Abstract
We evaluated whether ejaculatory dysfunction induced with a selective α1A-blocker influenced orgasm. Fifteen healthy male volunteers took silodosin or a placebo in a randomized, double-blind crossover design. We investigated the ejaculatory volume before and after administration of the agents. After each ejaculation, participants self-reported the answers to an original questionnaire, which was about discomfort on ejaculation, orgasm and satisfaction with the discomforting ejaculation. All participants on silodosin had a complete lack of seminal emission and expulsion. All participants felt orgasm in spite of a complete lack of seminal emission. Of the 15, 12 (80%) who had a somewhat uncomfortable feeling during orgasm were dissatisfied with this feeling, although 9 of the 12 reported that its degree was mild. Orgasm is preserved regardless of the loss of seminal emission with silodosin administration. Although most participants reported mild discomfort during orgasm, they were greatly dissatisfied with the loss of seminal emission.
Keywords: anejaculation, ejaculatory dysfunction, orgasm, sexual dysfunction, silodosin
Introduction
The use of α1-adrenoceptor antagonists (α1-blockers) is widely accepted as the first-line treatment for lower urinary tract symptoms suggestive of benign prostatic hyperplasia.1, 2, 3, 4, 5 Recent studies have highlighted the influence of α1-blockers on ejaculatory function.6, 7, 8, 9, 10 A loss of seminal emission has been recently postulated as one of the mechanisms of abnormal ejaculation.7, 8 Highly selective α1-adrenoceptor subtype-A antagonists (α1A-blockers) cause a high incidence of ejaculatory dysfunction. However, the orgasm and feeling during ejaculatory dysfunction with α1-blocker administration have not been fully assessed.11, 12, 13 In addition, even the general physiology underlying orgasm has not been completely elucidated.14 As an another new problem with regard to orgasm, climacturia after radical prostatectomy has been noticed recently, but its mechanism has not been completely elucidated.14, 15 Therefore, study of orgasm-related issues will be necessary to clarify the physiology of orgasm not only during normal, but also abnormal ejaculatory conditions.
Silodosin is a new highly selective α1A-blocker launched in 2006 in Japan and approved by the Food and Drug Administration in 2008 in the United States. The selectivity of silodosin towards the α1A-adrenoceptor vs the α1B-adorenoceptor subtype was reported to be 38 times higher than that of tamsulosin hydrochloride.16 We reported earlier that silodosin induced a complete lack of seminal emission in healthy volunteers.8 In this context, we evaluated the status of orgasm and feeling during ejaculatory dysfunction induced with the selective α1A-blocker in healthy volunteers.
Materials and methods
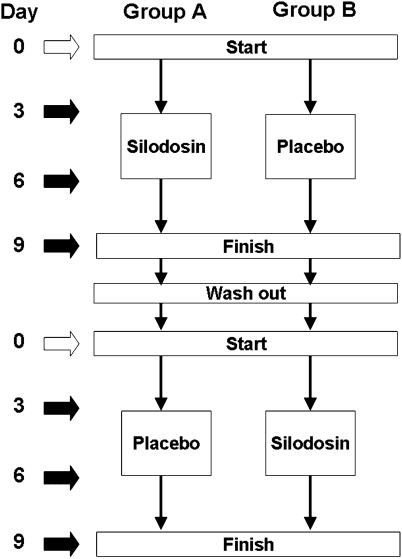
Fifteen healthy male urologists in our department voluntarily participated in the study. Details of ejaculatory profiles and methods of collection have been described earlier.8 In brief, this study used a double-blind, placebo controlled, randomized, crossover design (Figure 1). The median age of the participants was 32 years (range 26–47 years). We used silodosin, a new highly selective α1A-blocker launched in 2006 in Japan. It was purchased from Daiichi Sankyou Company, Ltd (Tokyo, Japan). Placebos were prepared by filling empty silodosin capsules with lactose in our laboratory. Baseline ejaculatory profiles were collected by masturbation after 72 h of abstinence (from Days 0 to 3) before administration of silodosin or the placebo. In the study, 4 mg of silodosin or a placebo consisting of lactose with a dosage identical to that of the drug was given twice daily to participants for 3 days. We evaluated the ejaculatory volume and sperm count in the urine after ejaculation. We defined anejaculation as a 100% decrease in ejaculation volume compared with the baseline ejaculatory profile as in earlier reports.6, 8 After each ejaculation, participants self-reported the answers to an original questionnaire composed of questions to evaluate ejaculatory function, including orgasm and feeling (see Appendix). All samples were collected by masturbation. All participants adhered to the schedule for ejaculation by masturbation and did not engage in intercourse during the study.
Figure 1.
