement activation in type 2 diabetic I/R injury may aid in the development of improved interventions to limit the severity of ischaemic heart disease and reperfusion injury in the setting of type 2 diabetes.
Acknowledgements
This work was supported in part by NIH HLBI Grants #58859 (McDonagh), the Hudson/Lovaas Endowment, and NIH HLBI Training Grant #07249 (Physiological Sciences GIDP) as well as an American Heart Association Predoctoral Fellowship #0610018Z (La Bonte). The authors would also like to thank Doug Cromey from the Southwest Environmental Health Sciences Center (SWEHSC) Cellular Imaging Core (NIEHS ES06694) and Norma Seaver for their technical expertise in this study. Dr. La Bonte is currently with the Center for Experimental Therapeutics and Reperfusion Injury in the Department of Anesthesiology, Perioperative and Pain Medicine at Brigham and Women’s Hospital and Harvard Medical School.
Abbreviations and acronyms
- AAR
area at risk
- AGE
advanced glycation end-product
- BSA
bovine serum albumin
- ELISA
enzyme-linked immunosorbent assay
- FLISA
fluorescence-linked immunosorbent assay
- GlcNAc
N-acetyl-D-glucosamine
- HOMA-IR
homeostatic model assessment – insulin resistance
- ICAM-1
intracellular adhesion molecule-1
- I/R
ischaemia/reperfusion
- LV
left ventricular
- mAB
monoclonal antibody
- MAC
membrane attack complex
- MBL
mannose-binding lectin
- NO
nitric oxide
- PMN
polymorphonuclear leukocyte
- VCAM-1
vascular cell adhesion molecule-1
- ZLC
Zucker Lean Control
- ZDF
Zucker Diabetic Fatty
Footnotes
Reprints and permission: http://www.sagepub.co.uk/journalsPermission.nav
References
- 1.Zhang L, Zalewsk iA, Liu Y. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–478. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]
- 2.Stockklauser-Farber K, Ballausen T, Laufer A, Rosen P. Influence of diabetes on cardiac nitric oxide synthase expression and activity. Biochim Biophys Acta. 2000;1535:10–20. doi: 10.1016/s0925-4439(00)00078-8. [DOI] [PubMed] [Google Scholar]
- 3.Tuttle HA, Davis-Gorman G, Goldman S, Copeland JG, McDonagh PF. Proinflammatory cytokines are increased in type 2 diabetic women with cardiovascular disease. J Diabetes Complicat. 2004;18:343–351. doi: 10.1016/S1056-8727(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 4.Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104:1413–1418. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- 5.Collard CD, Väkevä A, Morrissey MA, et al. Complement activation after oxidative stress. Role of the lectin complement pathway. Am J Pathol. 2000;156:1549–1556. doi: 10.1016/S0002-9440(10)65026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Bonte LR, Davis-Gorman G, Stahl GL, McDonagh PF. Complement inhibition reduces injury in the type 2 diabetic heart following ischaemia and reperfusion. Am J Physol Heart Circ Physiol. 2008;294:H1282–H1290. doi: 10.1152/ajpheart.00843.2007. [DOI] [PubMed] [Google Scholar]
- 7.Pinckard RN, O’Rourke RA, Crawford MH, et al. Complement localization and mediation of ischemic injury in baboon myocardium. J Clin Invest. 1980;66:1050–1056. doi: 10.1172/JCI109933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vakeva A, Laurila P, Meri S. Loss of expression of protectin (CD59) is associated with complement membrane attack complex deposition in myocardial infarction. Lab Invest. 1992;67:608–616. [PubMed] [Google Scholar]
- 9.Hokama JY, Ritter LS, Davis-Gorman G, Cimetta AD, Copeland JG, McDonagh PF. Diabetes enhances leukocyte accumulation in the coronary microcirculation early in reperfusion following ischaemia. J Diabetes Complicat. 2000;14:96–107. doi: 10.1016/s1056-8727(00)00068-4. [DOI] [PubMed] [Google Scholar]
