Abstract
Depression has been associated with reduced expression of brain-derived neurotrophic factor (BDNF) in the hippocampus. In addition, animal studies suggest an association between reduced hippocampal neurogenesis and depressive-like behavior. These associations were predominantly established based on responses to antidepressant drugs and alterations in BDNF levels and neurogenesis in depressive patients or animal models for depressive behavior. Nevertheless, there is no direct evidence that the actual reduction of the BDNF protein in specific brain sites can induce depressive-like behaviors or affect neurogenesis in vivo. Using BDNF knockdown by RNA interference and lentiviral vectors injected into specific subregions of the hippocampus we show that a reduction in BDNF expression in the dentate gyrus, but not the CA3, reduces neurogenesis and affects behaviors associated with depression. Moreover, we show that BDNF has a critical function in neuronal differentiation, but not proliferation in vivo. Finally, we found that a specific BDNF knockdown in the ventral subiculum induces anhedonic-like behavior. These findings provide substantial support for the neurotrophic hypothesis of depression and specify anatomical and neurochemical targets for potential antidepressant interventions. Moreover, the specific effect of BDNF reduction on neuronal differentiation has broader implications for the study of neurodevelopment and neurodegenerative diseases.
Keywords: brain-derived neurotrophic factor, lentiviral vector, short hairpin RNA, neurogenesis, hippocampus, depression
Introduction
Several studies have suggested that brain-derived neurotrophic factor (BDNF)1, 2, 3, 4 and neurogenesis5, 6, 7, 8 have an important function in the pathophysiology of depression, or at least in the mechanism of antidepressant action.1, 5, 9, 10 Postmortem analyses have reported decreased hippocampal levels of BDNF in suicide victims2, 4 and depressed individuals.11 In addition, a large volume of evidence derived from animal studies indicates that chronic stress, implicated as a risk factor for depression,12, 13 can decrease the expression of BDNF in the hippocampus,14 whereas other studies did not observe such effect.15 In addition, electroconvulsive seizures1, 16 and administration of antidepressant drugs can increase hippocampal BDNF levels.1 Moreover, infusion of BDNF into rat hippocampus results in antidepressant-like effects, as observed in the forced swim test (FST) and learned helplessness paradigms.3 In addition, a recent study by Adachi et al.10 showed that hippocampal BDNF is necessary for the action of antidepressant drugs in mice, but failed to show an effect of BDNF reduction on the behavior of untreated mice. Finally, a general reduction of BDNF expression within the forebrain of female (but not male) mice induced an increase in immobility in the FST.9 There is also accumulating evidence that hippocampal neurogenesis is decreased by stress8, 17, 18, 19 and increased by antidepressant treatment.6, 7, 8 Furthermore, Santarelli et al.5 showed that hippocampal neurogenesis is required for the behavioral effects of current antidepressants in the novelty-suppressed feeding paradigm in mice, but that reduced neurogenesis per se does not alter the mice latency to feed at the novelty-suppressed feeding. Some of these observations provide the basis for the neurotrophic hypothesis of depression which associates decreased BDNF expression with depression and increased BDNF expression with antidepressant action,20 effects possibly mediated by alterations in neurogenesis.7
Despite the substantial amount of data indicating an association between decreased hippocampal BDNF levels and depression, and the critical function of BDNF in antidepressant action, there is no direct evidence that localized reduction in BDNF protein levels can actually affect behaviors associated with depression, or perhaps reduced BDNF levels is merely a side effect of stress and depression. It also remains unknown whether BDNF expression in specific hippocampal subregions is more critical than in other subregions. Moreover, while intrahippocampal BDNF infusion facilitates neurogenesis,21 there is no direct evidence that a local reduction in the BDNF protein actually impairs adult hippocampal neurogenesis in vivo. To examine these issues, we sought to reduce BDNF levels in specific hippocampal subregions, and measure the consequential effects on behavior and neurogenesis. Reducing BDNF levels by producing conventional BDNF homozygous (−/−) knockout mice is not practical for this purpose, because such mice do not survive to adulthood22 and the use of BDNF heterozygous (+/−) mice have previously resulted in conflicting findings.23, 24, 25 Moreover, this approach does not allow for site-specific reduction in BDNF. Finally, as BDNF is reduced early in development in these transgenic mice, it is unknown what potential compensatory mechanisms can occur during neurodevelopment. We therefore made use of RNA interference (see Supplementary Appendix 1) to allow for BDNF knockdown in specific regions without interfering with normal embryonic neurodevelopment. To induce a stable knockdown, we generated lentiviral vector (LV) constructs26, 27 expressing short hairpin RNAs (shRNAs) complementary to the coding exon of the rat BDNF gene, common to all isoforms of this gene.28 Lentiviruses allow for long-term expression of these BDNF shRNAs (shBDNF), as they are capable of infecting non-dividing cells, and because the genes they transduce are integrated into the genome of the target hippocampal cells.29 This approach was utilized in this study to evaluate whether BDNF knockdown in specific hippocampal subregions can actually affect behaviors associated with depression and whether such a localized reduction in the BDNF protein can affect neurogenesis in vivo.
Materials and methods
In vitro
Design of shRNAs
Four shRNA sequences, each complementary to a unique segment of mRNA transcribed from the coding sequence common to all isoforms of the rat BDNF gene, were produced.28 One scrambled shRNA sequence was designed as a control (shSCR). One of the shRNA constructs (sh1) was designed using the publicly available siRNA Target Finder (Ambion, Austin, TX, USA), and three constructs (sh2, sh3 and sh4) were designed as described previously.30 The forward sequences of the shRNA contructs are as follows: sh1: GGTTATTTCATACTTCGGT, sh2: TCGAAGAGCTGCTGGATGA, sh3: TATGTACACTGACCATTAA and sh4: GAACTACCCAATCGTATGT. In a 5′ to 3′ order the shRNA constructs contained the following: a 19–nt sense sequence, a short spacer (ttcaagaga), a 19–nt reverse sequence complementary to the sense strand and five thymidines (RNA polymerase-3 transcriptional stop signal). These sequences were annealed and cloned between Hind III and BamHI restriction sites in the pSilencer 3.0–H1 vector (Ambion), downstream to the H1-RNA polymerase-3 promoter.
BDNF cloning
Rat total DNA was extracted from a C6 rat glioma cell line using the GenElute mammalian genomic DNA purification kit (Sigma-Aldrich, St Louis, MO, USA), and the coding sequence of the BDNF gene was subsequently amplified by PCR using an Expand High Fidelity DNA polymerase (Roche Diagnostics, Penzberg, Germany). The following primers were used: 5′-ATTTGCGGCCGCTTCCACCAGGTGAGAAGAGTG-3′ (forward) and 5′-TTTAGGATCCTATCTTCCCCTTTTAATG-3′ (reverse). NOT1 and BamH1 (Fermentas, Burlington, Canada) restriction sites were introduced, which allowed for the insertion of the BDNF sequence downstream to the cytomegalovirus promoter in a pcDNA3.1(-) expression vector (Invitrogen, Carlsbad, CA, USA).
