Abstract
Despite tremendous development in chemotherapy for ovarian cancer over the past few decades, the prognosis of advanced cases with massive peritoneal dissemination is still unsatisfactory, and novel treatment modalities that can combine with chemotherapy are urgently needed. We recently developed virotherapy for solid tumors using telomerase-specific replication-selective adenoviruses (Telomelysin: OBP-301), in which the human telomerase reverse transcriptase (hTERT) gene promoter has been inserted to direct tumor-specific E1 gene expression. In this study, we investigated the anti-tumor effects of OBP-301, combined with cisplatin (CDDP), on ovarian cancer cells. In vitro treatment of SKOV3 cells with OBP-301 at a multiplicity of infection (MOI) of 0.01–100 induced significant cell death in a dose-dependent manner, with moderate cytotoxicity at an MOI of 1–10 and maximal cytotoxicity at an MOI of 100. In contrast, OBP-301 treatment of normal human cells showed no significant cell death at an MOI of 1–10 and exhibited modest cytotoxicity at an MOI of 100. The effects of low-dose CDDP at 0.5–1 μM, which induced only 20% cell death, were significantly augmented by combination with OBP-301 at an MOI of 1–10, finally achieving 40% cell death. Such enhancement of CDDP sensitivity was also observed in CDDP-resistant ovarian cancer cells. The combinatorial effects were further tested using a xenograft mouse model of SKOV3 with peritoneal dissemination. After intraperitoneal administration of OBP-301, we confirmed that injected OBP-301 fused with the green fluorescent protein (GFP) gene (OBP-401) was preferentially localized to peritoneal disseminations, as determined by fluorescence imaging. Treatment of mice with CDDP at low dose (0.5 mg kg–1) had modest effects, showing a 10% decrease in disseminations, whereas combination with intraperitoneal administration of OBP-301 at an MOI of 10 led to enhanced effects, achieving an approximately 80% decrease in disseminations. Kaplan–Meier analysis showed improved overall survival of mice treated with CDDP plus OBP-301 compared with CDDP alone. These findings support the therapeutic potential of intraperitoneal administration of OBP-301 to sensitize ovarian cancer cells to CDDP.
Keywords: ovarian cancer, virotherapy, oncolytic adenovirus, telomerase, cisplatin
Introduction
Surgical tumor reduction and subsequent platinum-based chemotherapy has been considered as the standard therapy for advanced ovarian cancer over the past few decades.1 Despite advances in the development of chemotherapeutic agents, the prognosis of advanced ovarian cancer with massive peritoneal disseminations is still unsatisfactory.2, 3, 4, 5, 6 Hence, there is a great deal of interest in developing novel therapeutic agents, hopefully as chemosensitizers.
As such candidates, a variety of gene therapies have been tested by many laboratories researching on ovarian cancer. These studies have used specific transgenes, most of which induce apoptosis or cell cycle arrest, introduced into tumors through plasmid or viral vector systems using tumor-specific promoters.7, 8, 9 Despite these efforts, the levels of transgene expression have been insufficient to eradicate tumors, mainly because of the unfavorable characteristics of adenoviral vectors, in which the E1 gene is deleted to inhibit the replicative capacity of the vector. These non-replicative vectors have limited distribution within the tumor mass after injection and are therefore not suitable for advanced ovarian cancers, as this tumor type frequently has multiple disseminated lesions throughout the whole peritoneal cavity. To increase viral spread to neighboring tumor cells or even distant lesions, the use of replication-competent adenoviruses has become a reality.
Many efforts have been made to realize cancer-specific adenoviral replication using a variety of gene promoters, including the prostate-specific antigen, MUC1, osteocalcin, L-plastin, midkine and E2F-1 genes.10, 11, 12, 13, 14, 15 Unfortunately, these promoters have tissue-type specificity and exhibit transcriptional activity only in cells that express such tumor markers. Furthermore, the transcriptional activity is relatively low. We were prompted by these studies to use the human telomerase reverse transcriptase (hTERT) promoter and established an oncolytic adenovirus vector (Telomelysin, OBP-301), which contains the hTERT gene promoter upstream of the E1 gene in adenovirus type 5 genome.16 As hTERT expression is highly specific to cancer cells17, 18, 19 and the hTERT promoter has stringent cancer specificity,20 OBP-301 can express E1 genes preferentially in cancer cells and thereby replicate there with much higher efficiency than in normal cells.16
This virus system does not require any specific transgenes to deliver, because vigorous viral replication itself induces cell death as a result of viral toxicity. In previous studies, in vivo direct injection of OBP-301 into primary tumor sites led to efficient eradication of the tumor without significant adverse effects in various organs.16, 21, 22 Considering the superior infectivity of OBP-301 to not only primary tumors but also to the surrounding and even distant tumors, we sought to apply this virus to the treatment of ovarian cancers with multiple disseminations. As a major therapeutic protocol for ovarian cancer includes platinum-based chemotherapy, we have a special interest in whether OBP-301 has potential for additive or synergistic effects with cisplatin (CDDP). Here, using an in vitro and in vivo mouse model with peritoneal dissemination, we show the therapeutic efficacy of intraperitoneal administration of OBP-301 combined with cisplatin on ovarian cancer.