- 10.Ritter LS, McDonagh PF. Low flow reperfusion after myocardial ischaemia enhances leukocyte accumulation in coronary microcirculation. Am J Physiol. 1997;273:H1154–H1165. doi: 10.1152/ajpheart.1997.273.3.H1154. [DOI] [PubMed] [Google Scholar]
- 11.Tonnesen MG, Anderson DC, Springer TA. Adherence of neutrophils to cultured human microvascular endothelial cells. Stimulation by chemotactic peptides and lipid mediators and dependence upon the Mac-1, LFA-1, p150.95 glycoprotein family. J Clin Invest. 1989;83:637–646. doi: 10.1172/JCI113928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacks T, Moldow CF, Craddock PR, Bowers TK, Jacob HS. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes: An in vitro model of immune vascular damage. J Clin Invest. 1978;61:1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saddi S, Holzknecht RA, Patte CP. Endothelial cell activation by pore-forming structures: Pivotal role for Interleukin-1á. Circulation. 2000;101:1867–1873. doi: 10.1161/01.cir.101.15.1867. [DOI] [PubMed] [Google Scholar]
- 14.Kilgore KS, Flory CM, Miller BP. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol. 1996;149:953–961. [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh M, Bourcier T, Takahashi K, et al. Mannose binding lectin is a regulator of inflammation that accompanies myocardial ischaemia and reperfusion injury. J Immunol. 2005;175:541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 16.Hansen T, Tarnow L, Thiel S, et al. Association between mannose-binding lectin and vascular complications in type 1 diabetes. Diabetes. 2004;53:1570–1576. doi: 10.2337/diabetes.53.6.1570. [DOI] [PubMed] [Google Scholar]
- 17.Clark JB, Palmer CJ, Shaw WN. The diabetic Zucker Fatty Rat (41611) Proc Soc Exp Biol Med. 1983;173:68–75. doi: 10.3181/00379727-173-41611. [DOI] [PubMed] [Google Scholar]
- 18.Fujii S, Hitomi Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim Biophys Acta. 1981;661:342–345. doi: 10.1016/0005-2744(81)90023-1. [DOI] [PubMed] [Google Scholar]
- 19.Ikari N, Sakai Y, Hitomi Y, Fujii S. New synthetic inhibitor to the alternative complement pathway. Immunology. 1983;49:685. [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh MC, Shaffer LA, Guikema BJ, et al. Fluorochrome-linked immunoassay for functional analysis of the mannose binding lectin complement pathway to the level of C3 cleavage. J Immuno Methods. 2007;323:147–159. doi: 10.1016/j.jim.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeifer PH, Kawahara MS, Hugli TE. Possible mechanism for in vitro complement activation in blood and plasma samples: Futhan/EDTA controls in vitro complement activation. Clin Chem. 1999;45:1190–1199. [PubMed] [Google Scholar]
- 22.Homeister JW, Satoh P, Lucchesi BR. Effects of complement activation in the isolated heart. Role of the terminal complement components. Circ Res. 1992;71:303–319. doi: 10.1161/01.res.71.2.303. [DOI] [PubMed] [Google Scholar]
- 23.Kubota K, Mizoguchi H, Miura Y, Suda T, Takaku F. A new technique for the cytochemical examination of human hemopoietic cells grown in agar gel. Exp Hematol. 1980;8:339–344. [PubMed] [Google Scholar]
- 24.Lefer AM, Weyrich A, Buerke M. Role of selectins, a new family of adhesion molecules, in ischaemia-reperfusion injury. Cardiovasc Res. 1994;28:289–294. doi: 10.1093/cvr/28.3.289. [DOI] [PubMed] [Google Scholar]