Effectiveness of the various shRNA sequences in vitro
293T cells were cultured with Dulbecco's modified eagle's medium (Gibco-BRL, Invitrogen, Belgium) containing 10% fetal calf serum (GIBCO-BRL). Twenty-four hours before transfection, 293T cells were split 1:5 to reach 60% confluency. A quantity of 8.4 μg of either one or a mix of the shRNA vectors (sh1-sh4 or shSCR), or a control plasmid expressing green fluorescent protein (GFP) only, were transfected with 10 μg of BDNF expression vector into the 293T cells using the calcium phosphate method. Precipitates were formed by the addition of the plasmids to TE buffer (1 mM Tris, 0.1 mM EDTA, pH=8) supplemented with 250 mM CaCl2 in a final volume of 500 μl. Then, 500 μl of 2 × HEPES-buffered saline (281 mM NaCl, 100 mM HEPES, 1.5 mM Na2HPO4, pH=7.12) was added drop wise and the solution was vortexed. The resulting precipitate of each plasmid solution was immediately added to 293 T cell cultures. The cell media were replaced after 14 to 16 h, and collected at 24, 48 and 72 h post-transfection. Secreted BDNF levels were measured using DuoSet ELISA development system (R&D systems, Minneapolis, MN, USA) as we described earlier,31 and then normalized per 106 cell number.
Cloning lentiviral silencing vectors
Complete H1 promoter and shBDNF, or shSCR cassettes were amplified from pSilencer plasmid by PCR using Expand High Fidelity DNA polymerase and the following primers: 5′-GCGCTCGAGGTTTTCCCAGTCACGAC-3′ (forward) and 5′-ATCGAGTTAGCTCACTCATTAGGC-3′ (reverse). The forward primer was designed to include an XhoI restriction site. Next, the PCR product was cloned into a transfer plasmid of the LV, which express GFP, between the unique XhoI and EcoR5 restriction sites located downstream to the central polypurine tract (cPPT) of the transfer plasmid. LVs were named LV-shBDNF and LV-shSCR, respectively, and a LV expressing the GFP only was named LV-GFP.
High titer lentiviral preparation
293T cells were split into 15 cm plates, precoated with 20 μg ml−1 of Poly-L-lysin (Sigma-Aldrich, USA), to reach 60% confluency. Cells were transfected using polyethyleneimine as described previously.32 In brief, on the transfection day, medium was replaced with Dulbecco's modified eagle's medium (which did not include fetal calf serum). DNA mixture containing 26 μg of transfer plasmid, 16.9 μg of packaging plasmid (encoding viral Gag and Pol proteins (pCMVΔR8.91)), and 9.1 μg of envelope plasmid (encoding the envelope of vesicular stomatitis virus (pCI)) and 78 μl of 1 mg ml−1 polyethyleneimine in a total volume of 1.5 ml Dulbecco's modified eagle's medium was prepared. The mixture was incubated for 10 min at room temperature and then added drop wise to plates containing 293T cells. After 16 h transfection, the medium was replaced with Dulbecco's modified eagle's medium supplemented with 10% fetal calf serum. Forty eight and seventy two hours after transfection, medium was collected, cleared by low speed centrifugation, filtered through 0.45 μm-pore-size filter and ultracentrifuged at 25 000 r.p.m. for 2 h in 4 °C (Beckman Coulter BV, Mijdrecht, the Netherlands) to obtain high titer viral stocks. The pellet was resuspended in 0.1 M phosphate-buffered saline (PBS), aliquoted, and stored at −80 °C until further use. To determine titer of the viral stocks, 105 293T cells were infected with 5 μl or 20 μl of viral stock. After 48 h, cells were fixed and then analyzed with fluorescence-activated cell sorting and GFP-positive cells were detected. LV stock titers were expressed as transducing units (TU) per milliliter, and ranged in the order of 109 TU ml−1.
Validation of LV-shBDNF activity in vitro
C6 rat glioma cells were grown to 60% confluency and infected with LV-shBDNF, LV-shSCR or LV-GFP supplemented with polybrene (8 μg ml−1). Cells were allowed to grow to 100% confluency and medium was replaced. Twenty-four hours later, medium was collected and stored at −20 °C for further analysis, and cells were harvested, rinsed twice with ice-cold PBS, and lysed with RIPA buffer (140 mM NaCl, 20 mM Tris, pH=7.4, 10% glycerol, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride and leupeptin at 20 μg ml−1). Lysates were centrifuged at 14 000 r.p.m. for 10 min at 4 °C, the pellet was discarded, and supernatant was aliquoted and stored at −20 °C for further analysis. BDNF protein levels were determined using DuoSet ELISA development system (R&D systems). Results were normalized per cell number for medium samples, or per total protein concentration (Bradford assay, Bio-Rad, Hercules, CA, USA) for protein extraction samples.
In vivo
Animals
Ten-week-old Sprague–Dawley male rats were supplied by the Animal Breeding Center of the Weizmann Institute of Science (Rehovot, Israel). Animals were individually housed on a 12 h light/12 h dark cycle (lights on at 0700 h) with food and water ad libitum. All animals were handled according to the regulations formulated by the Institutional Animal Care and Use Committee, which are in complete accordance with the NIH guidelines for care and use of laboratory animals.
Intracranial surgery
Rats were anesthetized with ketamine (170 mg kg−1) and acepromazine (1.7 mg kg−1) and placed in a stereotaxic frame. Stainless steel guide cannulae (Plastics One, Roanoke, VA, USA) were implanted bilaterally into either the dorsal dentate gyrus (dDG) (n=61), CA3 (n=18) or ventral subiculum (vSUB) (n=22) subregions of the hippocampus using the coordinates described below (relative to bregma and according to the atlas of Paxinos and Watson, 1998).33 dDG: −3.8 anteroposterior, +2.4 mediolateral and −1.95 dorsoventral (from dura) at a lateral angle of 10° CA3: −3.8 anteroposterior, 4.47 mediolateral and −1.36 dorsoventral (from dura) at a lateral angle of 10° vSUB: −6.5 anteroposterior, +7.05 mediolateral and −3.7 dorsoventral (from dura) at a lateral angle of 16°.