Materials and methods
Cell culture
The human ovarian cancer cell line, SKOV3, and normal primary human fibroblasts (Takara, Tokyo, Japan) were cultured at 37 °C under 5% CO2 in Dulbecco's modified Eagle's medium, supplemented with 10% heat-inactivated fetal calf serum (Sigma-Aldrich, St Louis, MO), 100 μg ml–1 streptomycin and 100 IU ml–1 penicillin. KF28 cells were derived from serous cystadenocarcinoma of the ovary, from which CDDP-resistant KFr13 cells were established.23 Both cells were cultured in the above conditions.
Reagents and viruses
Cisplatin was provided by Bristol Pharmaceuticals KK (Tokyo, Japan). OBP-301 (Telomelysin) is a telomerase-specific replication-competent adenovirus, in which the hTERT gene promoter has been inserted upstream of the E1 gene in adenovirus type 5 genome.16 A replication-deficient variant of adenovirus type 5 (dl312) was used as the control for oncolytic activity.16 To visualize viruses infected in vitro and in vivo, the green fluorescent protein (GFP) gene was inserted into the OBP-301 genome (OBP-401) under control of the cytomegalovirus (CMV) promoter, so that OBP-401 expresses GFP in infected cells and this expression can be detected by fluorescence imaging.24, 25 The construction and features of these viruses have been described in detail in our previous studies.16, 24, 26, 27 The viruses were purified by CsCl2 linear gradient ultracentrifugation. The viral titers were determined by a plaque-forming assay using 293 cells.
Cell viability assay
The cytotoxicity of the viruses and/or CDDP was evaluated using WST-1 reagent (Roche Diagnostics, Tokyo, Japan). Briefly, 1 × 103 cells were seeded in 96-well plates 24 h before treatment. Cells were treated with virus alone at the indicated multiplicity of infections (MOIs) and concentrations, respectively, for 24, 48, 72 and 96 h. In combination assay, cells were infected with virus at various MOIs and exposed to CDDP at various concentrations for 48 h. Then, a total of 10 μl of WST-1 reagent was added to each well, and the cells were further incubated for 2 h at 37 °C. Absorbance was measured using a microscope reader at test and reference wavelengths of 450 and 655 nm, respectively, to evaluate the relative viability of the cells treated.
Animal experiments
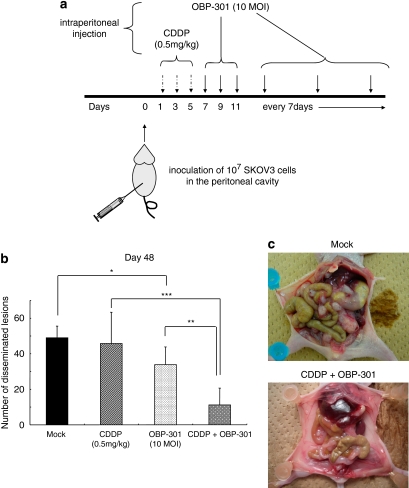
Female 7–9-week-old BALB/c nu/nu mice (SLC, Hamamatsu, Japan) were housed under specific pathogen-free conditions. Experiments proceeded according to the institutional guidelines. To confirm that we could produce a mouse model with peritoneal dissemination, SKOV3 cells (1 × 107 cells) were injected into the peritoneal space of the mice in 500 μl phosphate-buffered saline (PBS) and successful formation of disseminated cancer foci in the peritoneal cavity was apparent ∼2–3 weeks after injection. We then decided to test four groups of mice as follows: OBP-301 alone, CDDP alone, CDDP+OBP-301 and PBS only (the mock group). Disease-free BALB/c nu/nu mice were inoculated with 1 × 10 SKOV3 cells (day 0) and then 0.5 mg kg–1 CDDP in a total volume of 1 ml was injected into the peritoneal space once per day on days 1, 3 and 5. Then 108 pfu of OBP-301 was injected into the peritoneal space once per day on days 7, 9 and 11, followed by weekly injections of 108 pfu of OBP-301 until the killing. In the OBP-301 alone and CDDP alone groups, PBS was injected instead of CDDP or OBP-301, respectively. In the mock group only PBS was injected. Six mice in each group were treated according to this protocol and were killed 50 days after inoculation of SKOV3 cells to observe the peritoneal lesions. The effects of the treatment were evaluated by counting the number of peritoneal disseminations and evaluating the overall survival (OS).
