Abstract
It has been shown that there are many Chinese traditional herbals that can enhance sexual activity. Chuanxiongzine is a vasoactive ingredient that has been isolated and purified from Ligusticum chuanxiong Hort. In previous studies, it has been found that chuanxiongzine was effective in relaxing rabbit corpus cavernosum smooth muscle. We determined the effects of chuanxiongzine on relaxation of isolated corpus cavernosum strips in vitro and on increase of intracavernous pressure (ICP) in vivo in rabbits. Chuanxiongzine caused a concentration-dependent relaxation of phenylephrine precontracted isolated corpus cavernosum strips (EC50 1.58 × 10−4 mol l−1), which were endothelium independent and NO independent. However, the guanylyl cyclase inhibitor 1-H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one significantly shifted the chuanxiongzine concentration–response relationship to the right. Although there was no significant difference in the level of cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP) in isolated corpus cavernosum strips treated with chuanxiongzine or vehicle, chuanxiongzine caused a significant rise in the level of cGMP and cAMP in isolated corpus cavernosum strips pretreated with the activator of adenylyl cyclase forskolin and the source of NO sodium nitroprusside. In an in vivo study, chuanxiongzine dose-dependently raised ICP after the intracavernous injection of its cumulative doses (0.5, 1, 2 and 5 mg kg−1). The ICP increased from baseline to 19.1±3.7, 24.8±2.1, 30.2±4.8 and 39.7±6.1 mm Hg, respectively, and the duration of tumescence ranged from 8.5±2.8 to 22.9±7.3 min. Our results show that chuanxiongzine can relax isolated corpus cavernosum strips of rabbits in vitro and increase ICP of rabbits in vivo, which is neither endothelium dependent nor NO dependent, but may be partly mediated by the inhibition of cAMP phosphodiesterase or cGMP phosphodiesterase.
Keywords: chuanxiongzine, ligustrazine, rabbit corpus cavernosum, cyclic adenosine monophosphate, cyclic guanosine monophosphate, intracavernous pressure
Introduction
Erectile dysfunction is a common disorder affecting millions of the aged men worldwide. Feldman et al.1 have reported that men aged 40–70 years suffered from ED of various degrees by approximately 50%. Current pharmacological treatment for ED includes the oral, intracavernosal and intraurethral administration of erectogenic drugs.2, 3, 4, 5, 6 It has been proved that penile erection depends highly on the relaxation of penile arteries and erectile tissue such as the corpus cavernosum smooth muscle.7, 8, 9, 10 Some drugs such as inhibitors of PDE 5 or other vasodilators, which can promote the relaxation of corpus cavernosum smooth muscle, can be potentially used to treat ED.11, 12, 13, 14, 15, 16, 17, 18, 19 It has been shown that there are many Chinese traditional herbals that can enhance sexual activity.20, 21, 22, 23, 24 Recently, there is an increasing interest in investigating their effective components.
Chuanxiongzine is a vasoactive ingredient that has been isolated and purified from Ligusticum chuanxiong Hort (Umbelliferae) and is behind an ancient Chinese herbal remedy. Owing to its vasodilatory actions, chuanxiongzine has been used for the treatment of a variety of vascular diseases such as pulmonary hypertension secondary to chronic obstructive pulmonary disease.25 In previous studies, it has been found that chuanxiongzine was effective in relaxing rabbit corpus cavernosum smooth muscle.26 However, the relaxant effect of chuanxiongzine on cavernosal strips and its mechanism have not been fully elucidated. This study aims to investigate if chuanxiongzine can relax isolated corpus cavernosum strips in vitro and increase intracavernous pressure (ICP) in vivo. We report here that chuanxiongzine may improve the relaxation of isolated corpus cavernosum strips and enhance ICP through an increase in the levels of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP).