Microinjection of LV-shRNA
A week after surgery, rats were evaluated for their sucrose preference levels (see section ‘Sucrose preference test' for description) and divided into two groups with similar averaged preference. Two microliters of either LV-shBDNF (treatment) or LV-shSCR (control) were microinjected bilaterally into the specific hippocampal subregions over 4 min using a dual channel MAB 40 microdialysis pump (Microbiotech/SE AB, Stockholm, Sweden). Injectors were left in place for 2 min after completing the injection to ensure adequate diffusion from the injector tip. Microinjections were repeated three times every other day. One week after the last injection, the behavioral testing was initiated. All the behavioral assessments described below were performed for each rat, in the same order as written below.
Sucrose preference test
Sucrose preference was performed as we have previously described.31, 34 Rats had access to two drinking spouts positioned side-by-side at the rear of the cage. Fluid consumption was recorded by weighing the bottles every morning between 0930 and 1000 h. Sucrose solution (prepared in tap water) was placed in one bottle and tap water in the other. Rats were tested for 4 days, followed by 1 day of only tap water and then bottle positions were switched and rats were tested for an additional 4 days to control for side preference. Sucrose preference for each rat was defined as the average percentage of sucrose consumption of the total liquid consumption.
To increase the sensitivity of this method, different concentrations of sucrose solution were measured in order to examine which concentration of sucrose could reliably be distinguished (Supplementary Figure 6). On the basis of dose–response curve, we chose a high concentration (2%) at which rats strongly preferred the sucrose solution, and a low concentration (0.2%) at which rats show a significant, but moderate sucrose preference over plain water. We have previously shown that this method has increased sensitivity and allows for measurement of more subtle, but significant reductions of sucrose preference when applied to the chronic mild stress model for depression.31, 34
Home-cage locomotion
Chronic monitoring of locomotion was performed in the home cage using a computerized Inframot system (TSE, Bad Homburg, Germany) as described previously,31, 34 which is based on infrared sensors located above the home cage of each rat. Mobility during the dark period (over 12 h per night) was measured for 5 days. Increasing the measurement time in untouched animals in their home cages increases the stability and reproducibility of locomotion data and therefore enhances the probability of observing significant and replicable behavioral changes.
Exploration and novelty-induced behavior
Locomotion was performed as we have previously described.31, 34 Rats were placed in a 40 × 40 cm exploration box (ActiMot System Activity Chamber, TSE). Then, distance traveled and number of rearings were recorded automatically over 10 min. The distance traveled was estimated by beam breaks of 32 photo beam pairs, separated by 2.5 cm and placed 3 cm above the floor. The rearing activities of rats were detected by the number of beam breaks of 16 photo beam pairs, separated by 2.5 cm and placed 15 cm above the floor. The exploration box was thoroughly cleaned between tests.
Forced swim test
A modified FST was conducted in a cylindrical tank (40 cm high and 18 cm in diameter; constructed at the Weizmann Institute), as we have previously described.31, 34, 35 The water temperature was kept at 24 °C (2 °C above room temperature) and the water level was such that the rat could not touch the bottom with its hind paws. Rats were given a single 10 min exposure to the swim tank and were videotaped. Video films of each FST session were carefully analyzed by an observer blind to the treatment groups, using our locally developed software for a computer-attached joystick.35 The computerized analysis for FST was developed in our laboratory to continuously follow the limbs of the rats35 and detect fine alterations in mobility throughout the test. This allowed for a sensitive analysis of more information than the standard scoring protocols. In addition, immobility time was measured manually.
Morris water maze
The Morris water maze was performed as we described previously.36 Briefly, rats were trained and tested for 4 consecutive days and tested again on the fifth day. In the training sessions, rats were placed in a water tank (diameter of 1.4 m) with a hidden platform placed 1.5 cm below the water surface. Visual cues for enhanced orientation were available in the testing room and the water tank. The latency from placement of the rat in the water until it found the platform was recorded. Rats were allowed to remain on the platform for 15 s, and were then placed back in their home cages. On the fifth day, the platform was removed, and the time spent in each quarter of the water tank was recorded.
BDNF measurements, histology and neurogenesis. Punches and BDNF ELISA: The enzyme-linked immunosorbent assay (ELISA) was performed on a different group of rats that did not undergo behavioral tests, in order to verify the efficiency of BDNF knockdown in vivo. Rats were divided into three different groups microinjected with either LV-GFP, LV-shSCR or LV-shBDNF to the dDG. One week after LV infections, rats were decapitated and their brains were extracted, immediately frozen in isopropanol and stored at −80 °C. Bilateral tissue punches were obtained from the coronal sections generated by a manual cut within the cryostat environment (at −20 °C) and according to a rat brain atlas (Paxinous and Watson, 1998).33 The coronal sections used for punches of the dDG were taken from −2.3 to −4.3 mm from bregma. Protein extraction and sandwich ELISA were performed as we previously described,31 and BDNF concentration was normalized per total protein or per tissue weight. Similar results were obtained when normalized in both ways, and results are presented after normalization to total protein levels.
BrdU administration: Following the behavioral tests, rats were injected intraperitoneally with 50 mg kg−1 bromodeoxyuridine (BrdU; Sigma-Aldrich) at 12 h intervals for 2 days. Twenty four hours or a week after the last BrdU injection, rats were deeply anesthetized and transcardially perfused using PBS supplemented with 4 U ml−1 heparin (Merck Biosciences, Darmstadt, Germany), followed by fresh 2.5 % paraformaldehyde (J.T. Baker, Deventer, Holland) supplemented with 5% sucrose (J.T. Baker) in PBS. Brains were removed, postfixed overnight, and allowed to sink in 2.5% paraformaldehyde supplemented with 30% sucrose in PBS solution. The coronal sections (30 μm) were collected using a cryostat (Leica CM 3050 S, Nussloch, Germany) and placed into 24-well plates containing 0.01% azide (Sigma-Aldrich) in PBS and were stored at 4 °C until further use.
Immunostaining: GFP detection: Free-floating sections were mounted on SuperFrost Plus glass slides (Menzel Glaeser, Braunschweig, Germany), and GFP photomicrographs were acquired using a Nikon E800 microscope (Tokyo, Japan) equipped with filter for Cy-2 epifluorescence.
BDNF detection: After GFP photomicrographs were acquired, slices were allowed to dehydrate, immersed in citrate buffer (2.1 g l−1 citric acid monohydrate, pH=6) and boiled for 15 min in a microwave. Slices were then cooled to room temperature for 30 min and washed. Sections were incubated for 1 h with 20% normal horse serum (Vector, Burlingame, CA, USA) and 0.05% saponin (Sigma-Aldrich, USA). Sections were then covered with anti-BDNF antibodies (Alomone, Jerusalem, Israel) diluted 1:500 in PBS containing 0.05% saponin and 2% normal horse serum at room temperature. The following day, slices were washed and incubated for 1 h with 1:200 Cy-3-conjugated donkey anti-rabbit secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA). Sections were washed and were then covered with Hoechst solution (1:2000, Sigma-Aldrich) for 30–60 s to mark the nuclei of the cells. As a control for nonspecific staining, sections were incubated in identical conditions, without the corresponding primary antibody.
