Abstract
We studied seven genes that reflect events relevant to antidepressant action at four sequential levels: (1) entry into the brain, (2) binding to monoaminergic transporters, and (3) distal effects at the transcription level, resulting in (4) changes in neurotrophin and neuropeptide receptors. Those genes are ATP-binding cassette subfamily B member 1 (ABCB1), the noradrenaline, dopamine, and serotonin transporters (SLC6A2, SLC6A3 and SLC6A4), cyclic AMP-responsive element binding protein 1 (CREB1), corticotropin-releasing hormone receptor 1 (CRHR1) and neurotrophic tyrosine kinase type 2 receptor (NTRK2). Sequence variability for those genes was obtained in exonic and flanking regions. A total of 56 280 000 bp across were sequenced in 536 unrelated Mexican Americans from Los Angeles (264 controls and 272 major depressive disorder (MDD)). We detected in those individuals 419 single nucleotide polymorphisms (SNPs); the nucleotide diversity was 0.00054±0.0001. Of those, a total of 204 novel SNPs were identified, corresponding to 49% of all previously reported SNPs in those genes: 72 were in untranslated regions, 19 were in coding sequences of which 7 were non-synonymous, 86 were intronic and 27 were in upstream/downstream regions. Several SNPs or haplotypes in ABCB1, SLC6A2, SLC6A3, SLC6A4, CREB1 and NTRK2 were associated with MDD, and in ABCB1, SLC6A2 and NTRK2 with antidepressant response. After controlling for age, gender and baseline 21-item Hamilton Depression Rating Scale (HAM-D21) score, as well as correcting for multiple testing, the relative reduction of HAM-D21 score remained significantly associated with two NTRK2-coding SNPs (rs2289657 and rs56142442) and the haplotype CAG at rs2289658 (splice site), rs2289657 and rs2289656. Further studies in larger independent samples will be needed to confirm these associations. Our data indicate that extensive assessment of sequence variability may contribute to increase understanding of disease susceptibility and drug response. Moreover, these results highlight the importance of direct re-sequencing of key candidate genes in ethnic minority groups in order to discover novel genetic variants that cannot be simply inferred from existing databases.
Keywords: nucleotide polymorphism, antidepressant response, desipramine, fluoxetine, major depression
Introduction
Major depressive disorder (MDD) is a common, complex and recurrent disorder of gene–environment interactions. The estimated heritability may range from 0.36 to 0.66.1, 2 Following up on previous study on the pathophysiology of MDD and on the prevailing hypotheses for treatment response, we sought to identify genes that influence susceptibility for MDD or treatment response in the central nervous system pathways relevant to stress reactivity and to the pathways of action of antidepressant drugs. Current data point out to roles for genes involved in drug transport, serotonin neurotransmission, neurotrophin signaling and response to stress. Promising linkage results are located in several chromosomes,3 which highlight the multilocus nature of the genetic vulnerability to MDD.
Recently, rapid technological advances have started unraveling the contributions of common (frequency >1%) and rare genetic variants in complex disorders. In a topical review, Bodmer and Bonilla4 have synthesized current views, implications and integration of the competing hypotheses of common disease–common variant and common disease–rare variant. For most common variants, the disease-associated variant is unlikely to be functionally relevant; it may be closely linked to the functional variant, and it will cause a small increase in disease risk (odds ratio smaller than 2, generally between 1.1 and 1.4). In contrast, rare variants generally have functional and large phenotypic effects; in many cases they are missense variants that reflect amino-acid changes relevant to protein–protein interactions. Diverse scenarios may occur in the pathophysiology of common complex disorders: Common variants may be modifiers of genes with rare variant effects, such as recently described for the MC4R gene.5 Moreover, areas near common variants may contain candidate genes in which there are rare variants. The identification of rare variants may significantly affect our understanding of complex disease etiology.
We re-sequenced seven candidate genes of importance in the pathophysiology of MDD.6 Conceptually, we sought a group of genes that reflects a sequence of events relevant to drug action at four levels: (1) entry into the brain, (2) binding to monoaminergic transporters, and (3) distal effects at the transcription level, resulting in (4) changes in neurotrophin and neuropeptide receptors. Specifically, we studied a blood–brain barrier drug transporter pump (ACCB1, also called MDR1), which regulates drug entry into the brain (level 1), the norepinephrine, dopamine, and serotonin transporters (SCL6A2, SLC6A3 and SL6A4) (level 2), an antidepressant-regulated transcription factor (cyclic AMP-responsive element binding protein 1 (CREB1)) (level 3) and two receptors (level 4): neurotrophic tyrosine kinase type 2 receptor (NTRK2), important in synaptic function and neural plasticity, and corticotropin-releasing hormone receptor 1 (CRHR1), which regulates the response to stress at the behavioral, neuroimmune and neuroendocrine—hypothalamic–pituitary–adrenalaxis levels.
Materials and methods
Patients and controls
The study consisted of 272 patients (66% female, 34% male; mean age: 38±10) with MDD and 264 healthy control individuals (60% female, 40% male; average age: 36±11). MDD was defined as a DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) diagnosis of current, unipolar major depressive episode and a 21-item Hamilton Depression Rating Scale (HAM-D21) score of ⩾18 with item number 1 (depressed mood) rated ⩾2. All MDD patients were screened for the pharmacogenetic study of antidepressant treatment response as previously described.7 All MDD patients had comprehensive psychiatric and medical assessments in their primary language, on the basis of diagnostic and ratings instruments that had been fully validated in English and in Spanish. Exclusion criteria included active medical illnesses that could be etiologically related to the ongoing depressive episode, current or active suicidal ideation with a plan and strong intent, pregnancy, lactation, current use of medications with significant central nervous system activity, which interfere with electroencephalogram (EEG) activity (for example, benzodiazepines) or any other antidepressant treatment within the 2 weeks before enrollment, illicit drug use and/or alcohol abuse in the last 3 months or current enrollment in psychotherapy. All MDD patients were Mexican-Americans and had at least three grandparents born in Mexico.
All patients had an initial comprehensive psychiatric and medical assessment and, if enrolled in the pharmacogenetic study of antidepressant treatment response, had weekly structured follow-up assessments for 9 weeks. The study consisted of two phases: a 1-week single-blind placebo lead-in phase to minimize the impact of placebo responders followed, if subjects continued to meet the inclusion criteria after phase 1, by random assignment to one of the two treatment groups: fluoxetine 10–40 mg per day or desipramine 50–200 mg per day, administered in a double-blind manner for 8 weeks. Our primary clinical outcome measure was HAM-D21 score and clinical remission on antidepressants was defined as having a final (week 8) HAM-D21 score <8. In addition, the relative response change was also computed as the difference in HAM-D21 score between pre- and post-treatment divided by the pretreatment HAM-D21 score.
Age-, gender- and ethnicity-matched healthy control individuals were recruited from the same Mexican-American community in Los Angeles by the same bilingual clinical research team. Controls for our genomic studies were in general good health but were not screened for medical or psychiatric illness.
