Abstract
Androgen deficiency is a pervasive problem in the older male population and is thought to be responsible for many symptoms once considered to be the result of normal aging. Numerous methods have been proposed to facilitate the detection of men at risk for androgen deficiency. In this article, we propose a novel screening tool, the quantitative Androgen Deficiency in the Aging Male (qADAM) questionnaire and report its successful use in quantifying the severity of androgen deficiency in a group of older men. Fifty-seven males scheduled to undergo radical prostatectomy for prostate cancer completed the qADAM as well as the Sexual Health Inventory for Men (SHIM) and the Expanded Prostate Cancer Index Composite hormonal/sexual (EPICh/EPICs) questionnaires. Thirty-four men also had serum testosterone levels measured for comparison. The qADAM showed statistically significant correlation to the SHIM (P=0.001), EPICh (P=0.016), EPICs (P=<0.001), and serum testosterone (P=0.046). The qADAM represents a viable alternative to existing questionnaires used to detect androgen deficiency and to assess response to treatment.
Keywords: androgen deficiency, hypogonadism, testosterone
Introduction
Androgen deficiency is a serious problem in the older male population, thought to affect approximately 1 in 200 men.1 Although the precise extent to which this process occurs is controversial, the largest study conducted thus far involved 3220 European men and showed a decline in testosterone and free testosterone levels by 0.4 and 1.3% per year, respectively.2 The common symptoms of this decline in androgens have not been fully defined, as have those secondary to declining estrogen levels in women. Some symptoms that were earlier thought to be the effects of aging in males have been found to be more prevalent in patients with hypogonadism.3 These include changes in bone mineral density,3, 4, 5, 6 muscle strength,7, 8, 9 cognition,10 body composition,11, 12 and sexual function.13, 14, 15 These studies provide evidence of a correlation between decreasing testosterone levels and symptoms, although they fail to give clear evidence for causality.
The Saint Louis University Androgen Deficiency in the Aging Male (ADAM) questionnaire developed by Morley et al.16 has been widely used as a screening tool for detecting men at risk for androgen deficiency since its development in 2000. It was shown to have a sensitivity of 88%, emphasizing its utility as a screening test. It was shown to be less specific, however, at 60%. A recent study by Martínez-Jabaloyas et al.17 in Spain showed a lower sensitivity and specificity than earlier estimated, at 84 and 36.6%, respectively, in 230 patients over age 50. These results point to the need to modify the ADAM questionnaire in order to better facilitate the early diagnosis of patients with hypogonadism.
The standard ADAM questionnaire consists of 10 ‘yes or no' questions concerning symptoms of androgen deficiency. See Figure 1.16 These ‘yes' or ‘no' questions, though effective at identifying symptoms associated with androgen deficiency, offer no information about the severity of symptoms. Thus, there is no simple and non-invasive way to monitor the response to treatment using this tool alone, unless symptoms completely resolve. We propose a variant of the ADAM questionnaire using a graded response, which we call the quantitative ADAM questionnaire, or qADAM. See Figure 2. We believe this questionnaire will allow practitioners to more accurately screen for earlier unrecognized androgen deficiency, as well as monitor patients' responses to treatment.
Figure 1.
The Saint Louis University ADAM questionnaire.
Figure 2.
The quantitative ADAM questionnaire.
Materials and methods
Male patients who were scheduled to undergo a radical prostatectomy for prostate cancer and who had not undergone neoadjuvant hormonal therapy were eligible for this study. Institutional review board approval was obtained. Each patient was asked to complete the qADAM questionnaire preoperatively, as well as the Sexual Health Inventory for Men (SHIM) and the Expanded Prostate Cancer Index Composite hormonal and sexual domains (EPIC, EPICh, EPICs).18, 19 Patients were encouraged to fill out the questionnaires as honestly as possible, and to fill them out completely. All incomplete surveys were excluded from the study. Patients were asked to have serum testosterone levels drawn for comparison.
qADAM questionnaire
The qADAM questionnaire consisted the 10 questions of the original ADAM, with ‘yes' and ‘no' replaced by a Likert scale of 1–5, in which 5 represented the absence of a given symptom and 1 represented maximal symptoms.20 All questions were weighted equally. The summation of these responses yielded a total qADAM score between 10 and 50, with 10 being most symptomatic and 50 being least symptomatic.
Serum testosterone
Testosterone measurements were performed using the Beckman Access II platform assay (Beckman Coulter, Fullterton, CA, USA). Only testosterone levels that were drawn on the day that the questionnaires were completed were used in our analysis. Testosterone levels were drawn at random times during the day between 9 AM and 5 PM.