BrdU and DCX: detection Brain sections were incubated in 2 N HCl at 37 °C for 30 min, followed by 10 min incubation in 0.1 N boric acid. After washing, sections were incubated for 1 h with 20% normal horse serum and 0.3% Triton X-100 and then for 12 h at room temperature with 0.3% Triton X-100, 2% normal horse serum, rat anti-BrdU antibodies (1:200; Oxford Biotechnology, Oxford, UK), and goat anti-DCX (1:300; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Sections were then washed and incubated for 1 h with Cy-2-conjugated donkey anti-goat and Cy-3-conjugated donkey anti-rat (1:200; Jackson ImmunoResearch) secondary antibodies.
Quantification
A Nikon E800 microscope was used for analysis. Proliferation was assessed by bilateral counting of BrdU+ cells in the subgranular zone of the dentate gyrus (DG) (defined as the zone of the hilus, the width of two cell bodies, along the base of the granular layer). Neurogenesis was evaluated by counting the cells that were double labeled with BrdU and doublecortin X (DCX). For quantification, double-labeled cells were counted in six coronal sections (150 μm apart) per infected brain region. All counts were performed manually by an observer who was blinded to the experimental groups. To obtain an estimate of the total number of labeled cells per infected region, the total number of cells counted in the selected coronal sections from each brain was multiplied by the volume index (the ratio between the volume of the infected region and the total counted sections). For quantification of the infection spread in vivo, one in every six slices was collected from the anterior to the posterior edge of the injections region (until no GFP could be observed). The number of brain slices expressing GFP was multiplied by 30 μm (thickness of each slice) and by 6 (number of skipped slices). The medial-lateral and the dorsal-ventral spread were evaluated based on the central coronal section within the infected area. BDNF, GFP and Hoechst immunoreactivity were quantified using Image Pro Plus 4.5 software (Media Cybernetics, Bethesda, MD, USA) by measuring the intensity per unit surface area at the dDG, CA3 and vSUB. At least six hippocampal sections per rat were used for this analysis.
Statistical analysis
Results are expressed as mean±s.e.m. A two-tailed unpaired Student's t-test was used for analyses of the behavioral experiments, except for the learning curve of the Morris water maze test in which a two-way repeated measures analysis of variance was performed. One-way analysis of variance was used for the BDNF analysis in vitro and in vivo, followed by Fisher post hoc tests.
GenBank accession number
Brain-derived neurotrophic factor GenBank accession number NM_012513.
Results
Induction of localized BDNF reduction
We designed four different sequences of shRNAs complementary to the coding exon of the rat BDNF gene, that is common to all isoforms of this gene,28 and then tested which sequence most effectively reduces BDNF levels in vitro (Supplementary Figure 1). The most effective sequence was inserted into a lentiviral vector (LV-shBDNF) tagged with GFP.
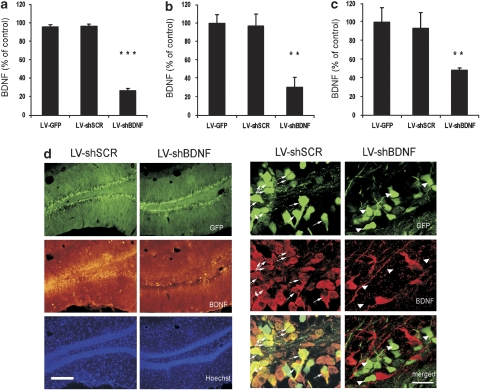
The LV-shBDNF was allowed to infect a C6 rat glioma cell line, which naturally expresses the BDNF protein. Forty-eight hours after infection, we observed a 75% reduction in BDNF secretion. LV expressing GFP and scrambled shRNA (LV-shSCR) or GFP only (LV-GFP) caused no reduction in BDNF secretion (Figure 1a). The measurements of BDNF protein extracted from C6 cells revealed similar results (Supplementary Figure 2). We then tested the ability of the same LV-shBDNF constructs to induce knockdown in vivo. Injection of the viral vectors into the DG of rats resulted in localized infection as shown by GFP expression (Figure 1d). The average radius of infection was 1.14±0.08 mm, and the viral infection was restricted primarily to the DG as observed by cells expressing GFP (Supplementary Figure 3). BDNF levels within slices taken from the site of DG infection were reduced by 69.37%±10.52 in rats injected with the LV-shBDNF relative to that of control rats injected with the LV-shSCR or the LV-GFP (Figure 1b). In addition, in a different group of rats, BDNF levels measured by ELISA on a tissue punch of the dDG infected with LV-shBDNF were reduced by 52.29%±2.37 relative to controls (Figure 1c). When the LV-shBDNF was injected into the CA3 or the vSUB, BDNF levels were reduced by 51.56%±1.78 and 52.48%±9.08, respectively. To verify that the decreased expression of BDNF was not caused by the damage of the tissue due to the viral injections, we examined the injection site by nuclear Hoechst staining. No change in cell density was observed (Figure 1d).
Figure 1.
Validation of LV-shBDNF infection and brain-derived neurotrophic factor (BDNF) knockdown (KD) in vitro and in vivo. BDNF protein expression was measured in a C6 rat glioma cell line and in vivo in response to infection with lentiviral vector (LV) expressing either green fluorescent protein (GFP) only (LV-GFP; infection control), scrambled shRNA (LV-shSCR; shRNA control) or shRNA complementary to the coding axon of the rat BDNF gene (LV-shBDNF; active sequence). Panel a presents BDNF levels measured in a C6 cell medium 48 h after infection and normalized per 106 cell number. Data are presented as percentages of BDNF secreted from noninfected control cells. One-way analysis of variance (ANOVA) reveals a significant main effect of treatment (F(3, 20)=374.756, P<0.001). Differences between the various LV treatments were assessed using Fisher post hoc analysis (***P<0.001; n=6 per treatment). Panel b presents BDNF levels in the dentate gyrus (DG) measured by immunohistochemistry and epifluorescence microscopy. The intensity per unit area was measured for each slice. BDNF levels are expressed as a percentage of control, by measuring the intensity in slices taken from infected rats relative to control slices taken from the noninfected rats. One-way ANOVA revealed a significant main effect of treatment (F(3, 17)=29.926, P<0.01). Differences between control and infected brains were assessed using Fisher post hoc analysis (**P<0.01; n=5 to 6 per group). Panel c presents BDNF levels in the dDG measured by ELISA and normalized per total protein. BDNF levels are expressed as a percentage of control. One-way ANOVA revealed a significant main effect of treatment (F(3, 14)=5.868, P<0.01). Differences between control and infected brains were assessed using Fisher post hoc analysis (**P<0.01; n=4 or 5 per group). Panel d presents representative micrographs of hippocampal slices including the dDG from the rats injected with LV-shSCR (control) or LV-shBDNF (BDNF KD). The micrographs in the left present the infection spread based on the GFP expression (green) within the sections. BDNF protein expression was visualized by immunohistochemical staining (middle lane). Note the significant reduction in BDNF expression induced by the LV-shBDNF microinjection. Cell nuclei were visualized using Hoechst staining (lower lane). The micrographs on the right show a higher magnification of an infected hippocampal region. The upper lane shows GFP expression as an infection marker, whereas the middle lane shows BDNF protein staining. The lower lane shows superimposition of the upper and middle lanes to highlight cells expressing both GFP and BDNF. The arrows highlight that while the rat brain infected with LV-shSCR show BDNF expression within infected (GFP) cells, none of the infected cells in the LV-shBDNF rat brain show BDNF expression. Scale bar=200 μm at the left micrographs and 20 μm at the right micrographs.
