Abstract
Objective:
To determine whether changes in neonatal practice and morbidity since 2000 have improved the growth attainment of infants with bronchopulmonary dysplasia (BPD).
Study Design:
We compared the respective z-scores of the weight, length and head circumference of extremely low-gestational-age infants (aged <28 weeks) with BPD at birth, 40 weeks and 20 months corrected age (CA) during two time periods, namely period I, 1996–1999 (n=117) and period II, 2000–2003 (n=105), and examined the effects of significant changes in neonatal practice, morbidities and neurosensory outcome on 20-month growth outcomes.
Result:
During the most recent period (2000–2003), there was a significant increase in mean weight z-scores (−1.60 vs −1.22) and decrease in rates of subnormal weight (40 vs 21%), P<0.05 at 20 months CA but not in those of length or head circumference. Significant predictors of the 20-month weight z-score included time period (period I vs II), duration of ventilator dependence and 20-month neurosensory abnormality (all P<0.05).
Conclusion:
Despite an improvement in weight since 2000, poor growth attainment remains a major problem among infants with BPD.
Keywords: preterm, bronchopulmonary dysplasia, growth
Introduction
Despite advances in neonatal intensive care, bronchopulmonary dysplasia (BPD) remains one of the leading morbidities among extremely preterm children.1 Long-term sequelae associated with BPD include neurodevelopmental impairments and poor growth attainment resulting from both growth failure during the neonatal period and later during infancy.2, 3, 4, 5, 6
We recently reported on decreased rates of neurosensory abnormality at 20 months corrected age (CA) among extremely low-birth-weight and gestational age children with BPD who were born during the years 2000–2003 as compared with those born during 1996–1999. This improvement was associated with changes in neonatal practice and morbidity which included a decrease in postnatal steroid therapy and decreased rates of severe cerebral ultrasound abnormality.7 However, it is unclear as to whether these changes are also associated with improvements in the early childhood growth of children with BPD. Thus, we sought to use the data to examine whether the recent changes in neonatal therapy and associated decrease in neonatal morbidity and later neurosensory sequelae have been associated with improved 20-month growth attainment of children with BPD. We hypothesized that extremely low-gestational-age infants (<28 weeks) with BPD born during the years 2000–2003 would have a significant improvement in growth attainment at 20 months CA as compared with those born during the years 1996–1999.
Methods
Population
The population included infants admitted to the neonatal intensive care unit at Rainbow Babies and Children's Hospital during two 4-year periods, namely 1996–1999 (period I) and 2000–2003 (period II). During periods I and II, 315 and 298 extremely low-gestational-age infants, respectively, were admitted to the unit of whom 14 (4%) and 11 (4%), respectively, were excluded because of major congenital malformations. Of the remaining infants, 242 (80%) in period I and 228 (79%) in period II survived to 20 months CA, of whom 126 (52%) and 120 (53%), respectively, were oxygen dependent at 36 weeks CA and were defined as having BPD.8 This definition includes both moderate and severe disease categories of BPD according to the National Institutes of Health consensus definition.9 One infant in period II was excluded from the study as the infant had been transferred to another hospital and the total duration of oxygen dependence was unknown. Four infants in period I died after 36 weeks CA, yet before 20 months CA, of causes related to their severe chronic lung disease. Eight infants died in period II, five because of causes related to their chronic lung disease, one infant had late onset necrotizing enterocolitis, one had disseminated intravascular coagulation and the cause of death of one infant who died after neonatal discharge was unknown. A total of 117 (96%) of the 122 surviving infants with BPD in period I and 105 (95%) of the 111 infants surviving in period II were followed up until 20 months CA and had complete growth measurements, including weight, length and head circumference.
Method of follow-up
Infants were followed up until 18–20 months CA, according to the protocol of the follow-up program at Rainbow Babies and Children's Hospital. Weight, length and head circumference were measured by a trained research assistant at 40 weeks postmenstrual age (that is, the expected date of delivery) and at 20 months CA. Children were weighed unclothed on a Health-O-Meter scale (Pediatric Scale Model 322KG Health-O-Meter Professional, Alsip, IL, USA) calibrated annually according to the manufacturer's recommendations. Length was measured using a Harpenden infantometer (Holtain, Crymych, UK) with infants in a supine position. Head circumference was measured using a non-stretchable tape as the maximum occipito-frontal circumference. Perinatal data were extracted from the hospital charts at the time of discharge from the neonatal nursery. Demographic data included maternal age, marital status, education level and race. Birth data included birth weight, length and head circumference and associated z-scores, gestational age, sex of the infant, delivery method, multiple birth status, presence of chorioamnionitis and maternal pregnancy-induced hypertension. Gestational age was determined from the date of the mother's last menstrual period, and confirmed with obstetric measures and ultrasound in most cases. Perinatal therapies included antenatal and postnatal steroid use, surfactant and indomethacin therapy, as well as ligation of a patent ductus arteriosus. Postnatal steroid therapy was prescribed for infants with chronic lung disease and prolonged ventilator dependence at the discretion of the attending neonatologist. The duration of administration of parenteral nutrition was recorded. We participated in the National Institute of Child Health and Human Development Neonatal Research Multicenter Glutamine Nutrition Trial during period II at which time the administration of intravenous amino acids was advanced more rapidly than during period I.10 We do not have detailed information on the amount of nutrients each infant received; however, during period I, the intravenous amino acids were advanced to a maximum of 3 g kg per day over the first 2 weeks of life, whereas during period II, our revised nutritional protocol included advancing to a maximum of 3 g kg per day of amino acids over the first 3 days of life. However, this was not always possible.