Genomic DNA collection, amplification and sequencing
At the initial visit, after informed consent was obtained from the participating individuals, blood samples were collected into EDTA (K2EDTA) BD Vacutainer EDTA tubes (Becton Dickinson, Franklin Lakes, NJ, USA), and genomic DNA was isolated by using Gentra Puregene DNA purification kits (Gentra Systems, Indianapolis, IN, USA). DNA sequencing for seven genes was carried out in collaboration with the Sanger Institute by following ExoSeq protocol (http://www.sanger.ac.uk/humgen/exoseq/). Briefly, the known protein-coding regions, novel coding sequences and transcripts, exons and their flanking sequence were extracted from the Vega database (http://vega.sanger.ac.uk/index.html). Primers were designed automatically using Primer3 (http://frodo.wi.mit.edu/) to amplify DNA and primer pairs were checked for uniqueness before ordering and pre-screened to determine the optimum conditions for amplification. After amplification, a sample of the products were visualized on an agarose gel to confirm the size of the PCR product. The remaining PCR product was then cleaned up using two enzymes, Exonuclease 1 and Shrimp Alkaline Phosphatase. Bidirectional sequencing of amplicons was carried out using Big DyeTM chemistry (Big Dye Terminator, Version 3.1; Applied Biosystems, Foster City, CA, USA). Single nucleotide polymorphisms (SNPs) were called using ExoTrace http://www.sanger.ac.uk/humgen/exoseq/analysis.shtml, a novel algorithm developed in-house for the detection of heterozygotes in sequence traces , which processes the sense and antisense sequence reads separately and subsequently, and combines the results to allow SNP scoring. All polymorphisms reported here had a genotyping rate of ⩾80% and an average nucleotide call rate of 93%.
Genomic control genotyping
To detect potential bias due to population stratification, two approaches were used to test for hidden stratification in our data. First, 54 independent SNPs across 22 autosomal chromosomes were selected to analyze a combined sample using the genotype data download from three HapMap ethnic samples using STRUCTURE program (http://pritch.bsd.uchicago.edu/software.html)8, 9 and showed that three distinct clusters were well identified with an average proportion of at least 92% of individuals correctly assigned to the given ethnic populations (CEU, CHB+JPT, YRI). This panel of SNPs were then used as genomic control to test our sample and showed an almost equal proportion assigned to each clusters, given K=2, 3, 4 in both cases and controls. Second, genotype frequencies from each of the 54 unlinked SNPs were also compared between cases and controls using the method described by Pritchard and Rosenberg 10 and no significant difference was found based on an overall test statistic (χ2=100.50, d.f.=108, P=0.68). Therefore, no population stratification adjustment was necessary for our association analyses.
Nucleotide diversity, population differentiation and Hardy–Weinberg equilibrium
Nucleotide diversity (θ) and its standard deviation (S(θ)) were calculated under the assumption of an infinite neutral allele model,11, 12 and all calculations were based on n=946 for all the sites given that the average sample size was 473 individuals across all the polymorphisms. Population differentiation estimation was based on the pairwise FST values for the dbSNPs (single nucleotide polymorphism database hosted at the National Center for Biotechnology Information), which were both detected in our Mexican-American sample and reported in HapMap sample. FST values were calculated as described byWeir,13 Weir and Cockerham,14 and Weir and Hill.15 In order to compare allele frequencies and to be able to treat chromosomes as independent observations, the genotype frequencies must be in Hardy–Weinberg equilibrium (HWE).16 Exact testing of HWE was performed separately for healthy controls and MDD patients using the PLINK program Version1.00 (http://pngu.mgh.harvard.edu/~purcell/plink/).17 SNPs that were not in HWE in the healthy control group were excluded from the allele-based association analyses of cases and controls.
Statistical analysis
Data preparation and descriptive statistics were carried out with SAS software (SAS Version 9.1.3, SAS Institute, Cary, NC, USA). For SNP-based association analyses of case vs control or remitter vs non-remitter, Fisher's exact test (two-tailed) was performed to compare allele and genotype distributions between depressed and healthy individuals using PLINK. In the allelic association analysis, each polymorphism was tested in controls to ensure the fitting with HWE; the odds ratio on the 2 × 2 contingency table of allele counts and its 95% confidence interval were estimated using Woolf's method or fitting exact logistic regression model with SAS software when the frequency in a table cell is zero.18 In genotypic association analysis, SNP effects were tested under a codominant model on the 2 × 3 contingency table of genotype counts.
For the quantitative outcome (relative reduction % in HAM-D21 scores between pre- and post-treatment), the analyses based on dominant model were performed, separately, for the joint sample of patients treated with desipramine or fluoxetine and for medication-specific sample. General linear regression models were used to examine the association between genotype and relative HAM-D21 score reduction by controlling for age, gender and baseline (pretreatment) HAM-D21 score using the PLINK program. The Benjamini and Hochberg method was used to control for false discovery rate and the significance threshold was set at FDR_BH⩽0.0519.
For haplotype-based association analysis, Haploview (Version 4.1, Broad Institute of MIT and Harvard, http://www.broad.mit.edu/mpg/haploview/), was first used to identify the haplotype blocks by applying the Four Gamete Rule20 based on the SNPs with a minor allele frequency (MAF) ⩾0.01 in the combined sample of cases and controls and HWE exact test P>0.01 in controls. The PLINK program was then used to examine the association of specific haplotype with depression diagnosis, clinical remission, as well as quantitative outcome of antidepressant treatment.
Results
Identification of sequence variations
A total of 419 single nucleotide sequence variants (Table 1) were identified by re-sequencing of ∼105 kb of exonic sequence and their flanking regions in the selected seven genes in an ethnically homogeneous sample of 264 healthy controls and 272 MDD patients. Among the 419 SNPs, 204 (49%) are novel polymorphisms, not previously described, including 86 in introns, 72 in untranslated regions (UTRs), 19 (12 synonymous) in coding regions, 18 in upstream and 9 in downstream regions. Overall, 95% of the novel polymorphisms had a MAF lower than 5%, whereas the corresponding proportion was 57% for dbSNPs (Supplementary Table 1). Similar distribution of MAFs was seen between cases and controls for both SNPs in intronic and in exonic regions (Figure 1a). Among the 419 SNPs, the proportion of SNPs with HWE exact test P-value ⩾0.05 was 92% for controls and 91% for MDD cases (Supplementary Table 1).
Table 1.
Single nucleotide polymorphisms (SNPs) detected in seven candidate genes for depression in Mexican-Americans
| SNP type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene (Location) | SNPa | Downstream | 3′ UTR | Intronic | SYN | NS | 5′ UTR | Upstream | All | Sequence screened (kb) | Nucleotide diversity (s.d.) × 10−4 |
| CREB1 (2q34) | New | 2 | 5 | 5 | 0 | 0 | 0 | 0 | 12 | 7.5 | 3.2 (0.9) |
| dbSNP | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 6 | |||
| Total | 3 | 7 | 7 | 1 | 0 | 0 | 0 | 18 | |||
| SLC6A3 (5p15.3) | New | 0 | 2 | 6 | 2 | 1 | 0 | 7 | 18 | 12.3 | 5.3 (1.2) |
| dbSNP | 0 | 7 | 10 | 5 | 1 | 0 | 7 | 30 | |||
| Total | 0 | 9 | 16 | 7 | 2 | 0 | 14 | 48 | |||
| ABCB1 (7q21.1) | New | 0 | 0 | 20 | 1 | 3 | 4 | 0 | 28 | 28.5 | 3.8 (0.8) |
| dbSNP | 0 | 4 | 37 | 2 | 5 | 4 | 1 | 53 | |||
| Total | 0 | 4 | 57 | 3 | 8 | 8 | 1 | 81 | |||
| NTRK2 (9q22.1) | New | 0 | 43 | 10 | 2 | 2 | 0 | 0 | 57 | 23.6 | 5.1 (1.0) |
| dbSNP | 0 | 24 | 6 | 2 | 0 | 0 | 0 | 32 | |||
| Total | 0 | 67 | 16 | 4 | 2 | 0 | 0 | 89 | |||
| SLC6A2 (16q12.2) | New | 0 | 4 | 10 | 2 | 1 | 1 | 11 | 29 | 13.3 | 5.6 (1.2) |
| dbSNP | 0 | 4 | 9 | 2 | 2 | 0 | 9 | 26 | |||
| Total | 0 | 8 | 19 | 4 | 3 | 1 | 20 | 55 | |||
| SLC6A4 (17q11.1-q12) | New | 5 | 4 | 23 | 0 | 4 | 1 | 0 | 37 | 12.5 | 7.8 (1.6) |
| dbSNP | 4 | 1 | 23 | 4 | 1 | 2 | 0 | 35 | |||
| Total | 9 | 5 | 46 | 4 | 5 | 3 | 0 | 72 | |||
| CRHR1 (17q12-q22) | New | 2 | 8 | 12 | 0 | 1 | 0 | 0 | 23 | 6.9 | 10.9 (2.4) |
| dbSNP | 1 | 12 | 16 | 3 | 1 | 0 | 0 | 33 | |||
| Total | 3 | 20 | 28 | 3 | 2 | 0 | 0 | 56 | |||
| All seven genes | New | 9 | 66 | 86 | 7 | 12 | 6 | 18 | 204 | 104.5 | 5.4 (1.0) |
| dbSNP | 6 | 54 | 103 | 19 | 10 | 6 | 17 | 215 | |||
| Total | 15 | 120 | 189 | 26 | 22 | 12 | 35 | 419 | |||
Abbreviations: ABCB1, ATP-binding cassette subfamily B member 1; CREB1, cyclic AMP-responsive element binding protein 1; CRHR1, corticotropin-releasing hormone receptor 1; NS, non-synonymous; SYN, synonymous; NTRK2, neurotrophic tyrosine kinase type 2 receptor; UTR, untranslated region.