- 25.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischaemia and reperfusion. Role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 26.American Heart Association. 2005. Heart disease and stroke statistics – 2005 update. [Google Scholar]
- 27.Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 28.Østergaard J, Hansen TK, Thiel S, Flyvbjerg A. Complement activation and diabetic vascular complications. Clin Chim Acta. 2005;361:10–19. doi: 10.1016/j.cccn.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Jerath RS, Burek CL, Hoffman WH, Passmore GG. Complement activation in diabetic ketoacidosis and its treatment. Clin Immunol. 2005;116:11–17. doi: 10.1016/j.clim.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Scantlebury T, Sniderman AD, Cianflone K. Regulation by retinoic acid of acylation-stimulating protein and complement C3 in human adipocytes. Biochem J. 2001;356:445–452. doi: 10.1042/0264-6021:3560445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scantlebury T, Maslowska M, Cianflone K. Chylomicron-specific enhancement of acylation stimulating protein and precursor protein C3 production in differentiated human adipocytes. J Biol Chem. 1998;273:20903–20909. doi: 10.1074/jbc.273.33.20903. [DOI] [PubMed] [Google Scholar]
- 32.Muscari A, Massarelli G, Bastagli L, et al. Relationship of serum C3 to fasting insulin, risk factors and previous ischaemic events in middle-aged men. Eur Heart J. 2000;21:1081–1090. doi: 10.1053/euhj.1999.2013. [DOI] [PubMed] [Google Scholar]
- 33.Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G. Intensive insulin therapy exerts anti-inflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88:1082–1088. doi: 10.1210/jc.2002-021478. [DOI] [PubMed] [Google Scholar]
- 34.Engstrom G, Hedblad B, Eriksson KF, Janzon L, Lindgarde F. Complement C3 is a risk factor for the development of diabetes: a population-based cohort study. Diabetes. 2005;54:570–576. doi: 10.2337/diabetes.54.2.570. [DOI] [PubMed] [Google Scholar]
- 35.McMillan DE. Elevation of complement components in diabetes mellitus. Diabetes Metab. 1980;6:265–270. [PubMed] [Google Scholar]
- 36.Busche MN, Walsh MC, McMullen ME, Guikema BJ, Stahl GL. Mannose-binding lectin plays a critical role in myocardial ischaemia and reperfusion injury in a mouse model of diabetes. Diabetologia. 2008;51:1544–1551. doi: 10.1007/s00125-008-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry ML, Davidson LB, Wilson JE, et al. Whole blood aggregation and coagulation in db/db and ob/ob mouse models of type 2 diabetes. Blood Coagul Fibrinolysis. 2008;19:124–134. doi: 10.1097/MBC.0b013e3282f41e56. [DOI] [PubMed] [Google Scholar]
- 38.Paul W, Queen LR, Page CP, Ferro A. Increased platelet aggregation in vivo in the Zucker Diabetic Fatty rat: differences from the streptozotocin diabetic rat. Br J Pharmacol. 2007;150:105–111. doi: 10.1038/sj.bjp.0706957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiane AE, Videm V, Lingaas PS, et al. Mechanism of complement activation and its role in the inflammatory response after thoracoabdominal aortic aneurysm repair. Circulation. 2003;108:849–856. doi: 10.1161/01.CIR.0000084550.16565.01. [DOI] [PubMed] [Google Scholar]
- 40.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.de Vries B, Kohl J, Leclercq WKG, et al. Complement factor C5a mediates renal ischaemia-reperfusion injury independent from neutrophils. J Immunol. 2003;170:3883–3889. doi: 10.4049/jimmunol.170.7.3883. [DOI] [PubMed] [Google Scholar]