Materials and methods
Cavernosal tissue strips preparation
All animal experiments were carried out with the approval of the Institute for Animal Care and Use Committee of Tongji Hospital (Wuhan, China). Twenty sexually mature male New Zealand white rabbits (3.2±0.3 kg) were anesthetized by subcutaneously injecting xylazine (5 mg kg−1) and ketamine (20 mg kg−1, i.m.) into the ear vein. Subsequently, the entire penis was surgically excised and cleaned by removing the corpus spongiosum and urethra. Then it was immediately immersed in an organ chamber containing fresh 4 °C Krebs solution and the corpus cavernosum tissue was carefully dissected away from the surrounding tunica.27, 28 Three to four cavernosal strips of approximately equal size (2 × 2 × 8 mm) were obtained from each penis and were prepared for organ bath studies separately. Each cavernosal strip was tied with silk in one organ chamber with one end fixed to a tissue holder and the other secured to a force transducer. The latter was connected to an appropriately calibrated four-channel polygraph (PowerLab; ADInstruments, Sydney, Australia) in which the transducer output was recorded. Cavernosal strips were maintained in the organ baths with Krebs solution at 37 °C by a thermoregulated water circuit and by continuously bubbling with a mixture of 95% O2 and 5% CO2 during the study. Each cavernosal strip was stretched to an optimal isometric tension of 2.0 g, which was previously found to be optimal for measurement of changes in the tension of rabbit corpus cavernosal preparation and was equilibrated for 2 h. During the equilibration period, the tissues were washed out with fresh Krebs solution every 15 min and tension was adjusted, if necessary.
Drugs and solutions
The following drugs were tested: chuanxiongzine hydrochloride was obtained from the National Institute for the Control of Pharmaceutical and Biological Products. NG-nitro-L-arginine methyl ester (L-NAME), 1-H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one (ODQ), forskolin and sodium nitroprusside were purchased from Sigma Chemical Company (St Louis, MO, USA). The composition of Krebs solution was as follows: (mmol l−1) NaCl 119, KCl 4.7, MgCl2 1.2, CaCl2 2.5, NaHCO3 15, NaH2PO4 1.2, glucose 11 (pH 7.4).
Organ bath studies in vitro
A 10−5 mol l−1 phenylephrine (PE) was added to each organ bath containing cavernosal strips at optimal isometric resting tension after they were equilibrated for 2 h. Subsequently, PE resulted in cavernosal strips contraction and rapidly reached a steady state of active tension. When a steady state of contraction was achieved, cumulative drug vehicle doses (control) were added to each organ bath and dose–responses were recorded on the polygraph. Although the strips showed no more changes of tension in response to vehicle, they were washed out three times. After washout and reequilibration for 1 h, the same PE concentration (10−5 mol l−1) was added to induce cavernosal strips contraction. Similarly, cumulative doses (10−8 to 10−4 mol l−1) of chuanxiongzine were added to each organ bath and dose–responses were recorded.
The role of endothelium and NO was determined in endothelium-denuded isolated corpus cavernosum strips following incubation of the NO synthase inhibitor L-NAME. The role of cAMP was investigated in the presence of the guanylyl cyclase inhibitor ODQ. In the denuded endothelium group, the corporal cavernosum strips were removed and tested for functionally deprived endothelium. If they reacted poorly (<10% of maximal relaxation) to acetylcholine (10−5 mol l−1) in the tissues with endothelium removed, they were considered to be successfully denuded of endothelium and were used for this study.29
Influence of the level of cAMP and cGMP by chuanxiongzine
The denuded endothelium cavernosal strips were suspended in organ baths and equilibrated for 2 h. Chuanxiongzine (10−4 mol l−1) and vehicle were added to organ baths without the precontraction by PE. In another group, a PE-induced contraction was obtained as described above, and chuanxiongzine (10−4 mol l−1) and vehicle were added. When they were in a steady state, the tissues were immediately frozen in liquid nitrogen for further study of cAMP and cGMP levels. The test was repeated with the isolated corpus cavernosum strips pretreated with the guanylyl cyclase activator forskolin (10−6 mol l−1) and the NO supplier sodium nitroprusside (10−6 mol l−1), respectively, to increase the level of cAMP and cGMP.
cAMP and cGMP levels in corpus cavernosum were measured by 125I radioimmunoassay (Isotope Unit, Shanghai University of Chinese Medicine) according to the manufacturer's protocol. Briefly, the frozen 50 mg cavernosal tissue was homogenized with a microhomogenizer in 2 ml of acetate acid buffer. After centrifugation, the supernatant was extracted with water-saturated diethyl ether, and aliquots of the aqueous phase were lyophilized to dryness at 60 °C bathwater. The 100 μl samples were mixed with 5 μl acetylate, 100 μl 125I, 100 μl antiserum and kept at 4 °C overnight. Subsequently, 100 μl rabbit serum and 100 μl goat anti-rabbit IgG, respectively, were added into the samples. They were then incubated fully at 4 °C overnight. On the following day, the samples were centrifuged at 3000 r.p.m. for 15 min. The supernatant was discarded, and then the γ− radioactive intensity was measured. Finally, the contents of cGMP and cAMP in the samples were obtained according to a designed standard curve.