Behavioral effects of BDNF knockdown in the dDG and the CA3
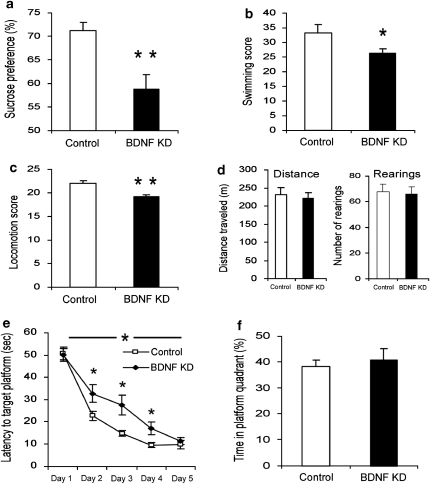
To investigate the effect of BDNF knockdown in specific brain sites on behavior, we used a battery of behavioral assays measuring home-cage locomotion, exploration of a novel environment, sucrose preference and activity in FST. In addition, we measured learning and memory in the Morris water maze. Local injection of LV-shBDNF into the dDG (Figure 1d) induced a significant reduction in sucrose preference over plain water (Figure 2a). This was evident when testing preference for low (0.2%) but not high (2%) concentrations of sucrose solutions (Supplementary Figure 4). In the FST, a significant reduction in the total activity score (measured by a computerized program35) was induced by BDNF knockdown in the dDG (Figure 2b). However, no significant differences were observed when only the immobility time was measured (Supplementary Figure 5). In addition, home-cage locomotion, but not exploration of a novel environment, was reduced by the BDNF knockdown in the dDG (Figure 2c,d). Finally, BDNF knockdown in the dDG induced a delay in spatial learning as measured in the Morris water maze (Figure 2e), but had no effect on performance in a subsequent memory probe test (Figure 2f).
Figure 2.
Behavioral analysis of rats subjected to brain-derived neurotrophic factor (BDNF) knockdown (KD) in the dorsal dentate gyrus (dDG). Lentiviral vector (LV)-shBDNF (knockdown) or LV-shSCR (control) constructs were injected into the dDG of mature rats. The battery of behavioral tests was initiated 3 weeks after recovery from the surgery and the following parameters were measured: sucrose preference (a), activity in a modified forced swim test (FST) (b), home-cage locomotion (c), exploration of a novel environment (d), latency to reach the platform in the water maze task (e) and percent of time spent at the target quadrant during the probe trial in the water maze task (f). Sucrose preference was assessed over a 10-day period using a 0.2% sucrose solution (t(43)=3.526, P=0.01). A modified FST was videotaped, and activity was scored and analyzed using our locally developed software which involves a computer attached joystick35 (t(38)=2.183, P<0.05). Locomotion was automatically measured over four consecutive nights in the home cage of rats (t(41)=2.855, P<0.01). The total distance traveled and number of rearings were automatically quantified in the test for exploration in a novel environment. The water maze task consisted of five training sessions over 5 days, and a test day 3 days later. A two-way repeated measures analysis of variance (ANOVA) revealed a significant Group X session interaction (F(1,26)=4.835, P<0.05). In the water maze task, the latency to find the platform was significantly lower in control rats than in BDNF KD rats at days 2, 3 and 4 (t(26)>2.115, P<0.05) but not at days 1 and 5. The probe trial was conducted 3 days after the training sessions, and the percentage of time spent at the target quadrant was measured during a 1-min test. The level of a random distribution between quadrants was 25%. Values are mean±s.e.m. (n=20 to 23 per group for all tests except for the water maze in which n=14 per group; *P<0.05, **P≤0.01).
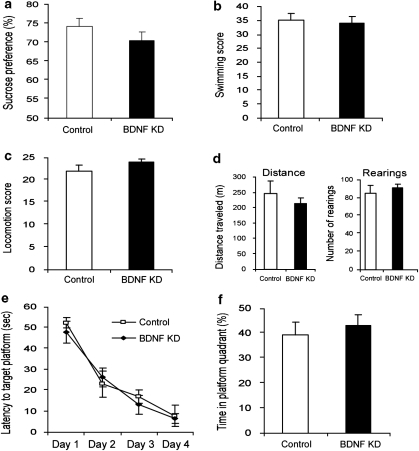
Although BDNF knockdown in the dDG induced several behavioral deficits, injection of LV-shBDNF into the CA3 did not induce any behavioral alterations as measured using the same battery of tests (Figure 3).
Figure 3.
Behavioral analysis of rats subjected to brain-derived neurotrophic factor (BDNF) knockdown (KD) in the CA3. Lentiviral vector (LV)-shBDNF (knockdown) or LV-shSCR (control) constructs were injected into the CA3 of mature rats. The battery of behavioral tests was initiated 3 weeks after recovery from the surgery and the following parameters were measured: sucrose preference (a), activity in a modified forced swim test (FST) (b), home-cage locomotion (c), exploration of a novel environment (d), latency to reach the platform in the water maze task (e) and percent of time spent at the target quadrant during the probe trial in the water maze task (f), as described in Figure 2. Values are mean±s.e.m. (n=8 per group).