In vitro and In vivo fluorescence imaging
SKOV3 and normal human fibroblasts were infected with OBP-401 at an MOI of 10 and GFP expression was assessed and photographed ( × 200) by an Eclipse TS-100 fluorescent microscope (Nikon, Tokyo, Japan) 24 h after infection. In vivo GFP fluorescence imaging was acquired by illuminating the animal with a Xenon 150-W lamp after an intraperitoneal injection of 108 pfu of OBP-401. The re-emitted fluorescence was collected through a long pass filter on a Hamamatsu C5810 three-chip color cooled charged-coupled device (CCD) camera (Hamamatsu Photonics Systems, Hamamatsu, Japan). High-resolution image acquisition was accomplished using an EPSON (Tokyo, Japan) personal computer. Images were processed for contrast and brightness with the use of Adobe Photoshop 4.0.1J software.
Statistical analysis
To assess the statistical significance of differences of the number of disseminated lesions, a standard two-tailed Student's t-test was performed using Microsoft Excel 2004. Life tables were computed using the Kaplan–Meier method, and the Log-rank test was used to assess statistical significance. A P-value of <0.05 was considered to indicate statistical significance.
Results
In vitro effect of OBP-301 on human ovarian cancer cell line and normal cells
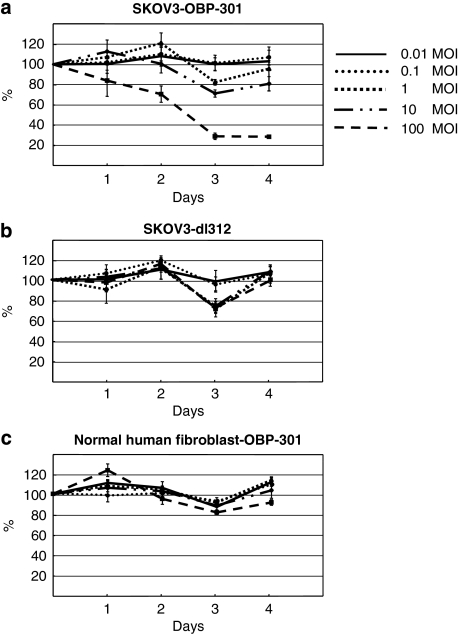
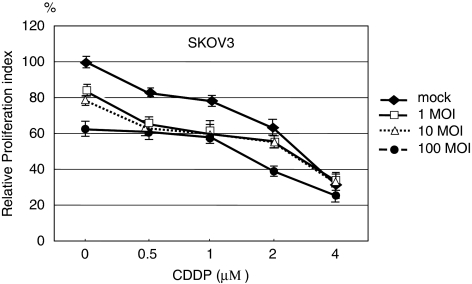
To examine the in vitro effect of OBP-301 on an ovarian cancer cell line, we infected the human ovarian cancer cell line SKOV3 and normal human fibroblast with OBP-301 and a replication-deficient variant (dl312) at various MOIs. Cell viability was assessed by the WST-1 assay 4 days after infection. Approximately 30–70% of SKOV3 cells were killed by OBP-301 at MOIs of 10–100, respectively (Figure 1a), whereas dl312 failed to kill any cells even at an MOI of 100 (Figure 1b), showing that the replicative capacity of the virus is crucial for the cytotoxic effect on ovarian cancer cells. In contrast, no cytotoxic effects were observed in normal fibroblasts 4 days after infection of OBP-301, even at an MOI of 100 (Figure 1c), clearly indicating the cancer-specific cytotoxicity of OBP-301. Next, we examined the combinatorial effect of OBP-301 with CDDP, which is a key drug for advanced ovarian cancer. OBP-301 was infected into SKOV3 cells at various concentrations, with or without 0–4 μM CDDP. Cell variability was measured 72 h after infection. In the absence of CDDP, ∼40% of SKOV3 cells were killed by OBP-301 at an MOI of 100, whereas 20% were killed at an MOI of 1–10 (Figure 2). The treatment of SKOV3 with 0–4 μM CDDP alone (mock) induced about 20% cell death at 0.5–1 μM and 40% death at 2 μM. When CDDP at 0.5–1 μM was combined with OBP-301 (1–100 MOI), the ratio of killed cells increased by 20%, resulting in a total of 40% cell death. When CDDP at 2 μM was combined with OBP-301 (100 MOI), the ratio of killed cells increased again by another 20%, resulting in 60% cell death. The treatment with CDDP alone at 4 μM resulted in ∼70% cell death, and the additive effects of OBP-301 were limited, with <10% increase in cell death even at 100 MOI. These findings suggest that OBP-301 has potent cytotoxic activity against ovarian cancer cells in vitro at an MOI of 1–100, and significantly enhances the effects of low-dose CDDP, which otherwise showed lesser cytotoxicity.
Figure 1.
In vitro cytotoxic effects of OBP-301 on cancer and normal cells. Human ovarian cancer cell line, SKOV3 cells (a), and normal human fibroblasts (c) were infected with OBP-301 at various multiplicity of infections (MOIs), and the cell viability was assessed daily by WST-1 assay until 4 days after infection. SKOV3 cells were also infected with replication-deficient adenoviral vectors (dl312) lacking the E1A gene (b) to test the specific effects of OBP-301. Bars indicate s.e.
Figure 2.