In vivo studies
Twelve mature male New Zealand white rabbits weighing 3.2±0.3 kg were used for in vivo studies. They were anesthetized with intraperitoneal pentobarbital sodium (30 mg kg−1) and maintained (10 mg kg−1) as needed. Animals were secured in the supine position and the common carotid artery on one side was exposed through a midline neck incision and cannulated for continuous monitoring of systolic arterial pressure (SAP), mean blood pressure (MBp) and heart rate (HR) through an ML0380 pressure transducer on a PowerLab polygraph. A 25-gauge needle filled with heparinized saline was inserted into the corpus cavernosum for ICP measuring through the MLT844 pressure transducer. The needle was connected to a three-way stopcock to administrate by intracavernosal injection. All the tubes were filled with heparinized saline to prevent clotting. Increasing concentrations of chuanxiongzine (0.5–5 mg kg−1) were injected intracavernosally in seven rabbits with the highest volume of less than 0.15 ml and normal saline of the same volume was administrated in five rabbits as a control group. ICP, duration of tumescence (DT) and SAP were recorded after the intracavernosal administration of cumulative doses of chuanxiongzine and the same volume of normal saline. To minimize the effect of the previous drug, we washed the cavernous body with 0.15 ml normal saline before each injection and the interval between the two injections was at least 1 h.29, 30
Data analysis
All data were expressed as mean±s.e.m. (standard error of mean). The relaxatory response induced by cumulative concentration of chuanxiongzine was expressed as the percentage of inhibitions of the initial tension of isolated corpus cavernosum strips induced by PE. The erectile responses were normalized by calculating the ratio of ICP/SAP because ICP was ultimately limited by SAP in vivo.31 Statistical significance was evaluated by Student's t-test. Statistical significance between different groups was analyzed by analysis of variance by means of SPSS 12.0 software (SPSS Inc., Chicago, IL, USA). EC50 values as the concentrations at which 50% relaxation occurred were calculated by using nonlinear curve fitting software (PHARM/PCS version 4.2, Springer-Verlag, New York, NY, USA). Results were considered as significant at P<0.05.
Results
Relaxatory response of isolated cavernosal strips by chuanxiongzine in vitro
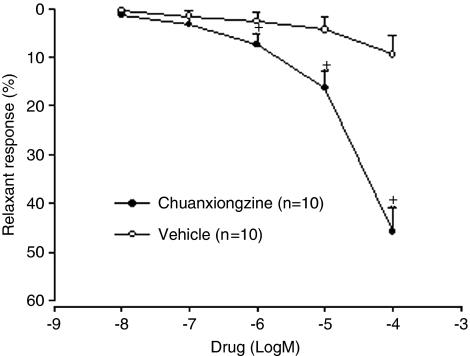
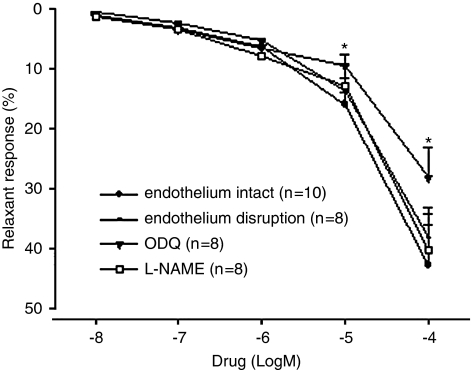
Chuanxiongzine caused a concentration-dependent relaxation of isolated rabbit cavernosal strips precontracted with PE (Figure 1, EC50 1.58 × 10−4 mol l−1, P<0.05 when compared with the vehicle). The presence of L-NAME or removal of endothelium had no significant effect on chuanxiongzine-induced relaxation in them, but the presence of ODQ caused a significant shift to the right of this relationship (Figure 2).
Figure 1.
Chuanxiongzine-induced relaxation in isolated corpus cavernosum strips precontracted with 10−5 mol l−1 phenylephrine. Symbols are mean±s.e.m. *P<0.05, when compared with that by the vehicle.
Figure 2.
Effects of L-NAME (NG-nitro-L-arginine methyl ester, 10−4 mol l−1), ODQ (1-H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one, 10−4 mol l−1) and removal of endothelium on chuanxiongzine-induced relaxation of isolated corpus cavernosum strips precontracted with phenylephrine (10−5 mol l−1). Symbols are mean±s.e.m. *P<0.05, when compared with L-NAME and removal of endothelium.