New: not reported in NCBI dbSNP database as of 30 June 2008.
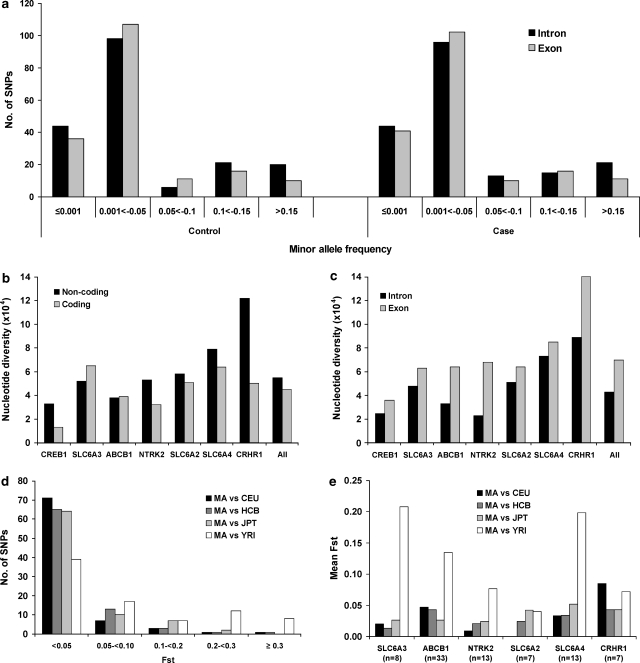
Figure 1.
Minor allele frequency (MAF), nucleotide diversity and FST measure in seven candidate genes in Mexican-American major depressive disorder (MDD) patients and controls. Histograms show the total number of single nucleotide polymorphisms (SNPs) detected in intronic (black bar) and exonic (gray bar) regions in the seven genes by MAF in 272 MDD patients and 264 healthy controls (a); the nucleotide diversity in noncoding (black bar) and coding (gray bar) (b) or intronic (black bar) and exonic (gray bar) regions (c) by gene in the combined sample of 272 MDD patients and 264 healthy controls; the total number of SNPs shared by Mexican-American (MA) sample and HapMap samples by pairwise FST value (d) or represent the average FST by gene (e) in MA vs CEU (black bar), MA vs HCB (dark gray bar), MA vs JPT (gray bar) and MA vs YRI (White bar).
Nucleotide diversity was estimated for each gene by correcting for both sample size and length of the screened site (Table 1). Nucleotide diversities were comparable in SLC6A3 (0.00053±0.00012), NTRK2 (0.00051±0.0001) and SLC6A2 (0.00056±0.00012), but were lower in CREB1 (0.00032±0.00009) and ATP-binding cassette subfamily B member 1 (ABCB1) (0.00038±0.00008) and appeared higher in SLC6A4 (0.00078±0.00016) and CRHR1 (0.00109±0.00024). This led to an overall nucleotide diversity of 0.00054 for all the seven genes investigated. When the nucleotide diversity was estimated separately for coding and noncoding sequence, five out of seven genes (except for SLC6A3 and ABCB1) showed higher nucleotide diversity in noncoding regions when compared with coding segments (Figure 1b). However, when the nucleotide diversity was estimated separately for exonic and intronic sequence, all the seven genes showed higher nucleotide diversity in exonic regions than in intronic segments (Figure 1c). This is because of the high nucleotide diversity in untranslated regions (0.00088±0.00017).
Among the 215 dbSNPs detected, 83 were reported in all four HapMap ethnic groups: CEU (Caucasian), YRI (African), CHB (Han Chinese) and JPT (Japanese) in the NCBI database as of 25 June 2008. Pairwise FST values between Mexican Americans (MA) and each HapMap ethnic sample were computed for the shared 83 dbSNPs. Overall, the greatest difference in allele frequencies was found between Mexican Americans and Africans with a highest mean FST of 0.126, compared with mean FST of 0.035 in MA vs CEU, 0.033 in MA vs CHB and 0.032 in MA vs JPT (Figure 1d). For the gene-specific mean FST in MA vs YRI, larger mean FST values were observed for SLC6A3 (0.208) and SLC6A4 (0.198), but much lower for SLC6A2 (0.04) (Figure 1e).
SNP-based genetic association analyses of cases and controls
Single nucleotide polymorphism-based allelic and genotypic association analyses revealed that 16 polymorphisms were associated with MDD with a nominal P<0.05 in five genes (Table 2), including two common 3′ UTR polymorphisms in NTRK2 (rs7020204 and rs2013566) and one rare 5′ UTR polymorphism in SLC6A4 (rs28914831). Among the nine SNPs with a nominal P<0.05 in both allelic and genotypic tests, seven were uncommon polymorphisms with a MAF <0.03 in controls, including one in CREB1 (rs3732076), two in ABCB1 (rs4728697, rs58898486) and four in SLC6A4 (rs7212502, rs28914831, NT_010799.14_3288789 and rs56355214) (Table2 and Supplementary Table 1). Three SLC6A4 common polymorphisms (rs7224199 and rs3813034 in upstream and rs140701) showed genotypic association, but with a small allelic odds ratio <1.3 and allelic test nominal P>0.05. No associated SNPs remained significant after adjusting for multiple tests with an FDR_BH ⩽0.05.
Table 2.