Study design for analysing ejaculation. Black arrows indicated a given point of ejaculation for semen analysis. White arrows indicated a given point of ejaculation for ensuring the same abstinence period.
Results of ejaculatory profiles are expressed as mean±s.d. and were considered to be statistically significant if P<0.05. The changes of parameters were tested using the Wilcoxon signed-ranks test. Statistical analyses were performed using StatView 5.0 for Windows (SAS Institute, Cary, NC, USA).
This study was approved by the Ethics Committee of Sapporo Medical University on 18 June 2006. Written informed consent was obtained from all participants.
Results
Ejaculatory dysfunction
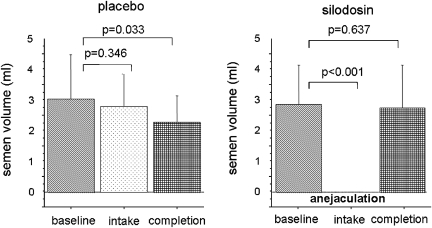
All participants on silodosin had a complete lack of seminal emission and expulsion of semen from the urethra (semen volume at baseline: 2. 9±1.3 ml, and after silodosin administration: 0 ml, P<0.001) (Figure 2). On the other hand, there was no significant change in ejaculation with placebo administration in any participant (semen volume at baseline: 3.0±1.5 ml, and after placebo administration: 2.8±1.1 ml, P=0.346). There were no sperms in urine after ejaculation under silodosin administration in any participant. Thus, the ejaculatory dysfunction caused by silodosin was a loss of seminal emission, not retrograde ejaculation. Three days after completion of silodosin, ejaculatory volume recovered to the baseline level (semen volume at the recovery: 2.7±1.4 ml vs that at the baseline: P=0.637). These results for ejaculatory dysfunction have been reported earlier.8
Figure 2.
The results of semen analysis. P-values were derived from statistical analysis using the Wilcoxon signed-ranks test.
Orgasm
All participants had sexual activity over the earlier month (Q1, Table 1). All participants who had silodosin felt discomfort on ejaculation, whereas none with the placebo did (Q2). In the questionnaire, participants gave details of orgasm if they felt discomfort during ejaculation (see Appendix). Therefore, only participants with silodosin answered the questions about satisfaction with ejaculatory discomfort, orgasm and the volume of semen (Q3–Q7).
Table 1.
Answers to the question about sexual activity and the felling of ejaculation with agent administration
| Q1. Did you have sexual activity over the past month? | ||
|---|---|---|
| Yes | No | |
| Placebo | 15 (100%) | 0 (0%) |
| Silodosin | 15 (100%) | 0 (0%) |
| Q2. Did you feel discomfort when you ejaculated during the sexual activity? | ||
| Yes | No | |
| Placebo | 0 (0%) | 15 (100%) |
| Silodosin | 15 (100%) | 0 (0%) |
All participants with silodosin were dissatisfied with the discomfort in terms of ejaculation, although the degree varied (Q3, Table 2). Although all participants with silodosin reported orgasm in spite of a complete lack of seminal emission and expulsion, 12 subjects (80%) had a somewhat unusual feeling during orgasm with ejaculatory dysfunction (Q4). However, the orgasm with some discomfort was not so big a problem during ejaculatory dysfunction (Q5). On the other hand, the complete loss of seminal volume (anejaculation) was judged to be a big problem by almost all participants (Q6 and Q7).
Table 2.
Answers to the questions about satisfaction with ejaculatory discomfort, orgasm and volume of semen with silodosin administration
| Q3. If you had to spend the rest of your life with your ejaculatory discomfort as it is now, how would you feel? | ||||||
| Very satisfied | Satisfied | Mostly satisfied | Neutral or mixed | Mostly dissatisfied | Dissatisfied | Very dissatisfied |
| 0 (0%) | 0 (0%) | 0 (0%) | 1 (6.7%) | 1 (6.7%) | 7 (46.7%) | 6 (40.0%) |
| Q4. Did you have a somewhat uncomfortable feeling during orgasm when you ejaculated? | ||||||
| Not at all | Slightly | Moderately | Very | |||
| 3 (20.0%) | 9 (60.0%) | 0 (0%) | 3 (20.0%) | |||
| Q5. How much has a feeling during orgasm with some discomfort been a problem for you? | ||||||
| No problem | Small problem | Moderate problem | Big problem | |||
| 3 (20.0%) | 6 (40.0%) | 4 (26.7%) | 2 (13.3%) | |||
| Q6. How is the volume of semen when you ejaculate? | ||||||
| Normal | Slightly decreased | Very decreased | None at all | |||
| 0 (0%) | 0 (0%) | 0 (0%) | 15 (100%) | |||
| Q7. How much has the volume of semen been a problem for you? | ||||||
| No problem | Small problem | Moderate problem | Big problem | |||
| 0 (0%) | 2 (13.3%) | 0 (0%) | 13 (86.7%) | |||
Discussion
Orgasm coincides with the moment of ejaculation in normal conditions and is the result of cerebral processing of pudendal nerve sensory stimuli. These stimuli are produced by increased pressure in the posterior urethra, sensory stimuli arising from the verumontanum and contraction of the urethral bulb and accessory sexual organs.17 Orgasm is enhanced by various events, such as the emission and ejaculatory processes.18 In this study, in spite of anejaculation, orgasm was preserved, although participants reported a slightly unusual feeling. When the orgasm with ejaculatory dysfunction induced by silodosin administration occurred, the increased pressure in the posterior urethra never happened because of the complete lack of seminal emission and expulsion. In addition, the sensory stimuli arising from the verumontanum did not occur because semen did not pass through the urethra. The complete lack of seminal emission suggested that the contraction of seminal vesicles was also suppressed. It is speculated that orgasm is induced by only the contraction of the urethral bulb, which may be maintained even under silodosin administration.