Influence of chuanxiongzine on the level of cAMP and cGMP
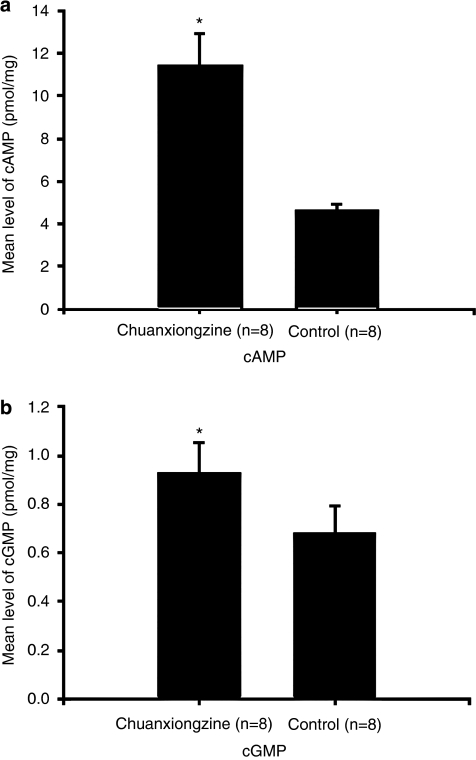
Without forskolin and sodium nitroprusside, the contents of cAMP and cGMP were measured in response to chuanxiongzine (10−4 mol l−1) or vehicle induced by PE or not. cAMP level exposed to only chuanxiongzine (10−4 mol l−1), vehicle, PE and chuanxiongzine, and PE and vehicle was 1.21±0.29, 1.17±0.14, 1.09±0.32 and 1.06±0.28 pmol mg−1 and the cGMP level exposed to them was 0.26±0.07, 0.23±0.09, 0.19±0.06 and 0.15±0.03 pmol mg−1, respectively. There were no significant differences on cAMP and cGMP in the groups above (P>0.05).
Conversely, in the presence of forskolin and sodium nitroprusside, chuanxiongzine increased the level of cAMP (11.47±1.48 pmol mg−1, P<0.05 when compared with the vehicle: 4.61±0.30 pmol mg−1; Figure 3a) and cGMP (0.93±0.12 pmol l−1, P<0.05 when compared with the vehicle: 0.68±0.11 pmol mg−1; Figure 3b).
Figure 3.
(a) Effects of chuanxiongzine on the levels of cyclic adenosine monophosphate (cAMP) in isolated corpus cavernosum strips precontracted with phenylephrine and pretreated with forskolin and sodium nitroprusside. *P<0.05, when compared with control. (b) Effects of chuanxiongzine on the levels of cyclic guanosine monophosphate (cGMP) in isolated corpus cavernosum strips precontracted with PE and pretreated with forskolin and sodium nitroprusside. *P<0.05, when compared with control.
Chuanxiongzine-induced rise of ICP in vivo
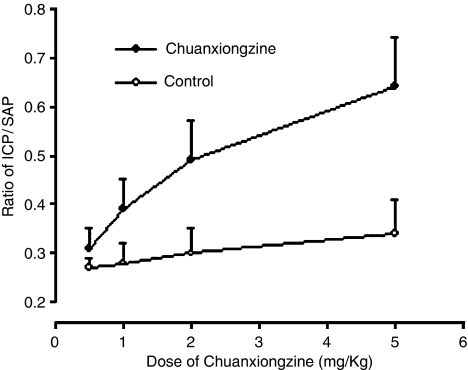
The response profile for chuanxiongzine in the organ bath studies formed the rationale for its further in vivo evaluation. In the rabbits, the recorded baseline ICP was 13.9±4.2 mm Hg and the mean SAP was 61.6±7.4 mm Hg. Intracavernosal injection of normal saline induced a transient rise in ICP in a volume-dependent manner. However, the rising ICP soon returned to the resting level within 1–2 min. In the chuanxiongzine group, the erectile responses were facilitated after cumulative doses were intracavernosally administered in seven rabbits. Chuanxiongzine increased ICP during erectile responses up to 6.5–25.8 mm Hg when compared with the group intracavernosal administration of normal saline. During the injection, the SAP, MBp and HR were unchanged. Intracavernosal administration of chuanxiongzine with cumulative doses (0.5, 1, 2 and 5 mg kg−1) caused a dose-dependent increase in the ICP. The ICP rose from the basal value to 19.1±3.7, 24.8±2.1, 30.2±4.8 and 39.7±6.1 mm Hg, respectively. DT ranged from 8.5±2.8 to 22.9±7.3 min (Figure 4). In three rabbits, a transient episode of slight decrease of SAP was recorded after the intracavernosal administration of high dose of chuanxiongzine (5 mg kg−1). However, there was no significant difference (P>0.05). At any doses of chuanxiongzine, its intracavernosal administration had no significant impact on SAP and HR in rabbits during the injections.