Neonatal morbidities recorded included patent ductus arteriosus, confirmed with echocardiography, sepsis, defined as a positive blood culture in the presence of clinical signs of infection and necrotizing enterocolitis, according to the definition of Bell et al.11 Duration of ventilator dependence was considered as a measure of the severity of chronic lung disease. Periventricular hemorrhage was categorized as grades I through IV, according to the definition of Papile et al.12 A severe cranial ultrasound abnormality was defined as the presence of a grade III or IV periventricular hemorrhage, periventricular leukomalacia and/or persistent ventriculomegaly at the time of discharge. Neurological abnormalities were documented at 20 months CA according to the Amiel-Tison13 method of muscle tone assessment. Major neurosensory abnormalities were defined as cerebral palsy, persistent hypertonia or hypotonia, shunt-dependent hydrocephalus without neurological abnormality, blindness and/or deafness requiring a hearing aid.
The study was approved by the Institutional Review Board of the University Hospitals of Cleveland, and signed parental consent was obtained for all study participants.
Statistical analysis
Growth outcomes examined included 20-month weight, length and head circumference and their respective z-scores. A z-score is calculated from the mean value of the reference population divided by its s.d. Weight gestational age z-scores at birth and 40 weeks were computed using the sex-specific standards described by Kramer et al.14 z-scores for length and head circumference at birth and at 40 weeks were based on the intrauterine growth standards of Usher and McLean.15 For infants who were more than 43 weeks postmenstrual age at the initial post-discharge visit, and at the 20-month visit, weight, length and head circumference z-scores were computed on the basis of the the Center for Disease Control and Prevention normative data, which are sex specific.16 Subnormal z-scores were defined as −2z (that is, −2 s.d.).
The variables compared between the two periods are listed in Tables 1 and 3. The unpaired t-test or Wilcoxon rank sum test was used to compare continuous variables, the χ2 or Fisher's exact test was used to compare categorical variables, and the Cochran–Armitage trend test was used to compare distributions of ordinal categorical variables.17 Postnatal steroid therapy was considered as whether the infant received steroid therapy (yes or no) and by the number of days received, as a surrogate for dose effect.
Table 1. Maternal sociodemographic, obstetric and infant birth data.
| Period I (1996–1999) n=117 | Period II (2000–2003) n=105 | P-value | |
|---|---|---|---|
| Maternal sociodemographic factors | |||
| Age (mean±s.d.) | 27.5±6.7 | 27.3±7.2 | 0.817 |
| Married, n (%) | 54 (46) | 40 (38) | 0.225 |
| Black race, n (%) | 66 (56) | 65 (62) | 0.406 |
| Education, n (%) | |||
| Greater than high school | 57 (49) | 42 (40) | |
| Completed high school | 44 (38) | 39 (37) | 0.074a |
| Less than high school | 16 (14) | 24 (23) | |
| Perinatal risk factors | |||
| Pregnancy-induced hypertension, n (%) | 15 (13) | 16 (15) | 0.604 |
| Chorioamnionitis n (%) | 15 (13) | 12 (11) | 0.838 |
| Antenatal steroid therapy, n (%) | 84 (72) | 87 (83) | 0.050 |
| Cesarean section, n (%) | 64 (55) | 55 (52) | 0.729 |
| Infant born outside hospital, n (%) | 9 (8) | 10 (10) | 0.626 |
| Infant birth data | |||
| Gestational age, weeks (mean±s.d.) | 25.2±1.3 | 25.1±1.2 | 0.323 |
| Birth weight, g (mean±s.d.) | 767.4±153.0 | 750.1±143.6 | 0.386 |
| Length, cm (mean±s.d.) | 32.6±2.30 | 32.7±2.4 | 0.847 |
| Head circumference, cm (mean±s.d.) | 23.2±1.5 | 22.9±1.5 | 0.130 |
| Male sex, n (%) | 55 (47) | 52 (50) | 0.708 |
| Multiple births, n (%) | 15 (13) | 25 (24) | 0.033 |
In some instances, data were missing for one or two children.
Cochran–Armitage test.
We sought to examine the effect of the significant (P<0.05) changes in neonatal therapies and morbidities, that is, those that differed significantly between the two periods, on 20-month growth attainment. These were entered in a stage-wise manner into a model after adjusting for the time period (period I vs II), sociodemographic factors (maternal education and race, sex of the child) and significant differences in birth data. The significant neonatal therapies were then entered, followed by neonatal morbidities and finally by 20-month neurodevelopmental outcome.