Combinatorial effects of OBP-301 and cisplatin on SKOV3 cells. SKOV3 cells were infected with OBP-301 at various multiplicity of infections (MOIs) and exposed to cisplatin at the indicated concentrations for 72 h. Then, the cell viability was measured by WST-1 assay. Bars indicate s.e.
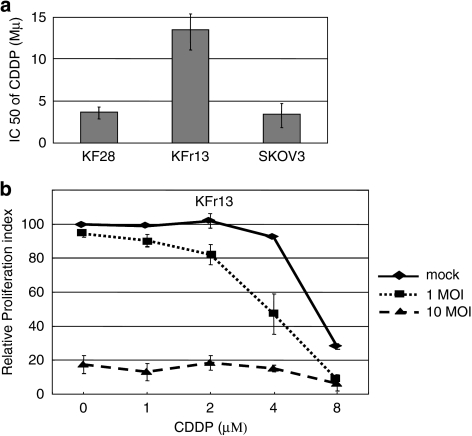
We further examined whether OBP-301 has cytotoxic activity or sensitizing effect on CDDP-resistant ovarian cancer cells as well. For this purpose, we used CDDP-resistant ovarian cancer cell line, KFr13 cells, established from KF28 cells derived from human serous cystadenocarcinoma of the ovary.23 The IC50 of CDDP was compared among KF28, KFr13 and SKOV3 cells and is shown in Figure 3a. KFr13 cells exhibited much higher IC50 than KF28 or SKOV3 cells, as expected. We then treated KFr13 cells with CDDP at various concentrations in the presence or absence of OBP-301 and examined the combinatorial effect. As shown in Figure 3b, the treatment of CDDP alone at 1–4 μM induced no significant death of this cell line. When CDDP at 1–4 μM was combined with OBP-301 at 1 MOI, the ratio of killed cells increased up to 10–40%, according to the concentration of CDDP. The treatment with CDDP alone at 8 μM resulted in ∼70% cell death, and the ratio of killed cells increased by 20% by the combination. Surprisingly, this cell line was very sensitive to OBP-301 because the treatment with OBP-301 alone at 10 MOI resulted in >80% cell death, irrespectively of the CDDP treatment. We also tested the effects of OBP-301 on another CDDP-resistant ovarian cancer cell, CaOV3.28 OBP-301 had similar cytotoxic effect on this cell line (data not shown). These findings indicate that OBP-301 has cytotoxic and sensitizing effect on CDDP-resistant ovarian cancer cells.
Figure 3.
Combinatorial effects of OBP-301 and cisplatin on CDDP-resistant KFr13 cells. (a) CDDP-sensitive KF28 and SKOV3 cells and CDDP-resistant KFr13 cells were treated with CDDP at various concentrations for 5 days. Then, the cell viability was measured by WST-1 assay, and IC50 of CDDP was calculated in each cell line. Bars indicate s.e. (b) CDDP-resistant KFr13 cells were infected with OBP-301 at various multiplicity of infections (MOIs) and exposed to cisplatin at the indicated concentrations for 72 h. Then, the cell viability was measured by WST-1 assay. Bars indicate s.e.
Visualization of infected viruses using the GFP gene in vitro and in vivo
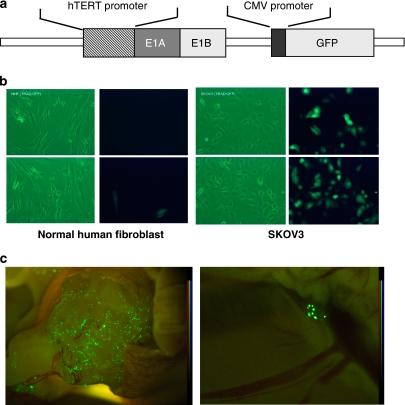
To confirm the specific effects of OBP-301 on ovarian cancer cells, we decided to confirm the localization of infected viruses, hopefully by visualizing the viruses. Thus, the GFP gene driven by the CMV promoter was inserted into OBP-301 so that the viruses could express GFP by efficient replication in infected cells (Figure 4a). This chimeric virus was named OBP-401.24, 25 We first infected OBP-401 into SKOV3 and normal human fibroblast at an MOI of 10 in vitro. Apparent expression of GFP was observed in SKOV3 cells (Figure 4b, right panel); however, only faint GFP signals were detected in normal human fibroblasts (Figure 4b, left panel), clearly indicating the cancer-specific replication of OBP-401 in vitro. Next, to assess the localization of intraperitoneally injected virus, we established an in vivo mouse model of ovarian cancer cells, in which 1 × 107 SKOV3 cells were inoculated into the peritoneal cavity of nude mice, leading to the formation of peritoneal disseminations. OBP-401 was then injected into the peritoneal cavity 4 weeks after the initial inoculation with the cancer cells. The mice were killed 72 h after OBP-401 injection and GFP expression was observed. GFP expression was found to be localized mainly at the surface of disseminated cancer lesions (Figure 4c, left panel). We even detected GFP expression in small lesions that were only detectable with a microscope, not by macroscopic observation (Figure 4c, right panel). These findings suggest that our viruses injected into the peritoneal cavity exhibit preferential and specific distribution in disseminated cancer foci.