Figure 4.
Chuanxiongzine-induced increase of ICP in rabbits. Symbols are mean±s.e.m., ICP: intracavernous pressure, SAP: systolic arterial pressure.
Discussion
It has been proved that NO–cGMP axis and/or cAMP signal transduction pathway have an important role in mediating penile erection.7, 32, 33 Agents that increase levels of cGMP and/or cAMP could be expected to enhance relaxation of corpus cavernosal smooth muscle and thereby may be applicable for the treatment of ED. Phosphodiesterase inhibitor is one such classical drug that prevents the hydrolysis of cGMP and/or cAMP and then elevates the levels of these cyclic nucleotides. There are several Chinese traditional herbals that relax the corpus cavernosum to contribute to penile erectile activity through enhancing the intracellular cyclic nucleotides.29, 34, 35, 36, 37, 38 As a vasodilator, chuanxiongzine has been clinically used for the treatment of various vascular diseases such as pulmonary hypertension and coronary artery diseases in China.25, 39 It has been shown to exert relaxant effects on vascular smooth muscle because of multiple actions such as affecting cellular Ca2+ homeostasis as a nonspecific calcium antagonist, enhancing NO synthesis or inhibiting the activity of phosphodiesterase leading to the subsequent enhancement of cAMP concentration.40, 41, 42, 43 This study was determined to explore its mechanism underlying the relaxatory actions of chuanxiongzine on corpus cavernosal smooth muscle.
Our studies have shown that chuanxiongzine was effective in relaxing isolated rabbit cavernosal strips and depressing the response to PE in a concentration-dependent manner in vitro. In the endothelium-deprived group, the response of cavernosal strips to chuanxiongzine was not affected. Therefore, an endothelium-dependent mechanism was excluded. Chuanxiongzine has a potent relaxant activity on cavernosal strips, which was endothelium independent. L-NAME did not execute significant effects on chuanxiongzine-induced relaxation in them, but ODQ caused a significant shift to the right of this relationship. Thus, we speculated that the relaxant effect of chuanxiongzine on cavernosal strips might not be related to the enhancement of activation of NO synthase, guanylyl cyclase and adenylyl cyclase. It was inferred that its relaxant properties might be attributable to other pharmacological actions such as prevention of cyclic nucleotide degradation. This experiment showed that chuanxiongzine caused a significant rise in the levels of cGMP and cAMP in isolated corpus cavernosum strips pretreated with the activator of adenylyl cyclase forskolin and the source of NO sodium nitroprusside.
In an in vivo study, the cumulative doses of chuanxiongzine (0.5–5 mg kg−1) from low (0.5 mg kg−1) to high (5 mg kg−1) doses were intracavernosally injected. In anesthetized rabbits, erectile responses were significantly facilitated after cumulative doses (0.5, 1, 2 and 5 mg kg−1) of chuanxiongzine were administered and induced a dose-dependent rise in the ICP. The maximal ICP was raised up to 25.8±5.9 mm Hg and DT lasted 22.9±7.3 min when a high dose of chuanxiongzine (5 mg kg−1) was intracavernosally injected. It has been found that optimal dosages of chuanxiongzine were 1–2 mg kg−1, which resulted in the most efficacious rise of ICP without significant changes in SAP and HR. Although three of seven rabbits showed a transient episode of slight decrease in SAP simultaneously after the 5 mg kg−1 chuanxiongzine intracavernosal administration during the injections, the SAP soon returned to the normal level. These results indicated that even if a high dosage (5 mg kg−1) of chuanxiongzine was intracavernosally injected, it did not lead to any significant hypotension and any other systemic hemodynamic changes. The possible reason is that chuanxiongzine was absorbed into blood slowly after it was injected into the corpus cavernosum. It is therefore reasonable to assume that chuanxiongzine has the potential to be used as a drug for intracavernous injection therapy for ED that was not influenced by any systemic hemodynamic changes. Jian and Hao44 reported that the intracavernous injection of phentolamine, papaverine in combination with chuanxiongzine (40 mg) could persist longer in erection duration and reinforce harder than phentolamine and papaverine alone in the patients of ED. In China, chuanxiongzine has been clinically used to treat pulmonary hypertension with the usual dosage (1–2 mg kg−1) of intravenous administration. Zhang and Zhou45, Wang and Cheng46 have confirmed that chuanxiongzine could decrease the tension and pressure of the pulmonary artery through increasing cAMP and cGMP levels. The experimental dosage of chuanxiongzine used in this study is compatible with the usual clinical dosage used for pulmonary hypertension. In a word, the usual clinical dosage of chuanxiongzine resulted in a dose-dependent rise in ICP after intracavernosal administration without significant impact on systemic hemodynamic changes.