Results
Comparison of sociodemographic, perinatal and infant birth data
Sociodemographic (Table 1) measures, including maternal age, race and level of education did not differ between the two periods (Table 1). There were no significant differences between periods in maternal obstetric factors or infant birth data other than an increase in multiple births during period II (P=0.033). Mean gestational age, birth weight, weight z-score and gender did not differ between the two periods.
Twenty-month growth outcomes
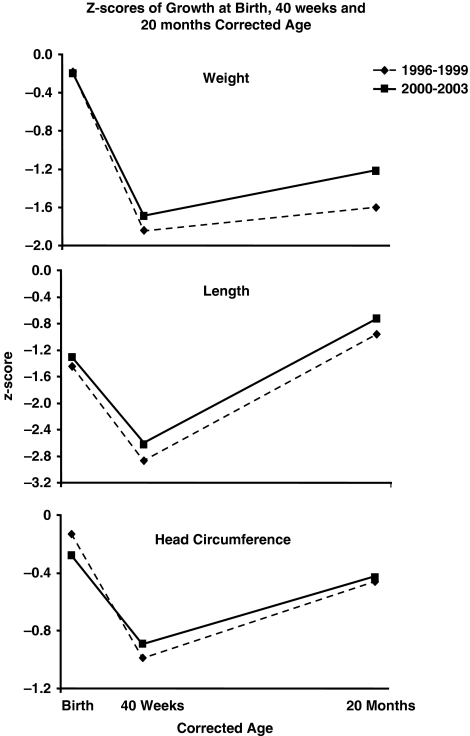
During period I as compared with period II at mean CAs of 19.2 and 19.4 months, respectively, the mean 20-month body weight was 10.00 vs 10.41 kg (P=0.049), the mean length was 79.5 vs 80.5 cm (P=0.088) and the mean head circumference was 46.8 vs 46.9 cm (P=0.634), respectively. Table 2 presents a comparison between the two periods of mean weight, length and head circumference z-scores at birth, 40 weeks and 20 months CA. These are illustrated in Figure 1. At the time of birth, the z-scores were similar. Between birth and 40 weeks, the expected term date of delivery, there was a deceleration in neonatal growth during both periods with a resultant decrease in the 40-week weight, length and head circumference z-scores as compared with the respective birth z-scores. Comparison of the 40-week growth parameters showed no significant differences between periods I and II. Catch-up growth occurred between 40 weeks and 20 months during both periods. Comparison of the body weight z-scores at 20 months showed a significantly higher weight z-score during period II. This increased catch-up growth in weight during period II was also evidenced by a decrease in the rate of subnormal weight attainment (z-score <−2 s.d.) from 38% at 40 weeks to 21% at 20 months, whereas during period I, the rates of subnormal weight attainment decreased from 46% at 40 weeks to 40% at 20 months, respectively. Twenty-month length and head circumference z-scores and their rates of subnormal growth attainment did not differ significantly between the two time periods (see Table 2). In Table 2, the very low length z-score at birth and 40 weeks in comparison with that of weight and head circumference is noteworthy. This is possibly due to inaccuracy in the measure of length in immature sick infants many of whom are on ventilators.
Table 2. Comparison of weight, length and head circumference z-scores and rates of subnormal scores (−2 s.d.) at birth, 40 weeks, 8 and 20 months corrected age between period I (1996–1999) and period II (2000–2003).
| Birth | 40 weeks | 20 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Period I | Period II | P-value | Period I | Period II | P-value | Period I | Period II | P-value | |
| n=117 | n=105 | n=117 | n=105 | n=117 | n=105 | ||||
| Weight | |||||||||
| z-scorea | −0.18±0.7 | −0.20±0.7 | 0.829 | −1.84±0.9 | −1.69±0.9 | 0.204 | −1.60±1.5 | −1.22±1.3 | 0.041 |
| z-score <−2 s.d. | 0 | 0 | 46% | 38% | 0.259 | 40% | 21% | 0.002 | |
| Length | |||||||||
| z-scorea | −1.46±1.3 | −1.31±1.1 | 0.370 | −2.87±1.4 | −2.63±1.4 | 0.184 | −0.96±1.3 | −0.73±1.1 | 0.150 |
| z-score <−2 s.d. | 31% | 29% | 0.721 | 77% | 66% | 0.073 | 16% | 9% | 0.086 |
| Head circumference | |||||||||
| z-scorea | −0.13±0.9 | −0.28±0.8 | 0.137 | −0.99±1.2 | −0.89±1.4 | 0.494 | −0.46±1.1 | −0.43±1.2 | 0.851 |
| z-score <−2 s.d. | 0.8% | 0% | 1.000 | 20% | 24% | 0.554 | 9% | 8% | 0.800 |
Figure 1.