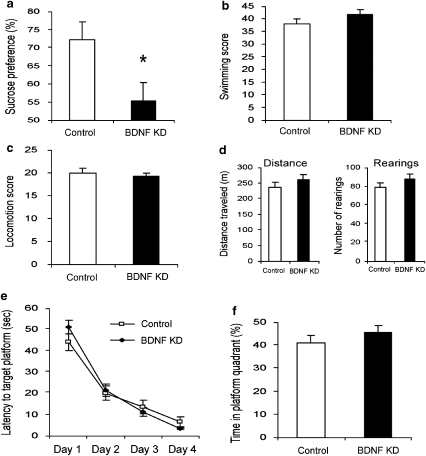
Behavioral effects of BDNF knockdown in the vSUB
Brain-derived neurotrophic factor knockdown in the vSUB reduced sucrose preference significantly as compared with control animals when sucrose concentration was 0.2% (Figure 4a). However, this reduction was not significant when rats were tested using a 2% sucrose solution (Supplementary Figure 4). Activity in FST or home-cage locomotion was not affected by BDNF knockdown in the vSUB (Figure 4b, c). Similarly, locomotor activity in a novel environment during the exploration test was not affected by BDNF knockdown in the vSUB (Figure 4d). Finally, BDNF knockdown in the vSUB had no effect on learning and memory, as measured in the Morris water maze paradigm (Figure 4e, f).
Figure 4.
The behavioral effect of brain-derived neurotrophic factor (BDNF) knockdown in the ventral subiculum (vSUB). Lentiviral vector (LV)-shBDNF (knockdown) or LV-shSCR (control) constructs were injected into the vSUB of mature rats. The battery of behavioral tests was initiated 3 weeks after recovery from the surgery and the following parameters were measured: sucrose preference (a), activity in a modified forced swim test (FST) (b), home-cage locomotion (c), exploration of a novel environment (d), latency to reach the platform in the water maze task (e) and percent of time spent at the target quadrant during the probe trial in the water maze task (f), as described in Figure 2. Values are mean±s.e.m. (* P<0.05, n=11 per group). A significant difference between the experimental groups was observed only in the sucrose preference test (t(20)=2.418, P<0.05).
BDNF knockdown in the dDG reduces neuronal differentiation in vivo
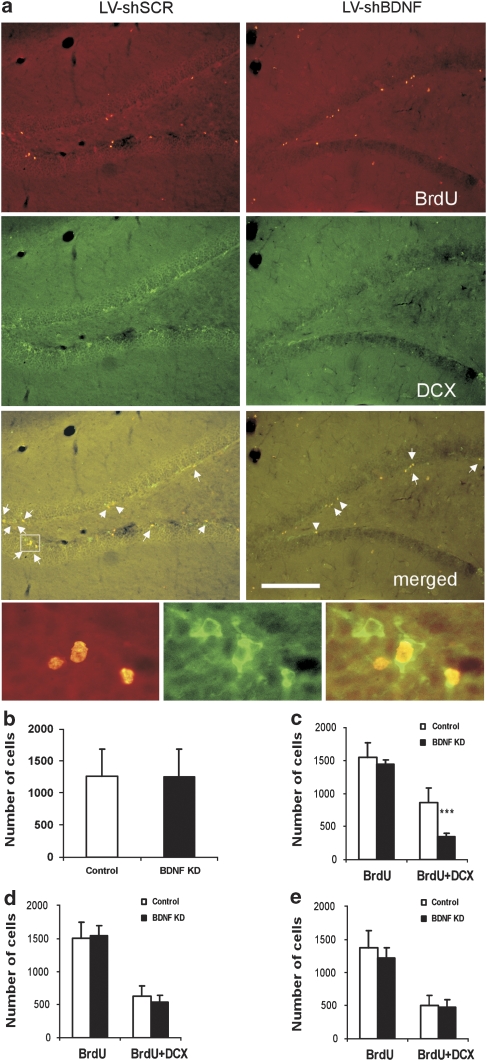
We tested whether local BDNF knockdown can actually reduce hippocampal neurogenesis in vivo. LV-shBDNF- and LV-shSCR-treated rats were injected with BrdU 24 h or 1 week before euthanasia, and neurogenesis was evaluated by immunohistochemistry. BDNF knockdown in the dDG did not affect proliferation, as measured by the number of BrdU stained cells in the DG (Figure 5a–c). However, neuronal differentiation was decreased by 60%, as measured by the number of cells double stained with BrdU and DCX (Figure 5a, c). Conversely, BDNF knockdown in either the CA3 or the vSUB did not affect proliferation or differentiation in the DG (Figure 5d, e).
Figure 5.
The effect of brain-derived neurotrophic factor (BDNF) knockdown on hippocampal neurogenesis. Neurogenesis in the subgranular zone (SGZ) was measured 2 months after the induction of BDNF knockdown (KD) in the dorsal dentate gyrus (dDG), CA3 or ventral subiculum (vSUB) of mature rats. Rats were injected intraperitoneally with 50 mg kg−1 bromodeoxyuridine (BrdU) at 12 h intervals for 2 days. Twenty-four hours or a week later rats were deeply anesthetized and transcardially perfused. Immunohistochemistry was performed to detect neuronal dividing cells by counting the cells that incorporated BrdU into their genome, and express the immature neuronal marker doublecortin X (DCX), as shown in the small micrographs. Representative micrographs of the dDG from rats injected with lentiviral vector (LV)-shSCR (Control) or LV-shBDNF (BDNF KD) into their dDG are presented in panel a. Proliferation was measured by counting BrdU-stained cells (upper lane), immature neurons were visualized by DCX (middle lane), and differentiation was measured by counting double-labeled BrdU and DCX cells (lower lane). Scale bar=200 μm. Panel b shows a quantitative analysis of proliferation comparing control and BDNF KD rats after LV infections in the dDG. This panel presents data from rats that were euthanized 24 h after BrdU injections. Panels c,d and e show a quantitative analysis of proliferation and differentiation comparing control and BDNF KD rats after LV infections in the dDG, CA3 or vSUB, respectively. These rats were injected with BrdU a week before euthanasia. A significant effect of BDNF KD was observed only in the dDG group and only in the number of differentiated neuronal cells (BrdU + DCX) (t(11)=5.672, P<0.001), but not in total proliferation (BrdU). Values are mean±s.e.m. (*** P<0.001, n=6–9 per group).