Results
A total of 57 patients completed the qADAM questionnaire. The mean age of the patients studied was 62±10.9 years. The results of the questionnaires were compared using one-way analysis of variance for repeated measurements.
qADAM and serum testosterone
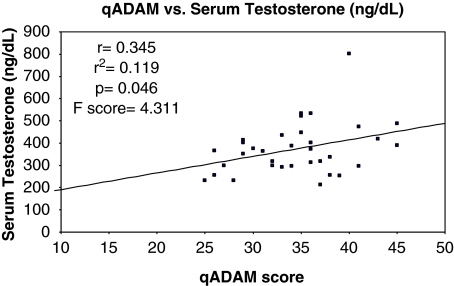
Of the 57 patients that completed the qADAM questionnaire, 34 (60%) consented to have serum testosterone values measured for analysis. Eleven of these patients were hypogonadal, defined as having serum testosterone values ⩽300 ng ml−1. The qADAM questionnaire positively correlated with the serum testosterone values with an r=0.345, r2=0.119, P=0.046, and an F-score of 4.311. See Graph 1.
Graph 1.
Serum testosterone levels versus the qADAM score.
qADAM and EPIC
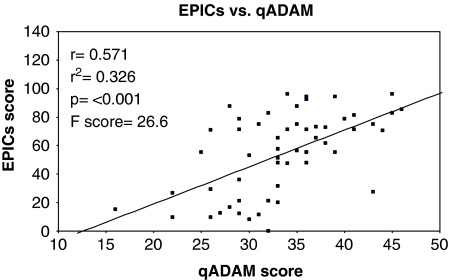
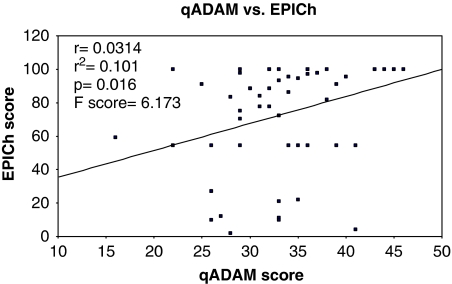
All of the patients in the study group had EPIC scores available for both the sexual (EPICs) and hormonal (EPICh) domains. There was a strong positive correlation between qADAM scores and the EPICs with r=0.571, r2=0.326, and a P-value of <0.001. The F-value was 26.6. See Graph 2. The EPICh also showed a positive correlation with the qADAM questionnaire, with r=0.0314, r2=0.101, P=0.016, and F=6.173. See Graph 3.
Graph 2.
The EPIC sexual domain score versus the qADAM score.
Graph 3.
The EPIC hormonal domain score versus the qADAM score.
qADAM and SHIM
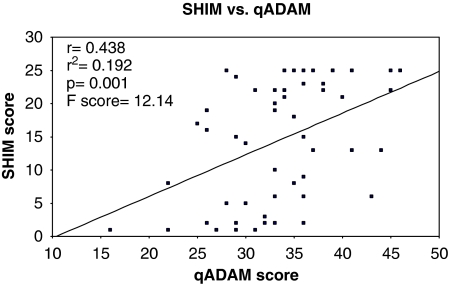
Fifty-three patients (93%) completed both the qADAM and the SHIM. The qADAM scores showed a strongly positive correlation with the SHIM questionnaire. A linear regression showed r=0.438, r2=0.192, F=12.14, and P=0.001. See Graph 4.
Graph 4.
The SHIM score versus the qADAM score.
Discussion
Although it is estimated that 40% of men over the age of 45 have low testosterone, our current screening tools lack the specificity to identify most of these patients. Often many of the symptoms of hypogonadism are subtle, with an insidious onset. Consequently, improved surveillance and implementation of routine screening of “at-risk” males could conceivably benefit otherwise unrecognized patients.
Currently, one of the most widely used screening tools is the Saint Louis University ADAM questionnaire. This questionnaire does not allow for the quantification of responses. As a result, it is difficult for clinicians to assess the degree of improvement in symptoms after initiating therapy. Heinemann, et al.21 have proposed another test, the Aging Male Symptom (AMS) rating, a battery of 17 questions graded on a Likert Scale of 1–5. Unlike the ADAM questionnaire, the AMS questionnaire allows one to quantify the degree of improvement in hypogonadal symptoms after initiating therapy. However, like the ADAM questionnaire, the AMS lacks specificity. Moore et al.22 conducted a study of 1174 androgen deficient males being treated with testosterone and used the AMS to assess response to treatment. The authors found a sensitivity of 96% but a specificity of only 30%. Additionally, some authors have criticized the AMS as excessively long and cumbersome, making its practical application difficult.23
We believe that the qADAM is a viable alternative to screening tools such as the ADAM and the AMS. The qADAM correlates with serum testosterone, indicating that it has potential as a screening as well as a diagnostic tool. Furthermore, the qADAM correlated with other instruments of sexual and hormonal function, such as the sexual and hormonal domains of the EPIC and the SHIM questionnaires.
Several aspects of our experimental design deserve further elaboration. We chose an experimental group composed of 57 men with prostate cancer scheduled to undergo radical prostatectomy. We acknowledge that the diagnosis of prostate cancer may have had an impact on these patients' symptoms of hypogonadism and their qADAM responses. Additionally, our decision to measure testosterone levels over the course of the day may draw some scrutiny. It is known that younger males often show variability in their testosterone level over the course of the day. It is believed by some authors, however, that the diurnal variability in testosterone levels seen in younger males is absent in older males.24 Given that our sample was composed entirely of men older than age 50, we did not feel that the variability in collection times altered the data significantly. Furthermore, we realize that the data presented do not validate the qADAM, but simply show significant correlations with serum testosterone and responses to other questionnaires, such as the ADAM, the SHIM, and the EPIC questionnaires.