Comparison between period I (1996–1999) and period II (2000–2003) of the mean z-scores of weight, length and head circumference at birth, 40 weeks corrected age (the expected date of delivery) and 20 months corrected age.
Comparison of neonatal therapies, morbidities and 20-month outcomes
A comparison of the neonatal therapies and morbidities are presented in Table 3. Significant changes in neonatal therapy between periods I and II included an increase in the rates of surfactant therapy, Indomethacin therapy and ligation of a patent ductus arteriosus. The rates of postnatal steroid therapy and duration of this therapy for those infants who received postnatal steroids decreased. The annual rates of steroid therapy for the years 1996, 1997, 1998 and 1999 were 88, 77, 61 and 71%, respectively, whereas the rates during 2000, 2001, 2002 and 2003 were 33, 38, 33 and 4%, respectively. There were no significant changes between periods in the rates of sepsis, patent ductus arteriosus, necrotizing enterocolitis, indirect hyperbilrubinemia, duration of parenteral nutrition and duration of overall hospitalization (Table 3). Significant changes in neonatal morbidity included a decrease in the rates of periventricular hemorrhage but not in those of periventricular leukomalacia and ventriculomegaly. The overall rate of severe cranial ultrasound abnormality decreased significantly during period II. The duration of ventilator dependence increased significantly in period II, although the duration of oxygen dependence did not change.
Table 3. Comparison of neonatal morbidities and therapies.
| Period I (1996–1999) n=117 | Period II (2000–2003) n=105 | P-value | |
|---|---|---|---|
| Neonatal therapies | |||
| Surfactant, n (%) | 101 (86) | 103 (98) | 0.001 |
| Indomethacin therapy, n (%)a | 65 (56) | 84 (80) | <0.001 |
| Surgical ligation of patent ductus arteriosus, n (%) | 31 (27) | 42 (40) | 0.032 |
| Postnatal steroid therapy, n (%) | 88 (75) | 30 (29) | <0.001 |
| Days postnatal steroids (median, range)b | 25 (2–102) | 8 (2–39) | <0.001 |
| Duration of ventilator dependence (median days, range) | 36 (2–86) | 40 (3–180) | 0.027 |
| Duration of oxygen dependence (median days, range)c | 105 (63–688) | 100 (64–727) | 0.357 |
| Neonatal morbidity | |||
| Sepsis, n (%) | 59 (50) | 55 (52) | 0.771 |
| Patent ductus arteriosus, n (%) | 78 (67) | 78 (74) | 0.215 |
| Necrotizing enterocolitis, n (%) | 6 (5) | 8 (8) | 0.446 |
| Indirect hyperbilirubinemia, n (%)d | 11 (9) | 12 (11) | 0.621 |
| Periventricular hemorrhage, n (%) | 51 (44) | 29 (28) | 0.013 |
| Periventricular leukomalacia, n (%) | 10 (9) | 7 (7) | 0.599 |
| Ventriculomegaly at discharge, n (%) | 16 (14) | 8 (8) | 0.147 |
| Severe cranial ultrasound abnormality, n (%)e | 30 (26) | 14 (13) | 0.022 |
Prophylaxis or treatment.
For those who received postnatal steroids.
Including oxygen given after discharge in the home.
Bilirubin >10 mg per 100 ml.
Includes grades III–IV intraventricular hemorrhage, periventricular leukomalacia and/or ventriculomegaly at discharge.
At 20 months, as reported previously, there was a significant decrease in deafness requiring a hearing aid (9 vs 2%) and in overall neurosensory abnormality (30 vs 16%) during period II.7 The specific rates of cerebral palsy and blindness did not change significantly.
Multivariable analysis of correlates of growth at 20 months CA
After adjusting for the time period (period I vs II) and sociodemographic factors (maternal education as well as race and sex of the child), variables that changed significantly between the time periods were then entered in the multiple stepwise regression analysis in a stage-wise manner. These included multiple birth status in stage I. Significant neonatal therapeutic changes entered in the second stage included surfactant, indomethacin and postnatal steroid therapy, as well as ligation of a patent ductus arteriosus. Significant changes in neonatal morbidity entered in stage III included severe cranial ultrasound abnormality and the duration of ventilator dependence, which was considered as a measure of severity of chronic lung disease. The presence of neurosensory abnormality at 20 months was entered in stage IV (Table 4). The results showed that the factors found to have an independent effect on 20-month weight included the time period of birth (period I vs II), longer duration of ventilator dependence and the presence of neurosensory abnormality at 20 months CA. Severe cranial ultrasound abnormality predicted 20-month weight before entering neurosensory abnormality in the stepwise regression analysis, but was not significant in the final analysis. Stepwise logistic regression analysis to examine the correlates of subnormal weight (<−2 s.d.) showed similar results (see Table 5). The fact that the time period that is, period I vs II) remained significant even after controlling for sociodemographic factors and identified neonatal therapeutic and morbidity factors that changed significantly between the two periods indicates that other unidentified factors contributed to the improvement in weight at 20 months.