Polymorphisms associated with depression in Mexican-Americans
| Risk allele frequency | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SNP | Chromosome | Position | SNP type | Risk/non- risk allele | Case | Control | ORa (95% CI) | Allelic | Genotypic |
| CREB1 | rs3730276 | 2 | 208140591 | INT | A/G | 0.998 | 0.977 | 11.45 (1.46–89.77) | 0.004 | 0.003 |
| SLC6A3 | rs8179029 | 5 | 1462985 | INT | C/T | 0.910 | 0.856 | 1.71 (1.12–2.58) | 0.02 | 0.02 |
| rs2550936 | 5 | 1464256 | INT | A/C | 0.839 | 0.799 | 1.31 (0.94–1.83) | 0.13 | 0.004 | |
| ABCB1 | rs4728697 | 7 | 86986874 | INT | A/G | 0.060 | 0.028 | 2.24 (1.18–4.28) | 0.01 | 0.02 |
| rs2032583 | 7 | 86998497 | INT | A/G | 0.936 | 0.899 | 1.64 (1.04–2.59) | 0.04 | 0.10 | |
| rs58898486 | 7 | 87063142 | INT | G/T | 0.998 | 0.984 | 8.40 (1.05–67.39) | 0.02 | 0.02 | |
| NTRK2 | rs7020204 | 9 | 86616007 | INT, 3′ UTR | C/T | 0.899 | 0.850 | 1.57 (1.07–2.32) | 0.02 | 0.05 |
| rs2013566 | 9 | 86616118 | INT, 3′ UTR | A/G | 0.876 | 0.828 | 1.47 (1.02–2.10) | 0.04 | 0.13 | |
| SLC6A4 | rs7224199 | 17 | 25547852 | Downstream | G/T | 0.411 | 0.369 | 1.19 (0.92–1.55) | 0.19 | 0.004 |
| rs3813034 | 17 | 25548930 | Downstream | A/C | 0.454 | 0.399 | 1.25 (0.98–1.60) | 0.08 | 0.040 | |
| rs140701 | 17 | 25562658 | INT | C/T | 0.454 | 0.400 | 1.25 (0.97–1.61) | 0.09 | 0.01 | |
| rs7212502 | 17 | 25573328 | INT | A/G | 1.000 | 0.987 | 8.56 (1.23–∞) | 0.01 | 0.01 | |
| rs28914831 | 17 | 25573988 | 5′ UTR | G/T | 1.000 | 0.988 | 8.37 (1.20–∞) | 0.02 | 0.01 | |
| rs2066713 | 17 | 25575791 | INT | A/G | 0.329 | 0.263 | 1.37 (1.04–1.80) | 0.03 | 0.004 | |
| NT_010799.14_3288789 | 17 | 25575922 | INT | C/G | 1.000 | 0.988 | 8.63 (1.24–∞) | 0.01 | 0.01 | |
| rs56355214 | 17 | 25576325 | INT | G/A | 1.000 | 0.988 | 8.47 (1.22–∞) | 0.01 | 0.01 | |
Abbreviations: ABCB1, ATP-binding cassette subfamily B member 1; CI, confidence interval; CREB1, cyclic AMP-responsive element binding protein 1; INT, Intronic; NTRK2, neurotrophic tyrosine kinase type 2 receptor; OR, odds ratio; SNP, single nucleotide polymorphism; UTR, untranslated region.
OR: calculation based on allele count and 95% CI based on exact logistic model when one cell count is 0.
SNP-based genetic association analysis of antidepressant response
In this study, there were 142 MDD patients who enrolled in the pharmacogenetic trial and completed 8-week antidepressant treatment (68 treated with desipramine and 74 treated with fluoxetine). For the discrete outcome (remission vs non-remission), SNP-based allelic or genotypic association analyses revealed that clinical remission status was associated with several polymorphisms in or near three genes, ABCB1, NTRK2 and SLC6A2 (Table 3). All of the nine associated NTRK2 SNPs were in 3′ UTR or coding regions except for rs2289658 at a splice site, whereas the two associated SLC6A2 SNPs were in intron or upstream region. For the ABCB1 gene, the associated SNPs included two in UTR, two in introns and one in coding sequence. No associated SNPs remained significant after adjusting for multiple tests with an FDR_BH ⩽0.05 in the discrete outcome analysis.
Table 3.
Polymorphisms associated with remission after 8-week antidepressant treatment with desipramine or fluoxetine
| Better allele frequency | Fisher's exact P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication | Gene | SNP | Chromo- some | Position | SNP type | Better/poor response allele | Remitter | Non-remitter | ORa (95% CI) | Allelic | Genotypic |
| Desipramine or fluoxetine (N=142) | ABCB1 | rs3842 | 7 | 86971302 | 3′ UTR | C/T | 0.203 | 0.094 | 2.44 (1.14–5.24) | 0.02 | 0.11 |
| rs17064 | 7 | 86971406 | 3′ UTR | A/T | 0.100 | 0.028 | 3.81 (1.08–13.5) | 0.03 | 0.11 | ||
| NTRK2 | rs45596934 | 9 | 86618439 | INT, 3′ UTR | A/G | 0.171 | 0.123 | 1.47 (0.74–2.94) | 0.31 | 0.002 | |
| NT_023935.17_16593339 | 9 | 86618627 | INT, 3′ UTR | T/C | 0.177 | 0.123 | 1.53 (0.77–3.05) | 0.24 | 0.002 | ||
| NT_023935.17_16593340 | 9 | 86618628 | INT, 3′ UTR | G/A | 0.181 | 0.123 | 1.58 (0.79–3.15) | 0.24 | 0.001 | ||
| rs1624327 | 9 | 86619110 | INT, 3′ UTR | A/G | 0.321 | 0.224 | 1.64 (0.95–2.83) | 0.08 | 0.021 | ||
| NT_023935.17_16651920 | 9 | 86677208 | INT, 3′ UTR | G/C | 0.961 | 0.880 | 3.35 (1.14–9.86) | 0.02 | 0.05 | ||
| rs2289658 | 9 | 86753190 | INT, splice site | T/C | 0.885 | 0.778 | 2.19 (1.12–4.28) | 0.03 | 0.05 | ||
| Desipramine (N=68) | ABCB1 | rs17064 | 7 | 86971406 | 3′ UTR | A/T | 0.132 | 0.018 | 8.39 (1.03–68.4) | 0.02 | 0.14 |
| NTRK2 | NT_023935.17_16593339 | 9 | 86618627 | INT, 3′ UTR | T/C | 0.157 | 0.113 | 1.47 (0.53–4.05) | 0.61 | 0.03 | |
| NT_023935.17_16593340 | 9 | 86618628 | INT, 3′ UTR | G/A | 0.167 | 0.113 | 1.57 (0.57–4.35) | 0.45 | 0.02 | ||
| rs11140793 | 9 | 86681300 | INT, 3′ UTR | A/C | 0.882 | 0.726 | 2.83 (1.12–7.14) | 0.03 | 0.05 | ||
| rs2289658 | 9 | 86753190 | INT, splice site | T/C | 0.909 | 0.742 | 3.48 (1.26–9.59) | 0.02 | 0.04 | ||
| rs2289657 | 9 | 86753280 | SYN | C/A | 0.955 | 0.823 | 4.53 (1.20–17.1) | 0.02 | 0.04 | ||
| rs56142442 | 9 | 86826085 | SYN | C/T | 0.957 | 0.906 | 2.31 (0.55–9.65) | 0.31 | 0.05 | ||
| SLC6A2 | rs5564 | 16 | 54283476 | INT | G/A | 0.141 | 0.000 | 11.92 (1.81–∞) | 0.004 | 0.04 | |
| Fluoxetine (N=74) | ABCB1 | rs1128503 | 7 | 87017537 | SYN | G/A | 0.522 | 0.304 | 2.49 (1.18–5.30) | 0.02 | 0.05 |
| rs10276036 | 7 | 87018134 | INT | T/C | 0.550 | 0.320 | 2.59 (1.24–5.44) | 0.01 | 0.04 | ||
| rs2235020 | 7 | 87037201 | INT | T/A | 0.539 | 0.318 | 2.50 (1.15–5.43) | 0.02 | 0.09 | ||
| NTRK2 | rs1624327 | 9 | 86619110 | INT, 3′ UTR | A/G | 0.348 | 0.212 | 1.99 (0.90–4.39) | 0.09 | 0.04 | |
| NT_023935.17_16651920 | 9 | 86677208 | INT, 3′ UTR | G/C | 0.972 | 0.864 | 5.52 (1.06–28.7) | 0.05 | 0.04 | ||
| SLC6A2 | rs1362621 | 16 | 54245985 | Upstream | A/G | 0.872 | 0.731 | 2.52 (1.06–5.96) | 0.04 | 0.03 | |
Abbreviations: ABCB1, ATP-binding cassette subfamily B member 1; CI, confidence interval; INT, intronic; NTRK2, neurotrophic tyrosine kinase type 2 receptor; OR, odds ratio; SNP, single nucleotide polymorphism; UTR, untranslated region; SYN, synonymous.