Although these initial results have been promising, some aspects of the qADAM should be explored more thoroughly. In particular, future study will be conducted to evaluate the ability of the qADAM to monitor response to treatment over time. Further work to define the sensitivity and specificity of the qADAM using a larger group of patients will also be needed.
Conclusions
The qADAM questionnaire offers a useful alternative to existing tools for screening and assessing hypogonadism. Unlike most other commonly used questionnaires, it provides results that may be quantified and, thus, more easily followed over time. We have shown that the qADAM correlates well with several existing screening tools, as well as with serum testosterone levels. In light of these results, we believe that the qADAM may be implemented safely and effectively.
Conflict of interest
Drs Khera and Lipshultz are both speakers for Auxilium. All other authors declare no conflict of interest.
References
- Morales A, Tenover JL. Androgen deficiency in the aging male: when, who, and how to investigate and treat. Urol Clin North Am. 2002;29:975–982. doi: 10.1016/s0094-0143(02)00084-8. [DOI] [PubMed] [Google Scholar]
- Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- Kiratli BJ, Srinivas S, Perkash I, Terris MK. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology. 2001;57:127–132. doi: 10.1016/s0090-4295(00)00895-5. [DOI] [PubMed] [Google Scholar]
- Stoch SA, Parker RA, Chen L, Bubley G, Ko YJ, Vincelette A, et al. Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J Clin Endocrinol Metab. 2001;86:2787–2791. doi: 10.1210/jcem.86.6.7558. [DOI] [PubMed] [Google Scholar]
- Stepan JJ, Lachman M, Zverina J, Zverina J, Pacovsky V, Baylink DJ. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. J Clin Endocrinol Metab. 1989;69:523–527. doi: 10.1210/jcem-69-3-523. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Wahner HW, Seeman E, Offord KP, Dunn WL, Mazess RB, et al. Changes in bone mineral density of the proximal femur and spine with aging. J Clin Invest. 1982;70:716–723. doi: 10.1172/JCI110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MP, Gardner GM, Mollinger LA, Sepic SB. Strength of isometric and isokinetic contractions: knee muscles of men aged 20–86. Phys Ther. 1980;60:412–419. doi: 10.1093/ptj/60.4.412. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, et al. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82:407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism. 1970;19:653–663. doi: 10.1016/0026-0495(70)90062-4. [DOI] [PubMed] [Google Scholar]
- Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- Kwan M, Greenleaf WJ, Mann J, Crapo L, Davidson JM. The nature of androgen action on male sexuality: a combined laboratory-self-report study on hypogonadal men. J Clin Endocrinol Metab. 1983;57:557–562. doi: 10.1210/jcem-57-3-557. [DOI] [PubMed] [Google Scholar]
- Davidson JM, Camargo CA, Smith ER. Effects of androgen on sexual behavior in hypogonadal men. J Clin Endocrinol Metab. 1979;48:955–958. doi: 10.1210/jcem-48-6-955. [DOI] [PubMed] [Google Scholar]
- Davidson JM, Chen JJ, Crapo L, Gray GD, Greenleaf WJ, Catania JA. Hormonal changes and sexual function in aging men. J Clin Endocrinol Metab. 1983;57:71–77. doi: 10.1210/jcem-57-1-71. [DOI] [PubMed] [Google Scholar]
- Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49:1239–1242. doi: 10.1053/meta.2000.8625. [DOI] [PubMed] [Google Scholar]
- Martínez-Jabaloyas JM, Queipo-Zaragozá A, Rodríguez-Navarro R, Quiepo-Zaragoza JA, Gil-Salom M, Chuan-Nuez P. Relationship between the Saint Louis University ADAM Questionnaire and sexual hormonal levels in a male outpatient population over 50 years of age. Eur Urol. 2007;52:1760–1767. doi: 10.1016/j.eururo.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Vroege JA. The sexual health inventory for men (IIEF-5) Int J Impot Res. 1999;11:177. doi: 10.1038/sj.ijir.3900401. [DOI] [PubMed] [Google Scholar]
- Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;140:1–55. [Google Scholar]
- Heinemann LA, Saad F, Zimmermann T, Novak A, Myon E, Badia X, et al. The Aging Males' Symptoms Scale: update and compilation of international versions. Health Qual Life Outcomes. 2003;1:15. doi: 10.1186/1477-7525-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males' Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol. 2004;46:80–87. doi: 10.1016/j.eururo.2004.01.009. [DOI] [PubMed] [Google Scholar]
- O'Leary MP. Development of an index to evaluate symptoms in men with androgen deficiency. Rev Urol. 2003;5 Suppl 1:S11–S15. [PMC free article] [PubMed] [Google Scholar]
- Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]