- 42.Acosta J, Hettinga J, Flückiger R, et al. Molecular basis for a link between complement and the vascular complications of diabetes. PNAS. 2000;97:5450–5455. doi: 10.1073/pnas.97.10.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito W, Schäfer JJ, Bhakdi S, et al. Influence of the terminal complement-complex on reperfusion injury, no-reflow and arrhythmias: a comparison between C6-competent and C6-deficient rabbits. Cardiovasc Res. 1996;32:294–305. doi: 10.1016/0008-6363(96)00082-x. [DOI] [PubMed] [Google Scholar]
- 44.Rossen RD, Swain JL, Michael LH, Weakley S, Giannini E, Entman ML. Selective accumulation of the first component of complement and leukocytes in ischemic canine heart muscle. A possible initiator of an extra myocardial mechanism of ischemic injury. Circ Res. 1985;57:119–130. doi: 10.1161/01.res.57.1.119. [DOI] [PubMed] [Google Scholar]
- 45.Dreyer WJ, Michael LH, West S, et al. Neutrophil accumulation in ischemic canine myocardium. Insights into time course, distribution, and mechanism of localization during early reperfusion. Circulation. 1991;84:400–411. doi: 10.1161/01.cir.84.1.400. [DOI] [PubMed] [Google Scholar]
- 46.Akatsu H, Abe M, Miwa T, et al. Distribution of rat C5a anaphylatoxin receptor. Microbiol Immunol. 2002;46:863–874. doi: 10.1111/j.1348-0421.2002.tb02774.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Qin G, Liang G, Li J, Barrington RA, Liu D. C5aR-mediated myocardial ischaemia/reperfusion injury. Biochem Biophys Res Comm. 2007;357:446–452. doi: 10.1016/j.bbrc.2007.03.152. [DOI] [PubMed] [Google Scholar]
- 48.Atsuumi T, Yaoita H, Shichishima T, Maehara K, Fujita T, Maruyama Y. Complement and polymorphonuclear leukocyte activation each play a role in determining myocardial ischaemia-reperfusion injury. Jpn Circ J. 2001;65:659–666. doi: 10.1253/jcj.65.659. [DOI] [PubMed] [Google Scholar]
- 49.Simpson PJ, Todd RF, III, Fantone JC, Mickelson JR, Griffin JD, Lucchesi BR. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mol, Anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988;81:624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDonagh PF, Hokama JY, Copeland JG, Reynolds JM. The blood contribution to early myocardial reperfusion injury is amplified in diabetes. Diabetes. 1997;46:1859–1867. doi: 10.2337/diab.46.11.1859. [DOI] [PubMed] [Google Scholar]
- 51.Ritter LS, Orozco JA, Coull BM, McDonagh PF. Leukocyte accumulation and hemodynamic changes in the cerebral microcirculation during early reperfusion after stroke. Stroke. 2000;31:1153–1161. doi: 10.1161/01.str.31.5.1153. [DOI] [PubMed] [Google Scholar]
- 52.Soriano SG, Coxon A, Wang YF, et al. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischaemia/reperfusion injury. Stroke. 1999;30:134–139. doi: 10.1161/01.str.30.1.134. [DOI] [PubMed] [Google Scholar]
- 53.Rinder CS, Fontes M, Mathew JP, Rinder HM, Smith BR Multicenter Study of Perioperative Ischaemia (McSPI) Research Group. Neutrophil CD11b upregulation during cardiopulmonary bypass is associated with postoperative renal injury. Ann Thorac Surg. 2003;75:899–905. doi: 10.1016/s0003-4975(02)04490-9. [DOI] [PubMed] [Google Scholar]
- 54.Booth G, Stalker TJ, Lefer AM, Scalia R. Elevated ambient glucose induces acute inflammatory events in the microvasculature: effects of insulin. Am J Physiol Endocrinol Metab. 2001;280:E848–E856. doi: 10.1152/ajpendo.2001.280.6.E848. [DOI] [PubMed] [Google Scholar]
- 55.Merin M. Global Health: addressing the world’s health challenges. Diabetes threatens lives worldwide. 2008 [Web Page]; http://www.america.gov/st/health-english/2008/April/20080410113749liameruoy0.5424921.html.