OR: calculation based on allele count and 95% CI based on exact logistic model when one cell count is 0.
For the quantitative outcome (relative reduction in HAM-D21 score) after controlling for age, gender and baseline HAM-D21 score, general linear regression analyses revealed that relative reduction of HAM-D21 scores was associated with six NTRK2 SNPs (three in 3′ UTR, two synonymous and one intronic at splice site) and one SLC6A3 intronic SNP rs8179029 in desipramine-treated patients, two SLC6A2 upstream SNPs in fluoxetine-treated patients and one SLC6A3 intronic SNP rs8179029 for combined sample, with a nominal P<0.01 (Table 4). Among the associated SNPs, only two NTRK2 synonymous SNPs, rs2289657 and rs56142442, remained statistically significant after correcting for multiple testing with an FDR_BH=0.05 in the sample of patients treated with desipramine. Desipramine-treated patients who are homozygous for C allele at synonymous SNP rs2289657 or at rs56142442 had higher levels of improvement with 27% larger reduction in HAM-D21 scores, compared with those who are not homozygous for C allele at rs2289657 or rs56142442.
Table 4.
Polymorphisms associated with relative reduction of HAM-D21 score after 8-week antidepressant treatment with desipramine or fluoxetine
| Medication | Gene | SNP | Chromo- some | Position | SNP type | Genotype | N | Mean (s.d.) | Deltaa (95% CI) | Pb | FDR_BHc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Desipramine or fluoxetine | ABCB1 | rs2214103 | 7 | 87062884 | INT | CC | 132 | 64.26 (21.35) | 21.45 (5.29–37.61) | 0.01 | 0.72 |
| CG | 8 | 46.44 (32.92) | |||||||||
| NTRK2 | rs9969765 | 9 | 86679605 | INT, 3′ UTR | CC | 46 | 69.48 (19.13) | 10.96 (3.11–18.81) | 0.007 | 0.72 | |
| GG/CG | 87 | 58.79 (23.81) | |||||||||
| rs2289657 | 9 | 86753280 | SYN | CC | 106 | 65.38 (20.35) | 12.74 (3.32–22.15) | 0.009 | 0.72 | ||
| AA/AC | 26 | 52.48 (29.11) | |||||||||
| SLC6A2 | NT_010498.15_9300464 | 16 | 54243766 | Upstream | TG | 15 | 77.25 (21.72) | 18.49 (6.60–30.39) | 0.003 | 0.72 | |
| TT | 121 | 60.69 (22.06) | |||||||||
| Desipramine | SLC6A3 | rs8179029 | 5 | 1462985 | INT | CC | 54 | 61.32 (20.05) | 29.79 (9.04–50.54) | 0.007 | 0.56 |
| TT/TC | 6 | 27.05 (39.26) | |||||||||
| NTRK2 | rs7038236 | 9 | 86678222 | INT, 3′ UTR | CC | 37 | 63.52 (18.98) | 16.97 (4.73–29.22) | 0.009 | 0.56 | |
| AA/AC | 18 | 48.02 (24.77) | |||||||||
| rs11140793 | 9 | 86681300 | INT, 3′ UTR | AA | 43 | 62.71 (23.47) | 18.30 (5.74–30.86) | 0.006 | 0.56 | ||
| CC/AC | 22 | 46.39 (23.30) | |||||||||
| rs2289658 | 9 | 86753190 | INT, splice site | TT | 44 | 62.94 (20.19) | 18.00 (5.32–30.69) | 0.007 | 0.56 | ||
| CC/TC | 20 | 43.57 (27.86) | |||||||||
| rs2289657 | 9 | 86753280 | SYN | CC | 51 | 62.11 (19.93) | 26.83 (13.39–40.27) | 0.0002 | 0.05 | ||
| AA/AC | 13 | 36.40 (29.89) | |||||||||
| rs56142442 | 9 | 86826085 | SYN | CC | 59 | 60.70 (19.89) | 33.17 (17.15–49.19) | 0.0001 | 0.05 | ||
| TT/TC | 8 | 28.92 (34.90) | |||||||||
| Fluoxetine | SLC6A2 | NT_010498.15_9300464 | 16 | 54243766 | Upstream | TG | 10 | 85.09 (13.13) | 20.55 (8.23–32.87) | 0.002 | 0.46 |
| TT | 60 | 65.69 (18.37) | |||||||||
| rs4783899 | 16 | 54244713 | Upstream | TT | 24 | 76.99 (15.15) | 15.02 (5.37–24.68) | 0.003 | 0.47 | ||
| GG/TG | 48 | 64.22 (20.09) |
Abbreviations: ABCB1, ATP-binding cassette subfamily B member 1; CI, confidence interval; HAM-D21, 21-item Hamilton Depression Rating Scale; INT, intronic; NTRK2, neurotrophic tyrosine kinase type 2 receptor; SNP, single nucleotide polymorphism; UTR, untranslated region; SYN, synonymous.
Delta: adjusted mean difference in relative reduction of HAM-D21 score.
P: based on general linear model after controlling for age, gender and baseline HAM-D21 score.
Benjamini-Hochberg false discovery rate after correcting for multiple testing.
Haplotype-based analyses
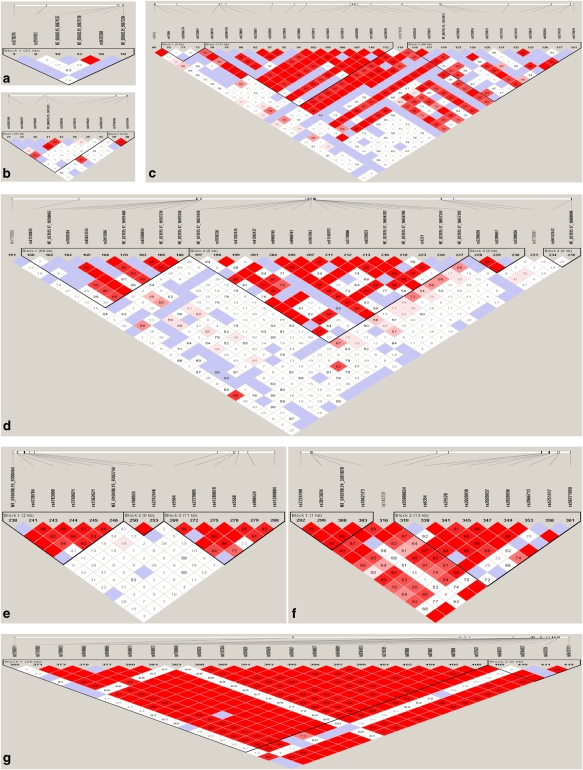
Haplotype analysis identified a total of 17 haplotype blocks in the seven genes using the Four Gametes Rules with the Haploview program, including one block in CREB1, two blocks in each of SLC6A3, SLC6A4 and CRHR1, three blocks in each of ABCB1 and SLC6A2, and four blocks in NTRK2 (Figure 2). For the association analysis of case and control, the diagnosis of depression was found to be associated with five haplotypes with a nominal P-value between 0.01 and 0.05 in CREB1, SLC6A3, ABCB1, NTRK2 and SLC6A2. Among the five depression-associated haplotypes, four included at least one SNP showing an association with depression in the single SNP-based analysis (Table 5). For the association of remitter and non-remitter, eight haplotypes were found to be associated with remission status, including two in ABCB1 (ACA in block 1 for desipramine-treated patients and GCGCACACGAGAC in block 2 for fluoxetine-treated patients), two in NTRK2 (TCG and CAG in block 3 for desipramine-treated patients), one in SLC6A2 (GCCAGT in block 4 for desipramine-treated patients) and three in SLC6A4 (TAGC and TAGA in block 1 and ATTGTAACCC in block 2 for the combined sample of desipramine- or fluoxetine-treated patients). Among the eight remission-associated haplotypes, three showed an association with a nominal P⩽0.01: TCG in block 3 of NTRK2 and GCCAGT in block 4 of SLC6A2 (P=0.009) for desipramine-treated patients, and TAGC in block 1 of SLC6A4 for fluoxetine-treated patients (P=0.004) (Table 5).