Figure 4.
Visualization of infected OBP adenoviruses by green fluorescent protein (GFP) expression. To visualize OBP-301 in vitro and in vivo, the GFP gene driven by the cytomegalovirus (CMV) promoter was inserted into the genome of OBP-301, named OBP-401 (a). SKOV3 and normal human fibroblasts were infected with OBP-401 at an multiplicity of infection (MOI) of 10 and GFP expression was assessed using a fluorescence microscope (b). SKOV3 cells (1 × 107 cells) were inoculated into the peritoneal cavity of BALB/c nu/nu mice and OBP-401 was injected into the peritoneal cavity 4 weeks later. The mice were killed and GFP expression was assessed 72 h after injection of OBP-401 (c).
In vivo effect of intraperitoneal administration of OBP-301 in combination with CDDP on peritoneal dissemination of ovarian cancer cells
As the use of platinum-based chemotherapeutic agents has commonly been accepted as the standard therapy for advanced ovarian cancer, we examined the in vivo effects of OBP-301 alone, CDDP alone or OBP-301 combined with CDDP using a mouse model with peritoneal dissemination (Figure 5a, see Materials and methods). The numbers of peritoneal disseminated lesions were then counted in each treatment group (Figure 5b). Administration of CDDP alone did not significantly reduce the number of disseminated lesions compared with the mock-treated mice, whereas treatment with OBP-301 alone led to a significant reduction in disseminated lesions (P<0.05). The combination of CDDP with OBP-301 resulted in a significant decrease in the number of disseminated lesions compared with CDDP or OBP-301 alone (P<0.001, P<0.01, respectively). A representative picture of the peritoneal cavities of two mice is shown in Figure 4c. Massive progression of the peritoneal disseminations can be seen in the mock-treated mouse, whereas markedly fewer and smaller disseminated tumors are evident in the CDDP/ OBP-301-treated mouse.
Figure 5.
In vivo effect of intraperitoneal administration of CDDP and/or OB-301 on peritoneal disseminations of SKOV3. (a) Protocol of intraperitoneal administration of CDDP and/or OB-301. After the inoculation of 1 × 107 SKOV3 cells (day 0), 0.5 mg kg–1 CDDP was injected into the peritoneal cavity once a day on days 1, 3 and 5. Then, 108 pfu of OBP-301 was injected into the peritoneal cavity once a day on days 7, 9 and 11 followed by weekly injection of 108 pfu of OBP-301 until the killing. Arrows indicate the day of treatment. (b) The numbers of peritoneal disseminated lesions were counted in each treatment group 50 days after inoculation of SKOV3 cells. Bars, s.d. (c) Representative picture of the control mice (upper panel) and mice treated with CDDP + OBP-301 (lower panel). Bars, s.d.; *P<0.05; **P<0.01; ***P<0.001.
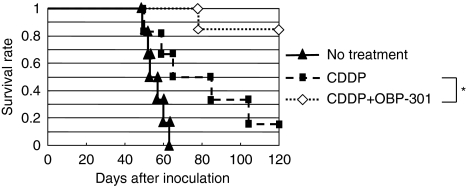
To clarify the survival effect of intraperitoneal administration of OBP-301, three groups of mice treated with PBS alone (mock), CDDP alone or CDDP combined with OBP-301 were further monitored for mortality until 120 days after inoculation of cancer cells (Figure 6). All mock-treated mice died around days 50–60, whereas CDDP alone extended survival, with a 50% OS rate at day 60 and 17% at day 120. Co-treatment with OBP-301 further extended survival, achieving 100% OS at day 80 and 83% at day 120. Thus, the prognosis was significantly improved by the combination of CDDP with OBP-301. Taken together, these findings suggest that intraperitoneal administration of OBP-301 sensitizes ovarian cancer cells to CDDP and may be a useful treatment option for advanced ovarian cancer with peritoneal dissemination.
Figure 6.
Survival of mice treated with intraperitoneal administration of CDDP and/ or OB-301. Kaplan–Meier plots of overall survival of the no treatment, CDDP alone and CDDP+OBP-301 groups. Each group consisted of six mice. *P<0.05.
Discussion
In this study, we showed the therapeutic potential of intraperitoneal administration of OBP-301 in combination with CDDP for advanced ovarian cancers with peritoneal dissemination. A series of our previous studies have shown the anti-tumor effects of OBP-301 in various types of cancers, including lung and colorectal cancers both in vitro and in vivo,16, 21, 22, 24, 25, 26, 27 and a clinical trial of OBP-301 therapy as monotherapy is currently underway based on these preclinical studies.29 However, these preclinical and clinical studies have been carried out mainly by direct injection of OBP-301 into primary tumor sites. This study is the first to test the effect of intraperitoneal administration of OBP-301.