Table 4. Results of stage-wise regression analysis predictors of weight z-scores at 20 months corrected age.
| Variable | Stage I | Stage II | Stage III | Stage IV | ||||
|---|---|---|---|---|---|---|---|---|
| B | (95% CI) | B | (95% CI) | B | (95% CI) | B | (95% CI) | |
| Time period (I vs II) | 0.35 | (−0.02,0.72) | 0.32 | (−0.11,0.76) | 0.48 | (0.04,0.93)* | 0.45 | (0.01,0.89)* |
| Sociodemographic status | ||||||||
| Maternal education <high school | 0.26 | (−0.23,0.75) | 0.31 | (−0.18,0.80) | 0.22 | (−0.26,0.70) | 0.22 | (−0.26,0.69) |
| Race | 0.11 | (−0.27,0.49) | 0.12 | (−0.26,0.50) | 0.18 | (−0.19,0.56) | 0.20 | (−0.17,0.57) |
| Sex of child | −0.12 | (−0.48,0.25) | −0.12 | (−0.48,0.25) | −0.12 | (−0.48,0.24) | −0.10 | (−0.46,0.25) |
| Multiple birth | −0.02 | (−0.51,0.47) | −0.10 | (−0.51,0.49) | −0.14 | (−0.63,0.35) | −0.10 | (−0.59,0.38) |
| Neonatal therapy | ||||||||
| Surfactant | −0.27 | (−0.97,0.43) | −0.22 | (−0.90,0.46) | −0.27 | (−0.94,0.40) | ||
| Postnatal steroids | −0.28 | (−0.70,0.14) | 0.02 | (−0.42,0.45) | 0.09 | (−0.35,0.52) | ||
| Indomethacin | −0.32 | (−0.75,0.11) | −0.29 | (−0.71,0.14) | −0.26 | (−0.68,0.15) | ||
| PDA ligation | 0.04 | (−0.38,0.45) | 0.14 | (−0.26,0.54) | 0.17 | (−0.23,0.57) | ||
| Neonatal morbidity | ||||||||
| Severity of lung diseasea | −0.01 | (−0.02,−0.003)** | −0.01 | (−0.02,−0.002)* | ||||
| Severely abnormal ultrasoundb | −0.60 | (−1.06,−0.13)* | −0.37 | (−0.86,0.13) | ||||
| Neurosensory outcome | ||||||||
| Neurosensory abnormality | −0.55 | (−1.01,−0.08)* | ||||||
| R2 | 0.03 | 0.06 | 0.12 | 0.14 | ||||
Abbreviations: B, unstandardized coefficient (95% CI); 95% CI, 95% confidence interval; PDA, patent ductus arteriosus.
Days on ventilator
Includes grades III–IV hemorrhage, periventricular leukomalacia and ventricular dilatation at discharge.
*P<0.05, **P<0.001.
Table 5. Predictors of subnormal weight (<–2 s.d.) at 20 months corrected age, results of stepwise logistic regression analysis.
| Variable | Stage I | Stage II | Stage II | Stage IV | ||||
|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Time period (I vs II) | 0.39 | (0.21,0.72)** | 0.34 | (0.17,0.71)** | 0.26 | (0.12,0.56)** | 0.26 | (0.12,0.58)** |
| Sociodemographic status | ||||||||
| Maternal education <high school | 0.86 | (0.38,1.96) | 0.81 | (0.35,1.88) | 0.96 | (0.41,2.26) | 0.97 | (0.40,2.31) |
| Race | 0.87 | (0.48,1.60) | 0.83 | (0.45,1.55) | 0.75 | (0.39,1.42) | 0.71 | (0.37,1.37) |
| Sex of child | 1.39 | (0.77,2.50) | 1.41 | (0.77,2.57) | 1.44 | (0.78,2.69) | 1.43 | (0.76,2.71) |
| Multiple birth | 1.21 | (0.56,2.62) | 1.17 | (0.52,2.63) | 1.42 | (0.61,3.30) | 1.32 | (0.56,3.12) |
| Neonatal therapy | ||||||||
| Surfactant | 1.97 | (0.59,6.64) | 2.02 | (0.58,7.12) | 2.40 | (0.65,8.83) | ||
| Postnatal steroids | 1.17 | (0.59,2.33) | 0.77 | (0.36,1.65) | 0.65 | (0.30,1.43) | ||
| Indomethacin | 1.95 | (0.95,3.98) | 1.94 | (0.93,4.05) | 1.85 | (0.88,3.90) | ||
| PDA ligation | 0.78 | (0.40,1.53) | 0.66 | (0.32,1.33) | 0.60 | (0.29,1.24) | ||
| Neonatal morbidity | ||||||||
| Severity of lung diseasea | 1.02 | (1.00,1.03)* | 1.01 | (1.00,1.03)* | ||||
| Severely abnormal ultrasoundb | 2.49 | (1.17,5.30)* | 1.56 | (0.68,3.61) | ||||
| Neurosensory outcome | 3.03 | (1.39,6.62)** | ||||||
Abbreviations: 95% C.I., 95% confidence interval; OR, odds ratio; PDA, patent ductus arteriosus.