Figure 2.
Linkage disequilibrium (LD) pattern in seven genes: cyclic AMP-responsive element binding protein 1 (CREB1) (a), SLC6A3 (b), ATP-binding cassette subfamily B member 1 (ABCB1) (c), neurotrophic tyrosine kinase type 2 receptor (NTRK2) (d), SLC6A2 (e), SLC6A4 (f) and corticotropin-releasing hormone receptor 1 (CRHR1) (g). Standard color scheme in Haploview program is used to display the level of logarithm of odds (LODs) and the D′. Shown in each box are estimated statistics of the D′, which indicates the LD relationship between each pair of single nucleotide polymorphisms (SNPs) and are not labeled if D′=1.00. Regions are shown in bright red, light blue, shades of pink/red and white for D′=1+LOD⩾2, D′=1+LOD<2, D′<1+LOD⩾2 and D′<1+LOD<2, respectively. Vertical lines on the long horizontal white indicate the relative positions of SNPs in the gene.
Table 5.
Haplotypes associated with depression or clinical remission after 8-week antidepressant treatment
| Sample | Gene | Block no. | Haplotypea | Frequency | χ2 | d.f. | P | |
|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||
| MDD patients and healthy controls | CREB1 | Block 1 | GCACGG | 0.003 | 0.018 | 5.71 | 1 | 0.02 |
| SLC6A3 | Block 1 | CGTGCGGT | 0.089 | 0.134 | 4.83 | 1 | 0.03 | |
| ABCB1 | Block 2 | GCACACACGAGAC | 0.060 | 0.028 | 6.12 | 1 | 0.01 | |
| NTRK2 | Block 1 | GTTAGGGCA | 0.094 | 0.133 | 3.96 | 1 | 0.05 | |
| SLC6A2 | Block 3 | ACCAGA | 0.004 | 0.017 | 3.98 | 1 | 0.05 | |
| Remitter | Non-remitter | |||||||
| MDD patients treated with desipramine or fluoxetine | ABCB1 | Block 1 | ACA | 0.101 | 0.029 | 4.72 | 1 | 0.03 |
| NTRK2 | Block 3 | TCG | 0.839 | 0.717 | 5.63 | 1 | 0.02 | |
| SLC6A4 | Block 1 | TAGC | 0.006 | 0.047 | 5.11 | 1 | 0.02 | |
| Block 1 | TAGA | 0.048 | 0.003 | 4.92 | 1 | 0.03 | ||
| Block 2 | ATTGTAACC | 0.028 | 0.081 | 4.00 | 1 | 0.05 | ||
| MDD patients treated with desipramine | ABCB1 | Block 1 | ACA | 0.132 | 0.018 | 5.31 | 1 | 0.02 |
| NTRK2 | Block 3 | TCG | 0.865 | 0.679 | 6.35 | 1 | 0.01 | |
| Block 3 | CAG | 0.045 | 0.177 | 5.74 | 1 | 0.02 | ||
| SLC6A2 | Block 3 | GCCAGT | 0.134 | 0.012 | 6.79 | 1 | 0.009 | |
| MDD patients treated with fluoxetine | ABCB1 | Block 2 | GCGCACACGAGAC | 0.503 | 0.682 | 4.08 | 1 | 0.04 |
| SLC6A4 | Block 1 | TAGC | 0.000 | 0.085 | 8.37 | 1 | 0.004 | |
Abbreviations: ABCB1, ATP-binding cassette subfamily B member 1; CREB1, cyclic AMP-responsive element binding protein 1; MDD, major depressive disorder; NTRK2, neurotrophic tyrosine kinase type 2 receptor; SNP, single nucleotide polymorphism.
Letters with underline indicate the SNPs also showing an association with a nominal P<0.05 in the corresponding SNP-based analysis.
For quantitative outcome analysis of antidepressant treatment, 15 haplotypes were found to be associated with the relative reduction in HAM-D21 score after controlling for age, gender and baseline HAM-D21 score (Table 6). Among the 15 associated haplotypes, 2 in SLC6A3 and 3 in NTRK2 showed a correlation with a nominal P<0.004 in desipramine-treated patients, and 2 in SLC6A2 showed an association with a nominal P<0.008 in fluoxetine-treated patients. The most significant association was found between NTRK2 haplotype CAG (rs2289658, rs2289657 and rs2289656) and relative reduction of HAM-D21 score with a nominal P=0.0002 and an effect size of squared R=0.20 (Table 6).
Table 6.
Haplotypes associated with relative reduction of HAM-D21 score after 8-week antidepressant treatment
| Medication | Gene | Block no. | Haplotypea | N | βb | R2 c | t | P |
|---|---|---|---|---|---|---|---|---|
| Desipramine or fluoxetine | SLC6A3 | Block 1 | CGCGAAGT | 133 | 40.95 | 0.07 | 3.14 | 0.002 |
| Block 1 | CGTGCGGT | 133 | 17.01 | 0.05 | 2.69 | 0.008 | ||
| ABCB1 | Block 3 | TCTTACCGATCG | 141 | 27.17 | 0.05 | 2.58 | 0.01 | |
| NTRK2 | Block 2 | GCACGCCATTGGCAC | 140 | −5.85 | 0.03 | −2.20 | 0.03 | |
| Block 3 | CAG | 132 | 14.58 | 0.07 | 3.16 | 0.002 | ||
| Block 3 | TCG | 132 | −7.49 | 0.04 | −2.35 | 0.02 | ||
| Block 4 | CA | 134 | −11.22 | 0.04 | −2.36 | 0.02 | ||
| SLC6A2 | Block 1 | GATGAT | 140 | −15.88 | 0.04 | −2.46 | 0.01 | |
| Block 1 | TAGGGT | 140 | 10.00 | 0.03 | 2.10 | 0.04 | ||
| Desipramine | SLC6A3 | Block 1 | CGTGCGGT | 65 | 36.51 | 0.15 | 3.29 | 0.002 |
| Block 1 | CGCGAGGT | 65 | −15.77 | 0.12 | −2.99 | 0.004 | ||
| NTRK2 | Block 2 | GAACCCGCTCGGTAC | 66 | 13.69 | 0.09 | 2.47 | 0.02 | |
| Block 2 | GCACGCCATTGGCAC | 66 | −9.68 | 0.08 | −2.30 | 0.02 | ||
| Block 3 | CAG | 64 | 24.02 | 0.20 | 3.90 | 0.0002 | ||
| Block 3 | TCG | 64 | −11.55 | 0.09 | −2.49 | 0.02 | ||
| Block 4 | CA | 65 | −20.49 | 0.15 | −3.29 | 0.002 | ||
| Block 4 | TA | 65 | 22.05 | 0.13 | 3.02 | 0.004 | ||
| SLC6A4 | Block 1 | GAGA | 67 | 10.65 | 0.08 | 2.37 | 0.02 | |
| Fluoxetine | NTRK2 | Block 2 | CCACCCCACCATCTC | 72 | 14.21 | 0.06 | 2.16 | 0.03 |
| SLC6A2 | Block 1 | GATGAT | 73 | −18.92 | 0.10 | −2.88 | 0.005 | |
| Block 1 | TAGGGT | 73 | 14.54 | 0.10 | 2.73 | 0.008 | ||
| Block 2 | GT | 67 | −13.66 | 0.06 | −2.03 | 0.05 | ||
| SLC6A4 | Block 1 | GAAA | 74 | 28.05 | 0.06 | 2.08 | 0.04 |
Abbreviation: ABCB1, ATP-binding cassette subfamily B member 1; HAM-D21, 21-item Hamilton Depression Rating Scale; NTRK2, neurotrophic tyrosine kinase type 2 receptor; SNP, single nucleotide polymorphism.