Our in vitro data showed that OBP-301 alone has marked anti-tumor effects on SKOV3 cells at an MOI of 100, whereas modest to mild effects were observed at an MOI of 10 or lower (Figure 1). However, such low-dose OBP-301 did have a sensitizing effect on SKOV3 cells to CDDP in vitro (Figure 2), rendering the cytotoxicity of low-dose CDDP (0.5 μM) equivalent to that of high-dose CDDP (2 μM). We also confirmed that similar sensitizing effect was observed in CDDP-resistant cells (Figure 3). Of particular interest is that CDDP-resistant KFr13 cells seemed to have higher sensitivity to OBP-301 than CDDP-sensitive SKOV3 cells. These finding suggest the therapeutic potential of OBP-301, irrespectively of the CDDP sensitivity of target cells. The beneficial combined effect of OBP-301 with CDDP was further supported by the in vivo analysis. Although the number of disseminated lesions was not significantly altered by low-dose CDDP (0.5 mg kg–1) alone, the number significantly decreased by the addition of OBP-301 to CDDP (P<0.01) (Figure 5).
The molecular mechanism through which OBP-301 sensitizes cells to CDDP was not analyzed in this study, but it is likely that OBP-301 and CDDP produce their cytotoxic activities through different mechanisms. We also confirmed that the replicative efficacy of OBP-301 was not affected by CDDP treatment in vitro and that OBP-301 infection had no effect on cell cycle distribution (data not shown). Therefore, the anti-tumor mechanisms of the two agents seem to be independent of each other. We also confirmed that the nuclear morphology of cells infected with OBP-301 did not exhibit findings of apoptosis characterized by chromosome condensation, nuclear shrinkage and fragmentation (data not shown). Furthermore, western blot analysis showed no change in a family of cysteine proteases known as caspases on infection with OBP-301 (data not shown). The in vitro dose-response curves of CDDP combined with OBP-301 show that the interaction between the two agents is additive, not synergistic, suggesting that OBP-301 targets cells that have escaped death by CDDP, leading to an additive effect. Interestingly, intraperitoneal administration of OBP-301 in combination with CDDP induced a more pronounced growth inhibitory effect, that is, a synergistic effect, in vivo than in vitro (Figure 5), suggesting particular interactions between OBP-301 and CDDP in vivo. Similar synergistic effects of OBP-301 in vivo, but not in vitro, have been reported with other cytotoxic agents.21 One possible explanation for the augmented cytotoxicity observed in vivo might be that the blood supply to tumor lesions is disturbed by the actions of OBP-301, in which intraperitoneal administration of OBP-301 may attack endothelial cells of microvessels in disseminated lesions and thereby enhance the cytotoxic effects of CDDP. Although microvessels are mouse origin, hTERT promoter might function in such cells because it has very similar context of the sequences with the mouse TERT promoter30 and it can be activated in proliferation-competent normal cells, such as endothelial cells in tumor microvessels.31, 32, 33 Alternatively, CDDP treatment affects the immunological circumstances of the host, disturbing the functions of macrophages and other immune cells, which may provide some advantage to OBP-301 in its infectivity and replicative capacity in cancer tissues. Finally, we tested the change in expression of various genes involved in drug resistance, such as MDR1, MRP1, LRP and GST, 24 h after the treatment with OBP-301 at 100 MOI. However, reverse transcriptase–PCR assays failed to find any specific factors whose expression was significantly regulated by the treatment (data not shown). Further analyses are needed to identify the molecular mechanisms of the synergistic effects by both agents in vivo.
Although this study did not investigate the adverse effects on mice, our previous studies showed no significant toxicity of OBP-301 on normal human cells.16, 21, 22, 24, 25 This is likely because of the use of the hTERT promoter for tumor-specific expression of the E1 gene. Although the hTERT promoter is reported to be silent in normal cells, it may provide some transcriptional activity in some cell types with telomerase activity, such as stem or germ-line cells.31, 32, 33 It is therefore possible that these telomerase-positive normal cells might be targeted by OBP-301. Nevertheless, severe adverse effects due to OBP-301 have not been reported. One possible explanation of this favorable phenomenon is that cells with stem-like characteristics are usually non-epithelial in origin and are therefore not likely to be targeted by adenoviral vectors.34 Alternatively, the levels of telomerase activity in cells with stem-like characteristics are not as high as in cancer cells unless the cells are exposed to ectopic mitogenic stimuli.32, 33 Therefore, the cytotoxicity by OBP-301 might be limited or permissive in such cells.
Platinum-based chemotherapy has widely been accepted as the standard regimen for both neoadjuvant and adjuvant settings for advanced ovarian cancer.1 However, ∼30–40% of patients have resistance to platinum-based chemotherapy, and even sensitive patients frequently acquire resistance after repeated cycles of CDDP-based chemotherapy.2, 3, 4, 5, 6 In this study, we found that OBP-301 was useful for CDDP-resistant ovarian cancer cells, indicating the therapeutic potential of this agent, irrespectively of the CDDP sensitivity of target cells. Furthermore, we found that use of OBP-301 alone had cytotoxic effects in vitro at an MOI of 100, but exhibited more pronounced effects in vivo at an MOI of 10, decreasing the number of disseminations (Figure 5). Although we did not examine the survival effect of OBP-301 alone, patients who relapse with acquired CDDP resistance may be able to use OBP-301 alone.