It is known that prepubertal children feel orgasm even without ejaculation. Orgasm without ejaculation is in fact named coitus reservatus.19 In addition, patients who have undergone radical prostatectomy can feel orgasm without seminal emission. When the post-radical prostatectomy patient feels orgasm, pudendal nerve sensory stimulation resulting from only smooth muscle contraction of the urethral bulb occurs. Therefore, it is not strange that orgasm can occur during ejaculatory dysfunction.
In this study, all participants were greatly dissatisfied with the ejaculatory dysfunction induced by the α1A-blocker. Participants reported only mild discomfort with the orgasm during ejaculatory dysfunction. However, the reduced amount of semen provoked a big problem. As it has been reported that a strong decline in ejaculatory volume is associated with reduced sexual pleasure,19 it is speculated that the semen volume, but not the feeling of orgasm, mainly affects the satisfaction of ejaculation by masturbation.
In this study, we used the term ‘anejaculation' to indicate a complete loss of ejaculation volume, as defined in earlier reports.6, 8 However, the terminology of ejaculatory dysfunction has not been standardized, as ejaculatory dysfunction with selective α1A-blockers has been noticed. We should create new terminology for ejaculatory dysfunction induced by highly selective α1A-blockers.
These are some limitations in this study. One of the problems was that we used an original questionnaire, which was not fully validated. The four-item version of Male Sexual Health Questionnaire (MSHQ-EjD Short Form) is a validated questionnaire for ejaculatory dysfunction in English.20 Although the MSHQ-EjD Short Form includes three ejaculatory function items and one ejaculation bother item, there is no item about the quality of orgasm. Moreover, the MSHQ has not been validated in Japanese yet. In this study, we wanted to determine what ejaculatory problems induced the most impaired quality of ejaculation, for example the amount of semen. In this context, there is no appropriate questionnaire to evaluate the quality of orgasm associated with ejaculatory dysfunction. This is the reason for not using the validated questionnaire to evaluate the quality of orgasm in this study. However, we should certainly validate the questionnaire for assessing the status of orgasm used in this study in the future.
Conclusions
Orgasm is preserved regardless of anejaculation with silodosin administration. Although most participants reported mild discomfort during orgasm, they were greatly dissatisfied with their ejaculatory status mainly because of the reduced amount of semen. These findings suggest that the emission of semen and its passage through the urethra are not necessary for orgasm.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the Gohtaro Sugawara-Memorial Research Found for Urological Disease. The authors thank all the volunteers for their extensive cooperation, which made this study clinically and scientifically relevant.
Appendix
Questionnaire about ejaculatory dysfunction sexual activity includes intercourse, caressing, foreplay and masturbation
Q1. Did you have sexual activity over the past month? (Yes/No)
*Please answer Q2 only if you answered ‘YES' to Q1 .
Q2. Did you feel discomfort when you ejaculated during the sexual activity? (Yes/No)
*Please answer the following question if you who answered ‘YES' to Q2.
Q3. If you had to spend the rest of your life with your ejaculatory discomfort as it is now, how would you feel?
(Very satisfied, Satisfied, Mostly satisfied, Neutral or mixed, Mostly dissatisfied, Dissatisfied, Very dissatisfied).
Q4. Did you have a somewhat uncomfortable feeling during orgasm when you ejaculated?
(Not at all, Slightly, Moderately, Very).
Q5. How much has a feeling during orgasm with some discomfort been a problem for you?
(No problem, Small problem, Moderate problem, Big problem).
Q6. How is the volume of semen when you ejaculate?
(Normal, Slightly decreased, Very decreased, None at all).
Q7. How much has the volume of semen been a problem for you?