Letters with underline indicate the SNPs also showing an association with a nominal P<0.05 in the corresponding SNP-based analysis.
β: regression coefficient after controlling for age, gender and baseline HAM-D21 score.
R2: proportion variance explained.
Discussion
In this study, we analyzed the fine structure of seven genes that are relevant to the pathophysiology of MDD or to antidepressant response at four sequential levels: (1) entry into the brain, (2) binding to monoaminergic transporters, and (3) distal effects at the transcription level, resulting in (4) changes in neurotrophin and neuropeptide receptors. We observed new alleles in all seven genes in Mexican-Americans. We described a total of 204 novel SNPs (Table 1), which almost doubled the number of reported SNPs in these genes that was detected in these individuals (total of dbSNPs was 215). The number of novel SNPs identified in these Mexican-American subjects ranged from 12 to 57, and in the case of CREB1, the total number of SNPs tripled from 6 to 18. Most of the novel SNPs reported here had MAF lower than 5% (Supplementary Table 1). Higher nucleotide diversity was found in the exonic regions of these genes, particularly in UTRs (Figure 2b). Only a small number of the novel SNPs were in coding regions19 and of those <40% (7) were non-synonymous. Analyses of HapMap data on four ethnic groups found different allele frequencies, with the greatest differences between Mexican Americans and Africans (Figure 1d).
Our analyses revealed nominal associations of eight SNPs and four haplotypes with susceptibility for MDD; those SNPs and haplotypes were located in four genes, ABCB1, CREB1, NTRK2 and SLC6A3. In addition, eight SNPs in SLC6A4 and one haplotype in SLC6A2 were also associated with MDD (Tables 2 and 5). However, some of these SNPs were not very common (MAF <0.03 in controls).
Nominal associations with several polymorphisms were also found for treatment response of 142 MDD patients who completed 8-week antidepressant treatment with desipramine or fluoxetine. Discrete outcome analyses (remitters vs non-remitters) showed that SNPs and haplotypes in ABCB1 and NTRK2 were associated with response. Variation in SLC6A2 and one haplotype in SLC6A4 were also associated with remission status. Quantitative outcome analyses showed that SNPs and haplotypes in ABCB1, NTRK2 and SLC6A2 were associated with relative HAM-D21 score reduction, but only two SNPs and one haplotype in NTRK2 remained significant for desipramine treatment after correcting for multiple testing.
Our data show that variations in six out of seven genes were associated with MDD or antidepressant response. Briefly, (i) SNPs in ABCB1 (located at 7q21.1), which is also called multidrug resistance 1, were associated with MDD and antidepressant response. ABCB1 encodes a large transmembrane transporter protein that acts as an active efflux pump transporting a wide range of drugs from the brain to the blood. Polymorphisms in this gene have been reported to predict the response to antidepressant treatment to drugs that are substrates for this transporter.21 (ii) SNPs in the CREB1 gene were associated with MDD. CREB (cyclic AMP response element-binding protein, located at 2q32.2-q34) encodes a transcription factor that modulates key growth factors important for synaptogenesis and neurogenesis. Sequence variations in the promoter and intronic regions of the CREB1 gene have previously been described to be cosegregated with mood disorders in women.22 3) SNPs in NTRK2 (located at 9q22.1) were associated with susceptibility to MDD and antidepressant response. Furthermore, two SNPs and one haplotype in NTRK2 continued to be significantly associated with relative reduction of HAM-D21 scores in the desipramine-treated group, after controlling for age, gender and baseline HAM-D21 scores. NTRK2, also known as tyrosine kinase receptor B, and its ligand, brain-derived neurotropic factor, regulate short- and long-term synaptic functions and neural plasticity. NTRK2 variants have been recently associated with obsessive-compulsive disorder in female patients.23 (iv) SNPs in SLC6A2 (noradrenaline transporter, located at 16q12.2) were associated with remission status and relative reduction of HAM-D21 scores, and one haplotype in this gene (ACCAGA) was associated with MDD. SLC6A2 gene encodes a transporter, which regulates norepinephrine (noradrenaline) homeostasis and the reuptake of norepinephrine into presynaptic nerve terminals.24 SLC6A2 polymorphisms have been reported to be associated with depression25, 26 and response to antidepressants.27, 28 (v) SNPs or haplotypes in SLC6A3 (dopamine transporter or DAT1, located at 5p15.33), which encodes a transporter that is important in dopaminergic neurotransmission, were associated with risk for MDD or relative reduction of HAM-D21. This transporter mediates the active re-uptake of synaptic dopamine.29 Variations in this gene have already been implicated in susceptibility for mood disorders30, 31 and antidepressant action.32 Other neuropsychiatric conditions have also been associated with SLC6A3, such as parkinsonism,33 attention-deficit hyperactivity disorder,34, 35, 36 Tourette's syndrome and addictive behavior.37, 38 6) SLC6A4 (serotonin transporter, located at 17q11.1-q12) encodes a transporter, which mediates antidepressant action, and behavioral effects of cocaine and amphetamines. Sequence variations in SLC6A4 have been extensively queried and they may be associated with several neuropsychiatric conditions, including MDD,39, 40, 41 anxiety-related personality traits42 and antidepressant response.43, 44 Our findings support that variations in SLC6A4 are associated with MDD risk. Haplotypes in SCL6A4 have also been associated with remission status and reduction of HAM-D21 scores.
The analyses presented here have not shown that variations in CRHR1 (located at 17q12-q22) gene are associated with susceptibility to MDD or antidepressant response. It can be noted that the current analyses have not taken anxiety levels into consideration. CRHR1 encodes the receptor of CRH, a key stress hormone that regulates the response to stress at the behavioral, immune, autonomic and neuroendocrine levels, through the activation of the hypothalamic–pituitary–adrenalaxis. Polymorphisms in CRHR1 were reported to be associated with antidepressant response, but only when anxiety scores are taken in consideration,45, 46 and with seasonal pattern and early onset of first depressive episode.47
In summary, we show that substantial levels of sequence variation, especially those that are not very common (MAF >5%), are likely to be found in candidate genes in an ethnically defined and understudied group. In this population group, for example, half of the SNPs detected were novel. Therefore, deep sequencing data may be relevant to our understanding of common and complex disorders, such as major depression, particularly in minority populations. Our analyses showed that several sequence variations and haplotypes in six out of seven selected genes were nominally associated with MDD risk and/or antidepressant treatment response and that after controlling for age, gender and baseline HAM-D21 score, as well as correcting for multiple testing, there was a significant association of antidepressant response with two NTRK2-coding SNPs and one haplotype. Our findings suggest that these variants may be implicated in the pathophysiology of MDD. The Mexican Americans are the most rapidly growing population group in the United States, but remain under represented in research studies. These results highlight the importance of direct re-sequencing of key candidate genes in ethnic minority groups in order to discover novel genetic variants that cannot be simply inferred from existing databases.