Days on ventilator.
Includes grades III–IV hemorrhage, periventricular leukomalacia and ventricular dilatation at discharge.
*P<0.05, **P<0.001.
Significant correlates of the 20-month length and head circumference z-scores were similar to those of the 20-month weight, with the exception that the time period of birth was not predictive and that male sex was predictive of the length z-score (data not shown).
Discussion
We sought to examine whether changes in neonatal practice, morbidity and associated neurodevelopmental outcomes since 2000 were associated with 20-month growth attainment of infants with BPD born at <28 weeks gestation. Results showed a significant increase in weight attainment z-scores at 20 months during period II but not in those of length or head circumference. Factors significantly associated with 20-month weight that had changed significantly between the time periods included the period of birth, with the most recent period having a positive effect, and the duration of ventilator dependence and the presence of neurosensory impairment both of which exerted a negative effect on 20-month growth. However, it is notable that the time period remained significant in the multivariable model that controlled for duration of ventilator dependence and neurosensory impairment, indicating that changes in these factors did not completely explain the improvement in 20-month weight between time periods.
The increase in 20-month growth during the most recent period which extended from the year 2000 to 2003 is possibly related to improvements in neonatal and postdischarge nutrition, as well as to other unidentified factors and probably contribute to the fact that the time period was a significant factor in multivariable analyses. Although we have no data in this regard, it is likely that a more aggressive approach to parenteral nutrition and early oral intake, as well as increased caloric post discharge formulae instituted during the most recent years in our unit may have contributed to the improvement in 20-month weight z-scores. This occurred despite the negative effects of the increased duration of ventilator dependence and of neurosensory impairment on growth. Prolonged duration of mechanical ventilation has been associated with poor growth in very-low-birth-weight populations.4 We speculate that the longer duration of ventilator dependence, indicating more severe lung disease, is most likely due to the decreased use of postnatal steroids during period II, which was based on the evidence obtained from randomized controlled trials of increased rates of cerebral palsy associated with its use.18 The negative effects of postnatal steroid therapy on growth have been well documented.19, 20, 23 However, in our population, postnatal steroid therapy did not exert a significant effect on growth at 20 months after adjusting for other confounding factors. We speculate that the potential beneficial effects on growth of the decreased postnatal steroid use that occurred during period II were likely offset by the concomitant increase in duration of ventilation dependence, which also occurred during this time and was associated with growth failure in our population. We similarly found that although the rates of 20-month neurosensory impairment decreased from 31 to 16% during period II and might have contributed to the improvement in weight at 20 months, their presence continued to exert a negative effect on growth outcomes. Poor growth attainment in children with neurological abnormality has been well documented and may be due to both nutritional factors related to oral feeding impairments and non-nutritional factors.21, 22, 23
To our knowledge, this is the first report of growth outcomes of infants with BPD born since 2000. Our results indicate that neonatal growth failure remains a major problem among infants with BPD, and that, although some catch-up growth occurs between term gestation (40 weeks CA) and the second year of life, extremely preterm infants with BPD remain deficient in all growth parameters at 20 months. Despite the increase in weight attainment since the year 2000, 20% of infants in the BPD population remain subnormal in weight, 9% in length and 8% subnormal in head circumference at 20 months CA. Newer ventilation strategies in the delivery room may contribute to a decrease in BPD rates, but may not improve the growth of those who do develop BPD. The strengths of our study include the relatively large inborn patient population and high rates of follow-up. We examined the growth outcomes by gestational age rather than by birth weight, thus eliminating the confounding effects of the inclusion of small-gestational-age infants of >28 weeks gestation.24 The limitation of our study is the lack of detailed information on both neonatal and post-discharge nutrition, as well as the lack of information on other factors that may affect growth.
There have been very few studies specifically regarding the later growth of extremely preterm infants with BPD. Ehrenkranz et al. reported that between 1995 and 1999, infants with moderate BPD (oxygen dependence >28 days and a requirement of <30% oxygen at 36 weeks postmenstrual age), the rates of subnormal weight, length and head circumference (<10th percentile) at 18–22 months CA were 55, 38 and 27%, respectively. Among infants with severe BPD (oxygen dependence >28 days and requiring ⩾30% oxygen and/or positive pressure ventilation at 36 weeks postmenstrual age), the corresponding rates were 63, 46 and 39%, respectively.9 Huysman et al.25 compared the growth and body composition of infants with BPD to healthy term infants during the first year of life, and reported that the mean fat-free mass and total body fat was significantly lower in infants with BPD at both 6 weeks and 12 months CA.