(No problem, Small problem, Moderate problem, Big problem).
References
- Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, de la Rosette JJ. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines) Eur Urol. 2004;46:547–554. doi: 10.1016/j.eururo.2004.07.016. [DOI] [PubMed] [Google Scholar]
- AUA guideline on management of benign prostatic hyperplasia Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–547. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of α1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36:1–13. doi: 10.1159/000019919. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG, Schwinn DA. α1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol. 2004;171:1029–1035. doi: 10.1097/01.ju.0000097026.43866.cc. [DOI] [PubMed] [Google Scholar]
- Djavan B, Chapple C, Milani S, Marmerger M. State of the art on the efficacy and tolerability of α1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2004;64:1081–1088. doi: 10.1016/j.urology.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Hellstrom WJ, Sikka SC. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol. 2006;176:1529–1533. doi: 10.1016/j.juro.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hisasue S, Furuya R, Itoh N, Kobayashi K, Furuya S, Tsukamoto T. Ejaculatory disorder caused by α-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission. Int J Urol. 2006;13:1311–1316. doi: 10.1111/j.1442-2042.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Masumori N, Hisasue SI, Kato R, Hashimoto K, Itoh N, et al. Inhibition of seminal emission is the main cause of anejaculation induced by a new highly selective α1A-blocker in normal volunteers. J Sex Med. 2008;5:2185–2190. doi: 10.1111/j.1743-6109.2008.00779.x. [DOI] [PubMed] [Google Scholar]
- Zlotta AR, Teillac P, Raynaud JP, Schulman CC. Evaluation of male sexual function in patients with lower urinary tract symptoms (luts) associated with benign prostatic hyperplasia (BPH) treated with a phytotherapeutic agent (permixon), tamsulosin or finasteride. Eur Urol. 2005;48:269–276. doi: 10.1016/j.eururo.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Nagai A, Hara R, Yokoyama T, Jo Y, Fujii T, Miyagi Y. Ejaculatory dysfunction caused by the new α1-blocker silodosin: a preliminary study to analyze human ejaculation using color Doppler ultrasonography. Int J Urol. 2008;15:915–918. doi: 10.1111/j.1442-2042.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- McMahon CG, Abdo C, Incrocci L, Perelman M, Rowland D, Waldinger M, et al. Disorders of orgasm and ejaculation in men. J Sex Med. 2004;1:58–65. doi: 10.1111/j.1743-6109.2004.10109.x. [DOI] [PubMed] [Google Scholar]
- Lue TF, Giuliano F, Montorsi F, Rosen RC, Andersson KE, Althof S, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2004;1:6–23. doi: 10.1111/j.1743-6109.2004.10104.x. [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis K, Hatzichristou D. Sexual dysfunctions: classifications and definitions. J Sex Med. 2007;4:241–250. doi: 10.1111/j.1743-6109.2007.00409.x. [DOI] [PubMed] [Google Scholar]
- Choi JM, Nelson CJ, Stasi J, Mulhall JP. Orgasm associated incontinence (climacturia) following radical pelvic surgery: rates of occurrence and predictors. J Urol. 2007;177:2223–2226. doi: 10.1016/j.juro.2007.01.150. [DOI] [PubMed] [Google Scholar]
- Lee J, Hersey K, Lee CT, Fleshner N.Climacturia following radical prostatectomy: prevalence and risk factors J Urol 20061762562–2565.discussion 2565 [DOI] [PubMed] [Google Scholar]
- Kawabe K, Yoshida M, Homma Y. Silodosin, a new α1A-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006;98:1019–1024. doi: 10.1111/j.1464-410X.2006.06448.x. [DOI] [PubMed] [Google Scholar]
- McMahon CG, Abdo C, Incrocci L, Perelman M, Rowland D, Stuckey B, et al. Disorders of orgasm and ejaculation in men Sexual Medicine: Sexual Function in Men and Women 2004Health Publications: Paris; 409–468.In: Lue TF, Basson R, Rosen R, Giuliano F, Khoury S, Montorsi F (eds) [Google Scholar]
- Korenman SG.Sexual dysfunction Williams Textbook of Endocrinology 1992WB Saunders Company: Philadelphia; 1033–1048.In: Wilson JD, Foster DW (eds)8th edn. [Google Scholar]
- Jannini EA, Lenzi A. Ejaculatory disorders: epidemiology and current approaches to definition, classification and subtyping. World J Urol. 2005;23:68–75. doi: 10.1007/s00345-004-0486-9. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Catania JA, Althof SE, Pollack LM, O'Leary M, Seftel AD, et al. Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology. 2007;69:805–809. doi: 10.1016/j.urology.2007.02.036. [DOI] [PubMed] [Google Scholar]