In conclusion, this study has shown that chuanxiongzine can relax isolated corpus cavernosum strips of rabbits in vitro and increase ICP of rabbits in vivo, which is neither endothelium dependent nor NO dependent, but may be partly mediated by the inhibition of cAMP phosphodiesterase or cGMP phosphodiesterase. Its relaxant properties were attributed to the increase in intracellular cyclic nucleotide levels, which were not due to the activation of NO synthesis, guanylyl cyclase or adenylyl cyclase, but to the prevention of cyclic nucleotide degradation. Further studies are needed to investigate whether chuanxiongzine induced relaxation by inhibiting some of those phosphodiesterases, which could increase cGMP and cAMP concentration.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank Professor Ji-Hong Liu for his excellent technical support. This study was supported by the National Natural Science Foundation of China (no. 30471736) and the Natural Science Foundation of Hubei Province (no. 2004ABA187).
References
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- Montorsi F, Salonia A, Deho' F, Cestari A, Guazzoni G, Rigatti P, et al. Pharmacological management of erectile dysfunction. BJU Int. 2003;91:446–454. doi: 10.1046/j.1464-410x.2003.04093.x. [DOI] [PubMed] [Google Scholar]
- Lue TF, Lee KL. Pharmacotherapy for erectile dysfunction. Chin Med J (Engl) 2000;113:291–298. [PubMed] [Google Scholar]
- Padma-Nathan H, Christ G, Adaikan G, Becher E, Brock G, Carrier S, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2004;1:128–140. doi: 10.1111/j.1743-6109.2004.04021.x. [DOI] [PubMed] [Google Scholar]
- Brant WO, Bella AJ, Lue TF. Treatment options for erectile dysfunction. Endocrinol Metab Clin North Am. 2007;36:465–479. doi: 10.1016/j.ecl.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Xiao HJ, Liu JH. Advance in pharmacotherapy for erectile dysfunction. J Med Postgrad. 2003;16:855–857. [Google Scholar]
- Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I, Angulo J, Cellek S, Gonzalez-Cadavid N, Heaton J, Pickard R, et al. Pathophysiology of erectile dysfunction. J Sex Med. 2005;2:26–39. doi: 10.1111/j.1743-6109.2005.20103.x. [DOI] [PubMed] [Google Scholar]
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379–395. doi: 10.1016/j.ucl.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wespes E. Smooth muscle pathology and erectile dysfunction. Int J Impot Res. 2002;14:S17–S21. doi: 10.1038/sj.ijir.3900792. [DOI] [PubMed] [Google Scholar]
- Stief CG, Uckert S, Becker AJ, Truss MC, Jonas U. The effect of the specific phosphodiesterase (PDE) inhibitors on human and rabbit cavernous tissue in vitro and in vivo. J Urol. 1998;159:1390–1393. [PubMed] [Google Scholar]
- Hallén K, Wiklund NP, Gustafsson LE. Inhibitors of phosphodiesterase 5 (PDE 5) inhibit the nerve-induced release of nitric oxide from the rabbit corpus cavernosum. Br J Pharmacol. 2007;150:353–360. doi: 10.1038/sj.bjp.0706991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LC, Adaikan PG. Mechanisms of direct relaxant effect of sildenafil, tadalafil and vardenafil on corpus cavernosum. Eur J Pharmacol. 2006;541:184–190. doi: 10.1016/j.ejphar.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Firoozi F, Longhurst PA, White MD. In vivo and in vitro response of corpus cavernosum to phosphodiesterase-5 inhibition in the hypercholesterolaemic rabbit. BJU Int. 2005;96:164–168. doi: 10.1111/j.1464-410X.2005.05588.x. [DOI] [PubMed] [Google Scholar]