The most notable finding of this study is that addition of intraperitoneal injection of OBP-301 improved the survival of mice. Our treatment modality is characterized by continuous weekly administration of OBP-301 after CDDP treatment. As one of the major purposes of this strategy is to reduce the total dose of CDDP delivered, thereby reducing its adverse effects, we did not test weekly combined administration of OBP-301 and CDDP. Our data indicate that continuous weekly OBP-301 administration after CDDP treatment has some survival effect and is a practical regimen for minimizing the adverse effects of CDDP.
We are currently investigating the combinatorial actions of OBP-301 with taxans, other key drugs for ovarian cancers. In fact, the combination therapy of taxans and CDDP/carboplatin is now a novel standard of chemotherapy for ovarian cancer. Therefore, it is necessary to investigate the best modality by which to combine OBP-301 with such standard regimens. Furthermore, it is of great interest to test the possibility of combination therapy of OBP-301 with the recently established molecular target therapies, such as signal transduction inhibitors, to provide relief to patients with ovarian cancers who have acquired resistance to standard therapy.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) and the Megumi Medical Foundation of Kanazawa University. We greatly appreciate Dr Yoshihiro Kikuchi (Ohki Memorial Kikuchi Cancer Clinic for Women, Tokorozawa, Saitama, Japan) for providing KF28 and KFr13 cells. We thank Mr H Kawamura (Oncolys BioPhama, Inc.) for his helpful assistance throughout the study.
References
- Bristow RE. Surgical standards in the management of ovarian cancer. Curr Opin Oncol. 2000;12:474–480. doi: 10.1097/00001622-200009000-00015. [DOI] [PubMed] [Google Scholar]
- Berek JS, Bertelsen K, du Bois A, Brady MF, Carmichael J, Eisenhauer EA, et al. Advanced epithelial ovarian cancer: 1998 consensus statements. Ann Oncol. 1999;10 suppl:87–92. doi: 10.1023/a:1008323922057. [DOI] [PubMed] [Google Scholar]
- du Bois A, Luck HJ, Meier W, Adams HP, Möbus V, Costa S, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- Engel J, Eckel R, Schubert-Fritschle G, Kerr J, Kuhn W, Diebold J, et al. Moderate progress for ovarian cancer in the last 20 years: prolongation of survival, but no improvement in the cure rate. Eur J Cancer. 2002;38:2435–2445. doi: 10.1016/s0959-8049(02)00495-1. [DOI] [PubMed] [Google Scholar]
- Neijt JP, Engelholm SA, Tuxen MK, Sorensen PG, Hansen M, Sessa C, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000;18:3084–3092. doi: 10.1200/JCO.2000.18.17.3084. [DOI] [PubMed] [Google Scholar]
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- Barnes MN, Pustilnik TB.Current strategies in gene therapy for ovarian cancer Curr Opin Obstet Gynecol 20011347–51.Review [DOI] [PubMed] [Google Scholar]
- Kimball KJ, Numnum TM, Rocconi RP, Alvarez RD.Gene therapy for ovarian cancer Curr Oncol Rep 20068441–447.Review [DOI] [PubMed] [Google Scholar]
- Casado E, Nettelbeck DM, Gomez-Navarro J, Hemminki A, Gonzalez Baron M, Siegal GP, et al. Transcriptional targeting for ovarian cancer gene therapy Gynecol Oncol 200182229–237.Review [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–2563. [PubMed] [Google Scholar]
- Kurihara T, Brough DE, Kovesdi I, Kufe DW. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Invest. 2000;106:763–771. doi: 10.1172/JCI9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Wada Y, Gardner TA, Egawa M, Park MS, Hsieh CL, et al. A conditional replication-competent adenoviral vector, Ad-OC-E1a, to cotarget prostate cancer and bone stroma in an experimental model of androgen-independent prostate cancer bone metastasis. Cancer Res. 2001;61:6012–6019. [PubMed] [Google Scholar]
- Peng XY, Won JH, Rutherford T, Fujii T, Zelterman D, Pizzorno G, et al. The use of the L-plastin promoter for adenoviral-mediated, tumor-specific gene expression in ovarian and bladder cancer cell lines. Cancer Res. 2001;61:4405–4413. [PubMed] [Google Scholar]
- Adachi Y, Reynolds PN, Yamamoto M, Wang M, Takayama K, Matsubara S, et al. A midkine promoter-based conditionally replicative adenovirus for treatment of pediatric solid tumors and bone marrow tumor purging. Cancer Res. 2001;61:7882–7888. [PubMed] [Google Scholar]
- Tsukuda K, Wiewrodt R, Molnar-Kimber K, Jovanovic VP, Amin KM. An E2F-responsive replication-selective adenovirus targeted to the defective cell cycle in cancer cells: potent antitumoral efficacy but no toxicity to normal cell. Cancer Res. 2002;62:3438–3447. [PubMed] [Google Scholar]