In general, studies of the postnatal growth of extremely low-birth-weight (<1 kg) infants born in the 1990s report that the majority, even those without BPD, develop growth failure in the neonatal period. This often persists into early childhood and later.2, 5, 6, 26, 27, 28 Neonatal correlates of growth failure in very-low-birth-weight (<1.5 kg) and extremely low-birth-weight infants included necrotizing enterocolitis, late-onset sepsis, postnatal steroid therapy, neurodevelopmental disability, BPD and feeding problems.2, 29 BPD was the main factor associated with growth failure between 40 weeks and 4 months CA in a cohort of very-low-birth-weight infants examined by Sices et al.30 There are many specific reasons for the growth failure of infants with BPD. Neonatal complications such as repeated episodes of infection during prolonged neonatal hospitalization may be associated with poor neonatal nutrition. Chronic hypoxia may result from frequent episodes of oxygen desaturation both at rest and during feeds;31, 32, 33 increased energy expenditure associated with the work of breathing may also affect growth.34 Feeding problems have also been described in infants with BPD and include swallowing dysfunction, oral aversion and gastroesophageal reflux, which may contribute to poor nutrition and associated growth failure.2, 35, 36, 37, 38
In conclusion, despite the improvement in 20-month weight since 2000, our results indicate that growth failure remains a major problem among infants who develop BPD. To improve growth outcomes in such infants, there is a need for strategies that prevent the severity of BPD and decrease associated neonatal morbidities and later neurodevelopmental impairment. Furthermore, neonatal and post-discharge nutrition needs to be optimized together with early recognition and management of feeding problems.39, 40
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We specially thank Angelia Williams, Alpher Torres and Bonnie Siner, RN for their assistance. This study was supported by grants T35 training grant from the National Institutes of Health, M01 RR00080, General Clinical Research Center and partly by grant HD21364 from the NICHD Neonatal Network.
References
- Lemons JA, Bauer CR, Oh W, Korones SB, Papille LA, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: growth and associated problems in children born at 25 weeks of gestational age or less. Arch Dis Child Fetal Neonatal Ed. 2003;88:F492–F500. doi: 10.1136/fn.88.6.F492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KA, Reichman B, Lusky A, Zmora E. Fetal growth and growth failure in very-low-birthweight infants. Acta Pediatr. 2006;95:236–242. doi: 10.1111/j.1651-2227.2006.tb02213.x. [DOI] [PubMed] [Google Scholar]
- Radmacher P, Looney S, Rafail S, Adamkin D. Prediction of extrauterine growth retardation (EUGR) in VVLBW infants. J Perinatol. 2003;23:392–395. doi: 10.1038/sj.jp.7210947. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz R, Younes N, Lemons J, Fanaroff A, Donovan EF, Wright LL, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104:280–289. doi: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003;111:986–990. doi: 10.1542/peds.111.5.986. [DOI] [PubMed] [Google Scholar]
- Kobaly K, Schluchter M, Minich N, Friedman H, Taylor HG, Wilson-Costello D. Outcomes of extremely low birth weight (<1 kg) and extremely low gestational age (<28 weeks) infants with bronchopulmonary dysplasia: effects of practice changes in 2000–2003. Pediatrics. 2008;121:73–81. doi: 10.1542/peds.2007-1444. [DOI] [PubMed] [Google Scholar]
- Shennan A, Dunn M, Ohlsson A, Lennox K, Hoskins E. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527–532. [PubMed] [Google Scholar]
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AL, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- Poindexter B, Ehrenkranz R, Stoll B, Wright LL, Poole K, Oh W, et al. Parenteral glutamine supplementation does not reduce the risk of mortality or late-onset sepsis in extremely low birth weight infants. Pediatrics. 2004;113:1209–1215. doi: 10.1542/peds.113.5.1209. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L. Neonatal necrotizing enterocolitis. Therapeutic decisions based on clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- Amiel-Tison C, Stewart AL. Follow-up studies during the first five years of life: a pervasive assessment of neurologic function. Arch Dis Child. 1989;64:496–502. doi: 10.1136/adc.64.4_spec_no.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:e35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- Usher R, McLean F. Intrauterine growth of live-born Caucasian infants at sea-level: standards obtained from measurements in 7 dimensions of infants born between 25 and 44 weeks of gestation. J Pediatr. 1969;74:901–910. doi: 10.1016/s0022-3476(69)80224-6. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC Growth Charts: United States Advance Data From Vital and Health Statistics No 314. National Center for Health Statistics: Hyattsville, MD; 2000. [PubMed] [Google Scholar]
- Agresti A. Categorical Data Analysis. John Wiley and Sons: New York, NY; 1990. pp. 100–102. [Google Scholar]