- Ghalayini IF. Nitric oxide–cyclic GMP pathway with some emphasis on cavernosal contractility. Int J Impot Res. 2004;16:459–469. doi: 10.1038/sj.ijir.3901256. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I, Angulo J, Cuevas P, Fernández A, Moncada I, Allona A, et al. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Int J Impot Res. 2001;13:282–290. doi: 10.1038/sj.ijir.3900726. [DOI] [PubMed] [Google Scholar]
- Oger S, Behr-Roussel D, Gorny D, Charles Tremeaux J, Combes M, Alexandre L, et al. Combination of alfuzosin and tadalafil exerts in vitro an additive relaxant effect on human corpus cavernosum. J Sex Med. 2008;5:935–945. doi: 10.1111/j.1743-6109.2007.00754.x. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Bernabe J, Alexandre L, Niewoehner U, Haning H, Bischoff E. Pro-erectile effect of vardenafil: in vitro experiments in rabbits and in vivo comparison with sildenafil in rats. Eur Urol. 2003;44:731–736. doi: 10.1016/s0302-2838(03)00377-4. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Nakane M, Hsieh GC, Moreland RB, Kolasa T, Sullivan JP. Activators of soluble guanylate cyclase for the treatment of male erectile dysfunction. Int J Impot Res. 2002;14:8–14. doi: 10.1038/sj.ijir.3900801. [DOI] [PubMed] [Google Scholar]
- Chiou WF, Huang YL, Chen CF, Chen CC. Vasorelaxing effect of coumarins from Cnidium monnieri on rabbit corpus cavernosum. Planta Med. 2001;67:282–284. doi: 10.1055/s-2001-12013. [DOI] [PubMed] [Google Scholar]
- Chen J, Liu JH. Advances in studies of Chinese herbs for improving relaxability of corporal smooth muscle. Zhonghua Nan Ke Xue. 2003;9:615–618. [PubMed] [Google Scholar]
- Liu WJ, Xin ZC, Xin H, Yuan YM, Tian L, Guo YL. Effects of icariin on erectile function and expression of nitric oxide synthase isoforms in castrated rats. Asian J Androl. 2005;7:381–388. doi: 10.1111/j.1745-7262.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- Choi YD, Xin ZC, Choi HK. Effect of Korean red ginseng on the rabbit corpus cavernosal smooth muscle. Int J Impot Res. 1998;10:37–43. doi: 10.1038/sj.ijir.3900300. [DOI] [PubMed] [Google Scholar]
- Park SW, Lee CH, Shin DH, Bang NS, Lee SM. Effect of SA1, a herbal formulation, on sexual behavior and penile erection. Biol Pharm Bull. 2006;29:1383–1386. doi: 10.1248/bpb.29.1383. [DOI] [PubMed] [Google Scholar]
- Peng W, Duan SF. The acute effects of ligustrazini on hemodynamics and right cardiac function in pulmonary arterial hypertension secondary to chronic obstructive pulmonary disease. J Tongji Med Univ. 1989;9:100–102. doi: 10.1007/BF02908935. [DOI] [PubMed] [Google Scholar]
- Xiao H, Liu J, Yin C, Wang T, Chen J, Fan L, et al. Effects of ligustrazine on the contraction of isolated rabbit corpus cavernosum strips. J Huazhong Univ Sci Technolog Med Sci. 2005;25:565–567. doi: 10.1007/BF02896019. [DOI] [PubMed] [Google Scholar]
- Utkan T, Yildirim MK, Yildirim S, Sarioglu Y. Effects of the specific phosphodiesterase inhibitors on alloxan-induced diabetic rabbit cavernous tissue in vitro. Int J Impot Res. 2001;13:24–30. doi: 10.1038/sj.ijir.3900626. [DOI] [PubMed] [Google Scholar]
- Campos AR, Cunha KM, Santos FA, Silveira ER, Uchoa DE, Nascimento NR, et al. Relaxant effects of an alkaloid-rich fraction from Aspidosperma ulei root bark on isolated rabbit corpus cavernosum. Int J Impot Res. 2008;20:255–263. doi: 10.1038/sj.ijir.3901624. [DOI] [PubMed] [Google Scholar]
- Chiou WF, Chen J, Chen CF. Relaxation of corpus cavernosum and raised intracavernous pressure by berberine in rabbit. Br J Pharmacol. 1998;125:1677–1684. doi: 10.1038/sj.bjp.0702249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff E. Rabbits as models for impotence research. Int J Impot Res. 2001;13:146–148. doi: 10.1038/sj.ijir.3900681. [DOI] [PubMed] [Google Scholar]