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
We thank our colleagues at the Sanger Institute, particularly Jane Rogers, for the sequencing of the seven genes. We are grateful to Israel Alvarado, Rita Jepson, Lorraine Garcia-Teague, Patricia Reyes, Isabel Rodriguez and Gabriela Marquez for their exemplary clinical work with the Mexican-American subjects. This work was supported by the NIH Grants GM61394, RR017365, MH062777, RR000865, RR16996, HG002500 and DK063240.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- Foley DL, Neale MC, Kendler KS. Reliability of a lifetime history of major depression: implications for heritability and co-morbidity. Psychol Med. 1998;28:857–870. doi: 10.1017/s0033291798006977. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Middeldorp CM, Hottenga JJ, Slagboom PE, Sullivan PF, de Geus EJ, Posthuma D, et al. Linkage on chromosome 14 in a genome-wide linkage study of a broad anxiety phenotype. Mol Psychiatry. 2008;13:84–89. doi: 10.1038/sj.mp.4002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W, Bonilla C. Common and rare variants in multifactorial susceptibility to common diseases. Nat Genet. 2008;40:695–701. doi: 10.1038/ng.f.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JC, Elliott P, Zabaneh D, Zhang W, Li Y, Froguel P, et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet. 2008;40:716–718. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- Perona MT, Waters S, Hall FS, Sora I, Lesch KP, Murphy DL, et al. Animal models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: prominent effects of dopamine transporter deletions. Behav Pharmacol. 2008;19:566–574. doi: 10.1097/FBP.0b013e32830cd80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Whelan F, Deloukas P, Whittaker P, Delgado M, Cantor RM, et al. Phosphodiesterase genes are associated with susceptibility to major depression and antidepressant treatment response. Proc Natl Acad Sci USA. 2006;103:15124–15129. doi: 10.1073/pnas.0602795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halushka MK, Fan JB, Bentley K, Hsie L, Shen N, Weder A, et al. Patterns of single-nucleotide polymorphisms in candidate genes for blood-pressure homeostasis. Nat Genet. 1999;22:239–247. doi: 10.1038/10297. [DOI] [PubMed] [Google Scholar]
- Li WH. Molecular Evolution. Sinauer Associates: Sunderland, MA; 1997. [Google Scholar]
- Weir BS. Genetic Data Analysis II. Sinauer: Sunderland, MA; 1996. [Google Scholar]
- Weir BS, Cockerham CC. Estinmating F-statistics for the analysis of population structure. Evoluation. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Weir BS, Hill WG. Estimating F-statistics. Annu Rev Genet. 2002;36:721–750. doi: 10.1146/annurev.genet.36.050802.093940. [DOI] [PubMed] [Google Scholar]
- Balding DJ, Martin Bishop M, Cannings C. Handbook of Statistical Genetics 2001JOHN WILEY & SONS, LTD: New York; (eds).3rd edn [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirji KF, Mehta CR, Patel NR. Computing Distributions for Exact Logistic Regression. JASA. 1987;82:1110–1117. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Uhr M, Grauer MT, Yassouridis A, Ebinger M. Blood-brain barrier penetration and pharmacokinetics of amitriptyline and its metabolites in p-glycoprotein (abcb1ab) knock-out mice and controls. J Psychiatr Res. 2007;41:179–188. doi: 10.1016/j.jpsychires.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Hughes HB, 3rd, Stiffler JS, Brechbiel A, Zubenko WN, Maher BS, et al. Sequence variations in CREB1 cosegregate with depressive disorders in women. Mol Psychiatry. 2003;8:611–618. doi: 10.1038/sj.mp.4001354. [DOI] [PubMed] [Google Scholar]
- Alonso P, Gratacos M, Menchon JM, Saiz-Ruiz J, Segalas C, Baca-Garcia E, et al. Extensive genotyping of the BDNF and NTRK2 genes define protective haplotypes against obsessive-compulsive disorder. Biol Psychiatry. 2008;63:619–628. doi: 10.1016/j.biopsych.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Kim CH, Hahn MK, Joung Y, Anderson SL, Steele AH, Mazei-Robinson MS, et al. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc Natl Acad Sci USA. 2006;103:19164–19169. doi: 10.1073/pnas.0510836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SH, Lee SH, Lee HJ, Cha JH, Ham BJ, Han CS, et al. Association between norepinephrine transporter gene polymorphism and major depression. Neuropsychobiology. 2004;49:174–177. doi: 10.1159/000077361. [DOI] [PubMed] [Google Scholar]
- Inoue K, Itoh K, Yoshida K, Shimizu T, Suzuki T. Positive association between T-182C polymorphism in the norepinephrine transporter gene and susceptibility to major depressive disorder in a Japanese population. Neuropsychobiology. 2004;50:301–304. doi: 10.1159/000080957. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Takahashi H, Higuchi H, Kamata M, Ito K, Sato K, et al. Prediction of antidepressant response to milnacipran by norepinephrine transporter gene polymorphisms. Am J Psychiatry. 2004;161:1575–1580. doi: 10.1176/appi.ajp.161.9.1575. [DOI] [PubMed] [Google Scholar]
- Kim H, Lim SW, Kim S, Kim JW, Chang YH, Carroll BJ, et al. Monoamine transporter gene polymorphisms and antidepressant response in Koreans with late-life depression. JAMA. 2006;296:1609–1618. doi: 10.1001/jama.296.13.1609. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15 3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Alexander M, Keck PE, McElroy S, Sadovnick AD, Remick RA, et al. Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder. Am J Med Genet. 2001;105:145–151. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1161>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Haeffel GJ, Getchell M, Koposov RA, Yrigollen CM, Deyoung CG, Klinteberg BA, et al. Association between polymorphisms in the dopamine transporter gene and depression: evidence for a gene-environment interaction in a sample of juvenile detainees. Psychol Sci. 2008;19:62–69. doi: 10.1111/j.1467-9280.2008.02047.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Goetz RM, Law CJ, Reith ME, et al. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science. 2007;317:1390–1393. doi: 10.1126/science.1147614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikova TD, Caron MG, Gainetdinov RR. DDD mice, a novel acute mouse model of Parkinson's disease. Neurology. 2006;67:S12–S17. doi: 10.1212/wnl.67.7_suppl_2.s12. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Stein MA, Ellison T, Unis AS, Leventhal BL. Attention deficit hyperactivity disorder and whole-blood serotonin levels: effects of comorbidity. Psychiatry Res. 1995;57:13–20. doi: 10.1016/0165-1781(95)02596-o. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Rowe DC, Abramowitz A, Kozel ST, Mohr JH, Sherman SL, et al. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet. 1998;63:1767–1776. doi: 10.1086/302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebebrand J, Dempfle A, Saar K, Thiele H, Herpertz-Dahlmann B, Linder M, et al. A genome-wide scan for attention-deficit/hyperactivity disorder in 155 German sib-pairs. Mol Psychiatry. 2006;11:196–205. doi: 10.1038/sj.mp.4001761. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Hakola P, Eronen M. Homicidal behaviour and mental disorders. Br J Psychiatry. 1995;167:821. doi: 10.1192/bjp.167.6.821a. [DOI] [PubMed] [Google Scholar]
- Lerman C, Caporaso NE, Audrain J, Main D, Bowman ED, Lockshin B, et al. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychol. 1999;18:14–20. doi: 10.1037//0278-6133.18.1.14. [DOI] [PubMed] [Google Scholar]
- Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, Goodwim GM, et al. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–733. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Lange M, Wolff B, Volzke H, Lucht M, Freyberger HJ, et al. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10:220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998;3:508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- Yu YW, Tsai SJ, Chen TJ, Lin CH, Hong CJ. Association study of the serotonin transporter promoter polymorphism and symptomatology and antidepressant response in major depressive disorders. Mol Psychiatry. 2002;7:1115–1119. doi: 10.1038/sj.mp.4001141. [DOI] [PubMed] [Google Scholar]
- Licinio J, O'Kirwan F, Irizarry K, Merriman B, Thakur S, Jepson R, et al. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry. 2004;9:1075–1082. doi: 10.1038/sj.mp.4001587. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Tang J, Liu W, et al. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett. 2007;414:155–158. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Papiol S, Arias B, Gasto C, Gutierrez B, Catalan R, Fananas L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Affect Disord. 2007;104:83–90. doi: 10.1016/j.jad.2007.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.