- American Academy of Pediatrics Committee on Fetus Newborn Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330–338. doi: 10.1542/peds.109.2.330. [DOI] [PubMed] [Google Scholar]
- Gibson AT, Pearse RG, Wales JK. Growth retardation after dexamethasone administration: assessment by knemometry. Arch Dis Child. 1993;69:505–509. doi: 10.1136/adc.69.5_spec_no.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman L, Hack M, Watts C, Borawski-Clark E, Bayley J, Amini S, et al. Twenty-month outcome in ventilator-dependent, very low birth weight infants born during the early years of dexamethasone therapy. J Pediatr. 1995;126:434–440. doi: 10.1016/s0022-3476(95)70464-7. [DOI] [PubMed] [Google Scholar]
- Sterling HM. Height and weight of children with cerebral palsy and acquired brain damage. Arch Phys Med Rehab. 1960;41:131–135. [PubMed] [Google Scholar]
- Sanders KD, Cox K, Cannon R, Blanchard D, Pitche J, Papathakis P. Growth response to enteral feeding by children with cerebral palsy. J Parent Ent Nutr. 1990;14:23–26. doi: 10.1177/014860719001400123. [DOI] [PubMed] [Google Scholar]
- Shapiro B, Green P, Krick J, Allen D, Capute AJl. Growth of severely impaired children: neurological versus nutritional factors. Dev Med Child Neurol. 1986;28:729–733. doi: 10.1111/j.1469-8749.1986.tb03924.x. [DOI] [PubMed] [Google Scholar]
- Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RHl. Very low birth weight: a problematic cohort for epidemiological studies of very small or immature neonates. Am J Epidemiol. 1991;134:604–613. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
- Huysman WA, de Ridder M, de Bruin N, Van Helmond G , Terpstra N, Van Goudoever JP, et al. Growth and body composition in preterm infants with bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. 2003;88:F46–F51. doi: 10.1136/fn.88.1.F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up. Semin in Perinatol. 2003;27:302–310. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- Finnstrom O, Otterblad P, Sedin G, Serenius F, Svenningsen N, Thiringer K, et al. Neurosensory outcome and growth at three years in extremely low birthweight infants: follow-up results from the Swedish national prospective study. Acta Paediatr. 1998;87:1055–1060. doi: 10.1080/080352598750031374. [DOI] [PubMed] [Google Scholar]
- Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics. 2003;112:e30–e38. doi: 10.1542/peds.112.1.e30. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz R, Dusick A, Vohr B, Wright L, Wrage L, Poole K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- Sices L, Wilson-Costello D, Minich N, Friedman H, Hack M. Postdischarge growth failure among extremely low birth weight infants: correlates and consequences. Paediatr Child Health. 2007;12:22–28. [PMC free article] [PubMed] [Google Scholar]
- Singer L, Martin RJ, Hawkins SW, Benson-Szekely LJ, Yamashita TS, Carlo WA. Oxygen desaturation complicates feeding in infants with bronchopulmonary dysplasia after discharge. Pediatrics. 1992;90:380–384. [PMC free article] [PubMed] [Google Scholar]
- Moyer-Mileur L, Nielson D, Pfeffer K, Witte M, Chapman D. Eliminating sleep-associated hypoxia improves growth in infants with bronchopulmonary dysplasia. Pediatrics. 1996;98:779–783. [PubMed] [Google Scholar]
- Thoyre SM, Carlson J. Occurrence of oxygen desaturation events during preterm infant bottle feeding near discharge. Early Hum Dev. 2003;72:25–36. doi: 10.1016/s0378-3782(03)00008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott L, Beghin L, Devos P, Pierrat V, Matian R, Gottrand F. Nutritional status at 2 years in former infants with bronchopulmonary dysplasia influences nutrition and pulmonary outcomes during childhood. Pediatr Res. 2006;60:340–344. doi: 10.1203/01.pdr.0000232793.90186.ca. [DOI] [PubMed] [Google Scholar]
- Gewolb I, Bosma J, Taciak V, Vice F. Abnormal developmental patterns of suck and swallow rhythms during feeding in preterm infants with bronchopulmonary dysplasia. Dev Med Child Neurol. 2001;43:454–459. doi: 10.1017/s0012162201000834. [DOI] [PubMed] [Google Scholar]
- Singer LT, Davillier M, Preuss L, Szekely L, Hawkins S. Feeding interactions in infants with very low birth weight and bronchopulmonary dysplasia. J Dev Behav Pediatr. 1996;17:69–76. [PMC free article] [PubMed] [Google Scholar]
- Pridham KF, Martin R, Sondel S, Tlucek A. Parental issues in feeding young children with bronchopulmonary dysplasia. J Pediatr Nurs. 1989;4:177–185. [PubMed] [Google Scholar]
- Johnson D, Cheney C, Monsen E. Nutrition and feeding in infants with bronchopulmonary dysplasia after initial hospital discharge: risk factors for growth failure. J Am Diet Assoc. 1998;98:649–656. doi: 10.1016/S0002-8223(98)00149-7. [DOI] [PubMed] [Google Scholar]
- Martin M, Shaw NJ. Feeding problems in infants and young children with chronic lung disease. J Hum Nutr Diet. 1997;10:271–275. [Google Scholar]
- Biniwale MA, Ehrenkranz RA. The role of nutrition in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:200–208. doi: 10.1053/j.semperi.2006.05.007. [DOI] [PubMed] [Google Scholar]