- Kawashima T, Kagawa S, Kobayashi N, Shirakiya Y, Umeoka T, Teraishi F, et al. Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res. 2004;10:285–292. doi: 10.1158/1078-0432.ccr-1075-3. [DOI] [PubMed] [Google Scholar]
- Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 1998;58:1558–1561. [PubMed] [Google Scholar]
- Kyo S, Kanaya T, Takakura M, Tanaka M, Inoue M. Human telomerase reverse transcriptase as a critical determinant of telomerase activity in normal and malignant endometrial tissues. Int J Cancer. 1999;80:60–63. doi: 10.1002/(sici)1097-0215(19990105)80:1<60::aid-ijc12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kyo S, Kanaya T, Takakura M, Tanaka M, Yamashita A, Inoue H, et al. Expression of human telomerase subunits in ovarian malignant, borderline and benign tumors. Int J Cancer. 1999;80:804–809. doi: 10.1002/(sici)1097-0215(19990315)80:6<804::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, et al. Cloning of human telomerase reverse transcriptase gene promoter and identification of proximal core promoter essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- Fujiwara T, Kagawa S, Kishimoto H, Endo Y, Hioki M, Ikeda Y, et al. Enhanced antitumor efficacy of telomerase-selective oncolytic adenoviral agent OBP-401 with docetaxel: preclinical evaluation of chemovirotherapy. Int J Cancer. 2006;119:432–440. doi: 10.1002/ijc.21846. [DOI] [PubMed] [Google Scholar]
- Hioki M, Kagawa S, Fujiwara T, Sakai R, Kojima T, Watanabe Y, et al. Combination of oncolytic adenovirotherapy and Bax gene therapy in human cancer xenografted models. Potential merits and hurdles for combination therapy. Int J Cancer. 2008;122:2628–2633. doi: 10.1002/ijc.23438. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Iwano I, Miyauchi M, Kita T, Oomori K, Kizawa I, et al. The mechanism of acquired resistance to cisplatin by a human ovarian cancer cell line. Jpn J Cancer Res. 1988;79:632–635. doi: 10.1111/j.1349-7006.1988.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeoka T, Kawashima T, Kagawa S, Teraishi F, Taki M, Nishizaki M, et al. Visualization of intrathoracically disseminated solid tumors in mice with optical imaging by telomerase-specific amplification of a transferred green fluorescent protein gene. Cancer Res. 2004;64:6259–6265. doi: 10.1158/0008-5472.CAN-04-1335. [DOI] [PubMed] [Google Scholar]
- Kishimoto H, Kojima T, Watanabe Y, Kagawa S, Fujiwara T, Uno F, et al. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat Med. 2006;12:1213–1219. doi: 10.1038/nm1404. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Urata Y, Tanaka N.Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter Curr Cancer Drug Targets 20077191–201.Review [DOI] [PubMed] [Google Scholar]
- Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;9:1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa J, Ohmichi M, Kurachi H, Ikegami H, Kimura A, Matsuoka T, et al. Inhibition of extracellular signal-regulated protein kinase or c-Jun N-terminal protein kinase cascade, differentially activated by cisplatin, sensitizes human ovarian cancer cell line. J Biol Chem. 1999;274:31648–31654. doi: 10.1074/jbc.274.44.31648. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Tanaka N, Nemunaitis JJ, Senzer N, Tong A, Ichimaru D, et al. Phase I trial of intratumorla administration of OBP-301, a novel telomerase-specific oncolytic virus in patients with advanced solid cancer: evaluation of biodistributon and immune response. J Clin Oncol. 2008;26:171s. [Google Scholar]
- Takakura M, Kyo S, Inoue M, Wright WE, Shay JW. Function of AP-1 in transcription of the telomerase reverse transcriptase gene (TERT) in human and mouse cells. Mol Cell Biol. 2005;18:8037–8043. doi: 10.1128/MCB.25.18.8037-8043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo S, Takakura M, Kohama T, Inoue M. Telomerase activity in human endometrium. Cancer Res. 1997;57:610–614. [PubMed] [Google Scholar]
- Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek MA, et al. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995;155:3711–3715. [PubMed] [Google Scholar]
- Kyo S, Inoue M. Complex regulatory mechanisms of telomerase activity in normal and cancer cells: How can we apply them for cancer therapy. Oncogene. 2002;21:688–697. doi: 10.1038/sj.onc.1205163. [DOI] [PubMed] [Google Scholar]
- Sakurai F, Mizuguchi H, Hayakawa T. Efficient gene transfer into human CD34+ cells by an adenovirus type 35 vector. Gene Therapy. 2003;10:1041–1048. doi: 10.1038/sj.gt.3301959. [DOI] [PubMed] [Google Scholar]