Discussion
This study provides direct evidence that reduced BDNF protein levels, in specific hippocampal subregions, can reduce neurogenesis in vivo and affect behaviors associated with depression. Specifically, BDNF knockdown in the dDG, but not in the CA3 or the vSUB, induced a reduction in neuronal differentiation and several behavioral impairments including reduced sucrose preference, locomotion and activity in the swim test. From another perspective, the behavioral effects of current antidepressant drugs were shown to be dependent on BDNF10 and neurogenesis.5
Given that the depressive behavior cannot be characterized by a single symptom in humans or in animals, we used a battery of behavioral tests associated with some symptoms of depression, including anhedonia, loss of interest and motivation, as well as fatigue and loss of energy (DSM IV, 2000). We also tested spatial memory, as BDNF has been implicated in long-term potentiation,37 and learning and memory mechanisms.38 Moreover, cognitive impairment is common in depressive individuals (DSM IV, 2000). The set of behavioral deficits induced after BDNF reduction in the dDG (but not the other sites) such as reduced sucrose preference, home-cage locomotion and activity in the FST can be associated with depressive-like symptoms. It is important to note that exploration of a novel environment was not affected in the same animals, indicating that the low scores measured in both the swim test and the home-cage locomotion tests were not due to motor impairment. Finally, in the Morris water maze, although acquisition was initially impaired, differences were not maintained and no effect was observed in the probe test for memory, suggesting that BDNF in this subregion does not have a critical function in spatial memory.
Importantly, the battery of behavioral tests used in this study was specifically designed to detect subtle behaviors. First, with regard to the sucrose preference test, we examined which concentration of sucrose could reliably be distinguished (Supplementary Figure 6). On the basis of the dose–response curve, we chose a high concentration (2%) at which all rats strongly preferred the sucrose solution, and a low concentration (0.2%) at which control rats showed a significant, but moderate sucrose preference over plain water. In the chronic mild stress model for anhedonia,39 we have previously found that when repeated ongoing measurements are used, the lower sucrose concentration provides a more sensitive distinction between control rats and those exposed to the chronic mild stress procedure.31, 34 In this study, when using the lower sucrose concentration we observed that BDNF reduction within the dDG and vSUB (but not the CA3) significantly impaired sucrose preference. Another methodological consideration is the use of a computerized analysis for FST that was developed in our laboratory to continuously follow the limbs of rats35 and detect fine alterations in mobility throughout the test. This allowed for analysis of more information than the standard scoring protocols. The computerized analysis revealed a significant effect of BDNF knockdown in the dDG (but not the vSUB or the CA3) on FST score. We also attempted to optimize our assessment of locomotion by measuring the home-cage locomotion of rats over a period of four consecutive nights in addition to the commonly used exploration test. Increasing the measurement time in untouched animals in their home cages increases stability and reproducibility of locomotion data and therefore enhances the probability of observing significant and replicable behavioral changes. Home-cage locomotion of rats with BDNF knockdown in the dDG (but not the CA3 or vSUB) was significantly lower than that of controls. On the other hand, a standard locomotion test in exploration boxes did not reveal alterations induced by BDNF knockdown in any of the hippocampal sites. Recently, the behavioral effect of BDNF knockout was measured in floxed BDNF mice, whose hippocampuses were injected with Cre recombinase-expressing viruses.10, 40 In these experiments, no differences were detected in sucrose preference, locomotor activity and percent of immobility in FST following BDNF knockout (but BDNF knockout impaired the behavioral effects of current antidepressant medications). In this study, BDNF knockdown in specific hippocampal subregions of rats showed similar results when using comparable parameters of high sucrose concentration (2%), locomotor activity in a novel environment and immobility time in FST. However, when testing the animals using a low sucrose concentration (0.2% on the basis of dose–response curve, Supplementary Figure 631, 34) continuous monitoring of locomotion in the home cages of rats31, 34 and a sensitive computerized analysis of FST31, 34, 35 a significant effect of BDNF knockdown in the dDG was detected.
Given the suggested function of neurogenesis in depressive-like behavior and the mechanism of action of antidepressant drugs in animal models,5, 6, 7, 8 we also measured the effect of localized BDNF knockdown on neurogenesis. In vitro studies have already established a strong association between BDNF and neurogenesis. Specifically, an addition of BDNF to central nervous system-derived neuronal precursors was shown to influence differentiation but not proliferation.41, 42 Moreover, it has previously been reported that the infusion of BDNF into the hippocampus of adult mice increases neurogenesis in vivo.21 However, no distinction was made between neuronal differentiation and proliferation. To clarify whether BDNF is necessary for hippocampal neurogenesis, it is essential to induce a localized knockdown of BDNF in vivo and measure both proliferation and differentiation. Studies which investigated this by generating heterozygous BDNF knockout (BDNF +/−) mice reported conflicting results; whereas one study reported that these animals exhibit decreases in the basal levels of proliferation and survival of newly generated neurons,43 another study reported an increase in the basal proliferation in such animals,44 while another group reported no difference in neural survival in these animals.45 Given that BDNF is reduced early in development in these mice, it is unknown what potential compensatory mechanisms can occur during neurodevelopment. Recently, a specific knockout of the tropomyosin-related kinase B (TrkB) receptor in hippocampal neural progenitor cells was performed by crossing mice harboring the TrkB flox alleles to transgenic mice expressing the Cre recombinase under the human glial fibrillary acidic protein promoter or the Synapsin 1 (Syn) promoter.46 In this study, specific deletion of TrkB in neural progenitor cells resulted in impaired proliferation and neurogenesis. In contrast, deletion of TrkB in differentiated neurons did not affect neurogenesis. Conflicting results were reported in a parallel study in which knockout of the catalytically active site of the TrkB receptor was performed specifically in the radial glia-like stem cells of the mice DG.47 In that study the composition of progenitors and new neurons that exit the cell cycle was not altered by deletion of TrkB. However, the survival of newborn neurons was shown to be critically dependent on the activation of the TrkB receptor. The apparent contradiction between these studies can be explained in several ways; however, in order to study the function of the BDNF protein itself in neurogenesis, a specific knockdown of BDNF should be performed. Indeed, TrkB is considered the high-affinity receptor for BDNF,48 however, it is also the high-affinity receptor for neurotrophin 4 (NT4).48 Although NT4−/− knockout mice do not show impaired neurogenesis,45 this might be attributed to compensatory mechanisms that could develop because such transgenic mice do not express NT4 from as early as their embryonic development. Moreover, BDNF may have effects that are not mediated by the TrkB receptor. Even though primary neurospheres derived from the DG of adult TrkB knockout mice and treated with BDNF displayed reduction in both number and size relative to those derived from the control mice,46 the effect of BDNF on neurogenesis in vivo was not measured in the TrkB knockout mice. Here we report that BDNF reduction in the dDG caused a significant reduction in neurogenesis. Specifically, BDNF was necessary for differentiation but not the proliferation of newborn neurons, as the only difference between BDNF knockdown and controls was the number of cells expressing both proliferation marker (BrdU) and immature neuronal marker (DCX), whereas the total number of proliferating cells, either 24 h (Figure 5b) or 7 days (Figure 5c) after BrdU injections, was unchanged. Consistent with our findings, an in vitro study showed that BDNF has a function in differentiation, but not proliferation, of neuronal precursors.41 In contrast to the remarkable effects of BDNF knockdown in the dDG on neuronal differentiation and most behavioral measures, BDNF reduction in the CA3 had no effect on neurogenesis or on any of the tested behaviors, whereas BDNF reduction in the vSUB affected sucrose preference (an indicator of anhedonic behavior49) only, but not any of the other tested behaviors or neurogenesis. This suggests that anhedonia is not necessarily an outcome of reduced hippocampal neurogenesis.