- Brugger N, Kim NN, Araldi GL, Traish AM, Palmer SS. Pharmacological and functional characterization of novel EP and DP receptor agonists: DP1 receptor mediates penile erection in multiple species. J Sex Med. 2008;5:344–356. doi: 10.1111/j.1743-6109.2007.00676.x. [DOI] [PubMed] [Google Scholar]
- Lin CS, Lin G, Lue TF. Cyclic nucleotide signaling in cavernous smooth muscle. J Sex Med. 2005;2:478–491. doi: 10.1111/j.1743-6109.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- Waldkirch E, Uckert S, Yildirim H, Sohn M, Jonas U, Stief CG, et al. Cyclic AMP-specific and cyclic GMP-specific phosphodiesterase isoenzymes in human cavernous arteries immunohistochemical distribution and functional significance. World J Urol. 2005;23:405–410. doi: 10.1007/s00345-005-0026-2. [DOI] [PubMed] [Google Scholar]
- Tian L, Xin ZC, Yuan YM, Fu J, Liu WJ, Wang LL. Effects of icariin on intracavernosal pressure and systematic arterial blood pressure of rat. Zhonghua Yi Xue Za Zhi. 2004;84:142–145. [PubMed] [Google Scholar]
- Chen J, Chiou WF, Chen CC, Chen CF. Effect of the plant-extract osthole on the relaxation of rabbit corpus cavernosum tissue in vitro. J Urol. 2000;163:1975–1980. [PubMed] [Google Scholar]
- Chiou WF, Chen CF. Pharmacological profile of evodiamine in isolated rabbit corpus cavernosum. Eur J Pharmacol. 2002;446:151–159. doi: 10.1016/s0014-2999(02)01762-4. [DOI] [PubMed] [Google Scholar]
- Chen J, Liu JH, Wang T, Xiao HJ, Yin CP, Yang J. Effects of plant extract neferine on cyclic adenosine monophosphate and cyclic guanosine monophosphate levels in rabbit corpus cavernosum in vitro. Asian J Androl. 2008;10:307–312. doi: 10.1111/j.1745-7262.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- Chiu JH, Chen KK, Chien TM, Chiou WF, Chen CC, Wang JY, et al. Epimedium brevicornum Maxim extract relaxes rabbit corpus cavernosum through multitargets on nitric oxide/cyclic guanosine monophosphate signaling pathway. Int J Impot Res. 2006;8:35–42. doi: 10.1038/sj.ijir.3901437. [DOI] [PubMed] [Google Scholar]
- Zhang F. Clinical analysis of ligustrazine hydrochloride for the treatment of coronary heart disease. J Chin Med Res. 2003;3:546. [Google Scholar]
- Kwan CY, Daniel EE, Chen MC. Inhibition of vasoconstriction by tetramethylpyrazine: does it act by blocking the voltage-dependent Ca2+ channel. J Cardiovasc Pharmacol. 1990;15:157–162. [PubMed] [Google Scholar]
- Pang PK, Shan JJ, Chiu KW. Tetramethylpyrazine, a calcium antagonist. Planta Med. 1996;62:431–435. doi: 10.1055/s-2006-957933. [DOI] [PubMed] [Google Scholar]
- Lin CI, Wu SL, Tao PL, Chen HM, Wei J. The role of cyclic AMP and phosphodiesterase activity in the mechanism of action of tetramethylpyrazine on human and dog cardiac and dog coronary arterial tissues. J Pharm Pharmacol. 1993;45:963–966. doi: 10.1111/j.2042-7158.1993.tb05636.x. [DOI] [PubMed] [Google Scholar]
- Peng W, Hucks D, Priest RM, Kan YM, Ward JP. Ligustrazine-induced endothelium-dependent relaxation in pulmonary arteries via an NO-mediated and exogenous L-arginine-dependent mechanism. Br J Pharmacol. 1996;119:1063–1071. doi: 10.1111/j.1476-5381.1996.tb15778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian HY, Hao JC. Comparing effect in the management of erectile dysfunction by using 2 sets of drugs of intracavernosal injection. Zhonghua Nan Ke Xue. 2003;9:200–201. [PubMed] [Google Scholar]
- Zhang F, Zhou RY. A study on the role of tetramethylpyrazine on pulmonary hypertension secondary to congenital heart disease. Acta Universitatis Medicinalis Anhui. 2005;40:241–244. [Google Scholar]
- Wang DA, Cheng SB. Effect of ligustrazine on pulmonary hypertension secondary to pulmonary heart disease. Chin J Gerontol. 2006;26:1428–1430. [Google Scholar]