Even though impairment of BDNF expression within the CA3 did not affect any of the tested behaviors, previous studies indicated that BDNF mRNA levels within the CA3 were increased by the antidepressant treatments.1, 50 It is important to consider the hippocampal circuitry, especially when interpreting our CA3 results. The mossy fibers of the DG granule cells project to the pyramidal neurons of the CA3 subregion51 and an anterograde transport and secretion of BDNF to the synapse has been reported.38 Therefore, although injection of the LV-shBDNF into the CA3 induced a significant reduction in local BDNF levels, secretion of BDNF from the DG can partially compensate for this loss.
Although alterations in BDNF levels in hippocampal subregions, such as the DG3, 14 and the CA3,1, 50 have been associated with depressive behavior, the function of subicular BDNF expression has not been studied in this regard. We postulate that the vSUB has a possible function in depressive behavior, as it strongly influences the reward system by direct projections to the nucleus accumbens52 and by excitatory input to dopamine (DA) neurons in the ventral tegmental area that can induce DA release in the nucleus accumbens.53 In our study, knockdown of BDNF in the rat vSUB caused a significant reduction of sucrose preference, an indicator of anhedonic-like behavior.49 Previous studies have shown that a sodium channel blocker injected into the vSUB causes a near-complete blockage of novelty-induced DA release of the mesolimbic DA pathway.54 Furthermore, when a glutamate receptor antagonist is applied to the nucleus accumbens, activation of DA neurons by stimulation of the vSUB is blocked.55 Comparably, BDNF reduction within the vSUB may impair glutamate secretion to the nucleus accumbens, as in vitro studies indicate that BDNF can rapidly potentiate glutamatergic transmission and release.56 Therefore, BDNF reduction in the vSUB can cause deficits in reward function and induce anhedonia. It is interesting to note that a selective ablation of the BDNF gene from the ventral tegmental area induced antidepressant-like effect in the social defeat stress paradigm,57 whereas in this study, BDNF reduction in the vSUB resulted in anhedonic-like behavior. Indeed, different roles were suggested for BDNF in the hippocampus and the ventral tegmental area, despite the interaction between these systems.58
In conclusion, this study strengthens the neurotrophic hypothesis of depression. Using LV and RNA interference we showed that hippocampal BDNF reduction affects several behaviors associated with depression. We also defined the dDG as the hippocampal subregion most affected by this reduction, and showed that BDNF reduction in the vSUB can induce anhedonic-like behavior. Finally, we showed that BDNF reduction impairs neuronal differentiation (but not proliferation) in the hippocampus.
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
We thank Raya Eilam, Roman Gersner, Hila Giladi, Avital Okrent, Michal Gropp, Eitan Galun, Shifra Ben-Dor and Adi Wilf for their contribution to the research. This research was supported by the Israel Science Foundation (ISF, Grant no. 1118/06).
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims the effects of antemortem diagnosis psychotropic drugs. Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science (New York, NY) 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- McGonagle KA, Kessler RC. Chronic stress acute stress, depressive symptoms. Am J Community Psychol. 1990;18:681–706. doi: 10.1007/BF00931237. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, McEwen BS. Effect of chronic restraint stress tianeptine on growth factors growth-associated protein-43 microtubule-associated protein 2 mRNA expression in the rat hippocampus. Brain Res. 1998;59:35–39. doi: 10.1016/s0169-328x(98)00130-2. [DOI] [PubMed] [Google Scholar]
- Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, Bullard J, et al. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci. 2004;24:2667–2677. doi: 10.1523/JNEUROSCI.5377-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, et al. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–1153. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science (New York, NY) 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Yu JY, DeRuiter SL, Turner DL. RNA interference by expression of short-interfering RNAs hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA. 2002;99:6047–6052. doi: 10.1073/pnas.092143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science (New York, NY) 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, et al. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Horbinski C, Stachowiak MK, Higgins D, Finnegan SG. Polyethyleneimine-mediated transfection of cultured postmitotic neurons from rat sympathetic ganglia and adult human retina. BMC Neurosci. 2001;2:2. doi: 10.1186/1471-2202-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, fourth edition. Academic Press: San Diego, 1998
- Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, et al. Vaccination as a novel approach for treating depressive behavior. Biol Psychiatry. 2009;65:283–288. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Gersner R, Dar DE, Shabat-Simon M, Zangen A. Behavioral analysis during the forced swimming test using a joystick device. J Neurosci Methods. 2005;143:117–121. doi: 10.1016/j.jneumeth.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not ‘natural' reinforcement. J Neurosci. 2007;27:14179–14189. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Patterson PH. Cytokine and growth factor involvement in long-term potentiation. Mol Cell Neurosci. 1999;14:273–286. [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Reynolds BA, Weiss S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J Neurosci. 1995;15:5765–5778. doi: 10.1523/JNEUROSCI.15-08-05765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memberg SP, Hall AK. Proliferation, differentiation, and survival of rat sensory neuron precursors in vitro require specific trophic factors. Mol Cell Neurosci. 1995;6:323–335. doi: 10.1006/mcne.1995.1025. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci USA. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Yancopoulos GD. Neurotrophin regulation of the developing nervous system: Analyses of knockout mice. Rev Neurosci. 1997;8:13–27. doi: 10.1515/revneuro.1997.8.1.13. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki K, Morinobu S, Duman RS. Administration of a cAMP phosphodiesterase 4 inhibitor enhances antidepressant-induction of BDNF mRNA in rat hippocampus. Neuropsychopharmacology. 2000;22:42–51. doi: 10.1016/S0893-133X(99)00084-6. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Legault M, Wise RA. Novelty-evoked elevations of nucleus accumbens dopamine: dependence on impulse flow from the ventral subiculum and glutamatergic neurotransmission in the ventral tegmental area. Eur J Neurosci. 2001;13:819–828. doi: 10.1046/j.0953-816x.2000.01448.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yamagishi S, Adachi N, Matsumoto T, Yokomaku D, Yamada M, et al. Brain-derived neurotrophic factor-induced potentiation of Ca(2+) oscillations in developing cortical neurons. J Biol Chem. 2002;277:6520–6529. doi: 10.1074/jbc.M109139200. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science (New York, NY) 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.